Abstract
Aims: This study was conducted to analyze the clinical significance of ASAP1 in epithelial ovarian cancer (EOC). Methods: A total of 95 patients with EOC were included in the study. The expression profile of ASAP1 in 10 pairs of ovarian cancer and normal ovary tissues were detected by Real-time PCR. The expression level of ASAP1 in 95 paraffin-embedded EOC specimens was measured by immunohistochemistry staining. Statistical analysis was performed to evaluate the clinicopathologic significance of ASAP1. Results: Levels of ASAP1 mRNA were higher in EOC than in normal ovary tissues. Patients with higher ASAP1 expression had shorter overall (P=0.019) and recurrence-free (P=0.030) survival time, whereas those with lower ASAP1 expression survived longer. In addition, high expression of ASAP1 was correlated with poor overall (P=0.044) and recurrence-free (P=0.006) survival in patients with advanced carcinomas. Moreover, statistical analysis displayed a signifi cant correlation in ASAP1 expression with pelvic metastasis (P=0.015). Multivariate analysis revealed that an elevated ASAP1 expression was a significant independent prognostic factor for the overall (P=0.039) and recurrence-free (P=0.028) survival of EOC patients. Conclusion: These results indicated that elevated expression of ASAP1 plays an important role in the progression and metastasis of ovarian cancer, and that ASAP1 may be used as a biomarker in predicting patient outcome in EOC patients.
Keywords: ASAP1, prognosis, epithelial ovarian cancer
Introduction
Ovarian cancer is one of the most common cancers in women and the leading cause of death among gynecologic tumors [1]. There are more than 225,500 new cases of ovarian cancer diagnosed worldwide and that nearly 140,200 women expected to die of this disease [2]. Over the past decades, significant advances have been made in both surgical techniques and chemotherapeutic treatments, while there has been very little change in prognosis for ovarian cancer [3]. Most patients have advanced tumor disease at the time of diagnosis, due to lacking of specific symptoms and effective methods for the early detection [4]. Tumor dissemination and metastasis of advanced stage tumors are directly linked to patient’s survival and account for the major causes of cancer deaths [5]. Thus, early identifi cation of high-risk patients is crucial for diagnosis and understanding the biological processes in ovarian carcinoma.
ASAP1, also known as ANK repeat and PH domain-containing protein 1, was originally identified as a phosphatidylinositol 4,5-bisphosphate-dependent Arf GTPase-activating protein (Arf GAP) specifi c for class 1 and 2 Arfs [6,7]. ASAP1 is located at 8q24, consists of 30 exons with a calculated molecular mass of 125 kDa. Two major splice variants of ASAP1 have been identified (ASAP1a and ASAP1b). They differ by the 57 amino acids within the proline-rich region [6]. ASAP1 functions as a GAP and as a scaffold to regulate focal adhesions (FAs) and invadopodia [8,9]. ASAP1 has also been found to serves as a scaffold to bring together the proteins necessary for transport to primary cilia [10]. Besides, it has also been demonstrated that ASAP1 could be recruited to the cell periphery by Arf1 therein, suggesting its possible role in membrane traffi cking [11]. Recently, ASAP1 has been implicated in regulating cell motility and tumor invasion. Onodera et al. reported that the expression of ASAP1 has been linked with breast cancer invasion [12]. Similarly, Ehlers et al. proposed that ASAP1 expression level was correlated with the invasive potential of uveal melanoma [13]. Nevertheless, the role of ASAP1 in ovarian carcinoma development and progression has not been elucidated.
In the present study, we found that ASAP1 was markedly up-regulated in surgical specimens of epithelial ovarian cancer (EOC). We also statistically analyzed the association between ASAP1 expression and clinical prognosis, and found that high level of ASAP1 was strongly associated with poor survival in EOC patients, especially in patients with advanced stage (FIGO stage III-IV) disease. Multivariate analysis reveals that ASAP1 expression level is an independent prognostic factor for the outcome of EOC patients. Taken together, our results suggest that ASAP1 plays an important role and could be used as a prognostic indicator in human epithelial ovarian carcinoma.
Materials and methods
Patients and tissue specimens
Ten pairs of EOC tissue samples and normal ovary specimens were obtained from patients who underwent surgical resection at Sun Yat-Sen University Cancer Center during 2011. All excised samples were obtained within 30 min after operation. They were then transferred into liquid nitrogen until further experiments. In addition, immunohistochemistry study was conducted on a total of 95 paraffi n-embedded EOC samples, which were histologically and clinically diagnosed at Sun Yat-Sen University Cancer Center between 2004 and 2007. None of them had received chemotherapy or radiation therapy before surgery. Clinical information of the 95 EOC samples was described in detail in Table 1. The disease stages of all the patients were classifi ed according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines for clinical staging [14]: 22 were allocated to stage I, 17 to stage II, 49 to stage III, and 7 to stage IV. The median age of the patients was 51.3 years (range, 22-75 years). In order to use these clinical materials for research purposes, informed consent from patients and approval from the Institute Research Ethics Committee was obtained.
Table 1.
Expression of ASAP1 expression in EOC patients according to clinicopathologic characteristics
| Characteristics | NO. | ASAP1 | p | |
|---|---|---|---|---|
|
| ||||
| Positive N (%) | Negative N (%) | |||
| Age (y) | 0.750 | |||
| ≤50 | 39 | 21 (53.8) | 18 (46.2) | |
| >50 | 56 | 32 (57.1) | 24 (42.9) | |
| FIGO Stage | 0.346 | |||
| I-II | 39 | 24 (37.8) | 15 (62.2) | |
| III-IV | 56 | 29 (67.2) | 27 (32.8) | |
| Differentiation | 0.191 | |||
| Grade 1/2 | 61 | 31 (50.8) | 30 (49.2) | |
| Grade 3 | 34 | 22 (64.7) | 12 (35.3) | |
| Pelvic Metastasis | 0.015 | |||
| - | 78 | 39 (50.0) | 39 (50.0) | |
| + | 17 | 14 (82.4) | 3 (17.6) | |
RNA extraction and Real-time PCR
Total RNAs from fresh ovarian cancer and normal ovary tissues were extracted using the Trizol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instruction. The RNAs were pretreated with RNAase-free DNase and 1 μg RNA from each sample was used for cDNA synthesis. Real-time PCR was then employed to determine the fold change of ASAP1 mRNA in the tissues, and GAPDH was used as an internal control. The primers selected were as the following: ASAP1, forward 5’-TGACTAGCAAAACGCAGAACC-3’, reverse 5’-ACACACATTATATCCCCCTCC-3’. GAPDH, forward 5’-GACTCATGACCACAGTCCATGC-3’; reverse, 5’-AGAGGCAGGGATGATGTTCTG-3’.
Immunohistochemistry
Resected EOC samples were longitudinally sliced into 4 μm sections, fi xed in 4% paraformaldehyde and embedded in paraffi n. The sections were then deparaffinized with xylene, rehydrated, and treated with 3% hydrogen peroxide to quench the endogenous peroxidase activity. Subsequent antigen retrieval was performed by heating in citrate buffer solution using a microwave oven. Non-specific binding was blocked by treating the slides with 1% fish skin gelatin, followed by incubation of the sections with a mouse monoclonal anti-ASAP1 antibody (Abcam, USA; 1:100). After washing, the slides were treated with biotinylated anti-mouse secondary antibody (Dako, Denmark; 1:200), followed by a further incubation with 3, 3-diaminobenzidine tetrahydrochloride (DAB). Finally, the sample sections were counterstained with 10% Mayer’s hematoxylin and mounted in Crystal Mount.
The degree of immunostaining of formalin-fixed, paraffin-embedded sections was reviewed and scored by two independent observers. The proportion of the stained cells and the extent of the staining were used as criteria of evaluation. For each sample, the intensity of the staining was graded into 4 levels: 0, negative staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown), and 3, strong staining (brown); and the percentage of stained cells was scored as: 0, 5% of the cells or less; 1, 5 to 10%; 2, 11% to 50%; 3, 51% to 80%; or 4, 80% or more positive cells. A final score was defined by multiplying the percentage of positive cells by the intensity. The final score was defi ned as low expression for score 0-4 and high expression for scores of 6-12.
Statistical analysis
All statistical analyses were carried out using the SPSS 15.0 statistical software package. Chi-square test was used to analyze the relationship between ASAP1 expression and clinicopathologic characteristics in archival samples from EOC patients. Survival curves were plotted by Kaplan-Meier method and compared by log-rank test. Survival data were evaluated using multivariate Cox regression analysis. P<0.05 in all cases was considered statistically significant.
Results
Overexpression of ASAP1 in ovarian cancer tissues
To examine the expression of ASAP1 in EOC tissues, Real-time PCR was performed. The expression level of ASAP1 in 10 EOC fresh tissues was compared to that of normal ovary tissues. In all the 10 human EOC samples, ASAP1 expression was significantly increased compared with the paired normal ovary tissues (Figure 1). The tumor/normal (T/N) ratio of ASAP1 message signals varied from approximately 3.3-to 10.7-fold in 10 paired specimens. Thus, ASAP1 is overexpressed in the examined EOC samples.
Figure 1.
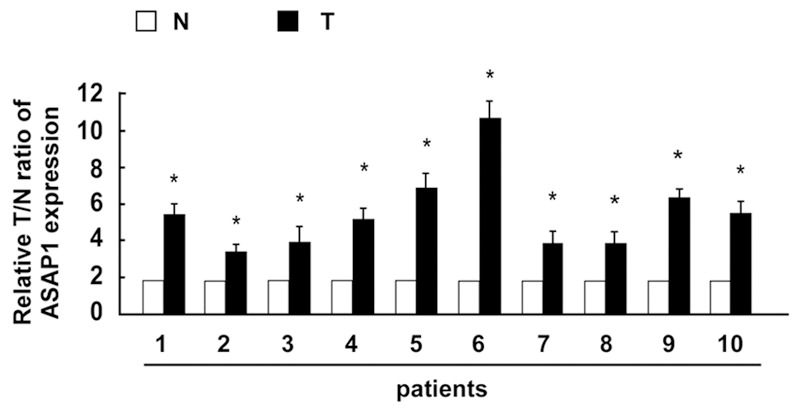
Real time-PCR analysis of ASAP1expression in each of the primary cervical cancer and paired normal ovary tissues. asterisks, P<0.05 (Student t test).
Correlation between ASAP1 expression and clinical features of EOC
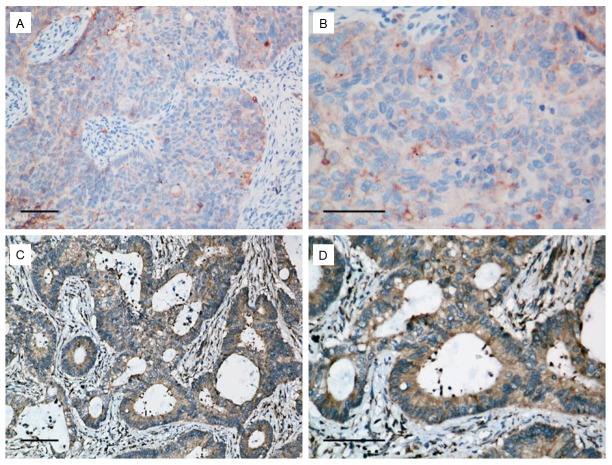
To investigate the potential roles of ASAP1 in the development and progression of EOC, immunohistochemistry was performed to measure ASAP1 expression in 95 paraffin-embedded EOC tissues. The representative immunostaining of ASAP1 in EOC was shown in Figure 2A-D. Besides, the correlations between ASAP1 expression and the patients’ clinicopathologic characteristics were examined. As presented in Table 1, ASAP1 expression was strongly correlated with pelvic metastasis (P=0.015). There was no significant relationship between ASAP1 expression and patient age, tumor stage, or differentiation. These observations suggested a correlation between increased ASAP1 expression and clinical development in ovarian cancer.
Figure 2.
Representative immunostaining of ASAP1 in ovarian cancer specimens (A and B: Low expression; C and D: High expression). Scale bar, 100 μm.
Correlation between ASAP1 expression and clinical outcomes in EOC patients
We further analyzed the association of the expression of ASAP1 with overall and recurrence-free survival in EOC patients. The results showed that the high ASAP1 expression group was associated with decreased overall (Figure 3A, P=0.019) and recurrence-free survival (Figure 3B, P=0.030) compared with the low ASAP1 group. Moreover, patients with tumors exhibiting high ASAP1 expression had significantly lowered overall (Figure 3C, P=0.044) and recurrence-free (Figure 3D, P=0.006) survival rates compared with those with low level expression of ASAP1 in the advanced disease group. Furthermore, multivariate analysis revealed that ASAP1 expression was an independent prognostic factor of patient overall and recurrence-free survival (Table 2).
Figure 3.
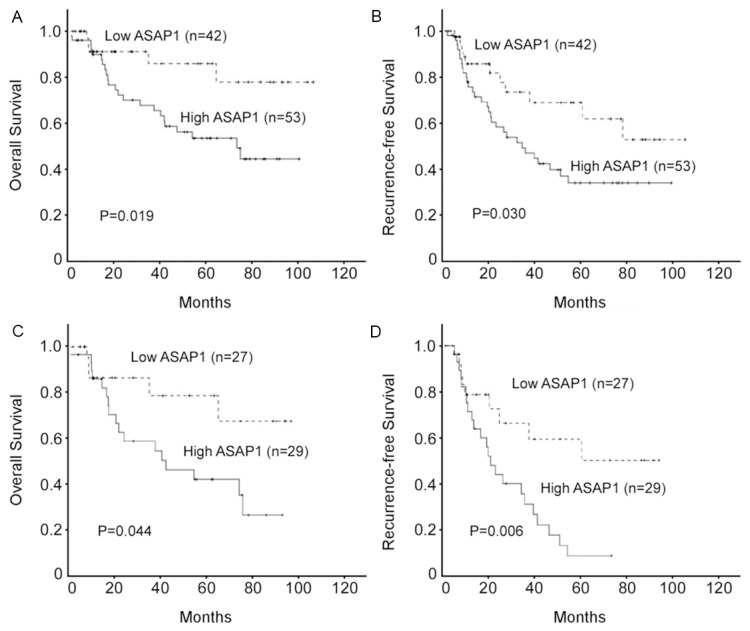
Kaplan-Meier analysis of overall survival and recurrence-free survival in relation to ASAP1 expression in 95 epithelial ovarian cancer (EOC) patients (A and B), and in 56 patients with FIGO stage III-IV EOC (C and D).
Table 2.
Multivariate Cox regression analysis of overall survival (OS) and Recurrence-free survival (RFS) in patients with EOC
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age (>50 vs ≤50) | 1.166 (0.484-2.807) | 0.732 | 1.311 (0.632-2.718) | 0.467 |
| FIGO Stage (III-IV vs I-II) | 3.236 (1.289-8.122) | 0.012 | 4.017 (1.828-8.829) | 0.001 |
| Differentiation (Grade 3 vs 1/2) | 1.078 (0.469-2.477) | 0.860 | 1.012 (0.500-2.048) | 0.973 |
| Pelvic Metastasis (+ vs -) | 2.201 (0.815-5.945) | 0.120 | 1.946 (0.778-4.868) | 0.154 |
| ASAP1 (+ vs -) | 3.224 (1.064-9.770) | 0.039 | 2.363 (1.098-5.085) | 0.028 |
Discussion
To our knowledge, this is the fi rst study revealing that ASAP1 is highly expressed in ovarian cancer and is closely correlated with clinical prognosis in patients with ovarian cancer. Statistical analysis showed that patients with higher levels of ASAP1 had poorer overall and recurrence-free survival, whereas patients with lower levels of ASAP1 had better survival. Collectively, our results indicate that ASAP1 is associated with ovarian cancer development and may represent an independent prognostic factor for the outcome in patients with epithelial ovarian cancer.
ASAP1 is a phospholipid-dependent Arf GAP that binds to c-Src tyrosine kinase and focal adhesion related kinase. As a multifunctional scaffold protein, ASAP1 has been shown to be involved in membrane-cytoskeleton interactions that affect membrane movements, cell appearance and motility [10,15]. ASAP1 has been found in perinuclear region, membrane ruffle, and focal adhesion [16], and is probably involved in exocytic membrane trafficking from the trans-Golgi network [17]. The BAR domain of ASAP1 mediates the formation of membrane tubular structures and dimerization of ASAP1, and functions as an inhibitor of its GAP activity [18]. Biochemically, ASAP1 has been shown to display GAP activity specifically toward the Arfs, a group of small GTP-binding proteins conserved throughout evolution of eukaryotic organisms [19]. ASAP1 enhances cell motility through deactivation of Arf1 [20], while siRNA-mediated knockdown of ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling [16]. Consistently, phosphorylation and inhibition of ASAP1 by proline-rich tyrosine kinase 2 (Pyk2) alters the Arf1 activity [21]. In addition, ASAP1 has been proposed to play an important role in regulating Arf4 mediated ciliary receptor targeting [10].
Recent studies have described that ASAP1 is associated with tumour development and invasion. The copy numbers of ASAP1 gene has been found to be increased in prostate cancer tissues compared with benign prostate tissue, and ASAP1 expression was correlated with the metastatic activity of prostate cancer [22]. Similarly, Onodera et al. reported that elevated expression of ASAP1 was involved in the invasive activities of breast cancer [12]. Using loss- and gain-of-function methods, Muller et al. found that ASAP1 stimulates colorectal cancer metastasis in vivo and promotes tumor cell motility and invasiveness in vitro [23]. In contrast, Liu et al. shown that reduction of ASAP1 by mislocalization or siRNA knockdown retards cell spreading and inhibits cell migration [16]. These studies indicated that ASAP1 plays crucial roles in the development and metastatic process in several malignancies. In line with these studies, we found that ASAP1 was significantly increased in EOC specimen compared with normal ovary.
To further investigate the biological roles of ASAP1 in ovarian cancer, we analyzed the correlation between ASAP1 expression and prognosis in EOC patients. We found that high level of ASAP1 was indicative of poor clinical survival in patients with EOC, especially in patients with advanced stage of this disease. Multivariate analyses unraveled that ASAP1 expression was regarded as an independent prognostic factor for EOC patient overall and recurrence-free survival. Moreover, ASAP1 expression was strongly correlated with pelvic metastasis, consistent with ASAP1-dependent regulation of cell adhesive structures. Taken together, our data not only imply a potentially promising application of ASAP1 as a valuable prognostic marker, but suggest a possible relationship between the molecular functions of ASAP1 and the carcinogenesis of ovarian cancer. However, further studies are needed to elucidate the mechanism underlying ASAP1 in the development and metastatic process of ovarian cancer, and to clarify whether ASAP1 could be used as a novel therapeutic target for ovarian cancer.
During the past years, several signalling pathways have been shown to be involved in ASAP1-mediated tumor invasion. ASAP1 overexpression has been reported to elevate the recycling-back of internalized EGFRs [24], which is frequently correlated with the progression of ovarian carcinoma [25]. Overexpression of ASAP1 has also been shown to enhance the cell motility and migration in response to PDGF-mediated signaling [16]. Moreover, it has been described that ASAP1 contains an SH3 domain and proline rich SH3-binding motifs, which allow it to bind specifi cally and strongly to the Src family members c-Src [6,26], indicating a close relationship of ASAP1 and Src signaling pathway. This is supported by Bharti and colleagues, who suggested that Src-dependent phosphorylation of ASAP1 leads to the formation of podosomes [27]. In our previous study, we found that phospho-Src expression was associated with the prognosis in ovarian cancer patients [28], which, together with our present findings, further suggests the potential roles of ASAP1 in integrating signals from Src tyrosine kinase signaling pathway.
In conclusion, this is the fi rst study highlighting the clinical signifi cance of ASAP1 in ovarian carcinoma. ASAP1 expression is associated with tumor progression and pelvic metastasis of ovarian cancer. Additionally, ASAP1 might be used as a valuable prognostic marker of poor overall and recurrence-free survival in EOC patients. However, further studies are needed to clarify the mechanism by which ASAP1 is involved in the development and metastasis of ovarian cancer.
Acknowledgements
This work was supported by grant from the Guangdong Medical Science Foudation (2012B031800385).
Disclosure of conflict of interest
None.
References
- 1.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Park NH, Chung HH, Kim JW, Song YS, Kang SB. Significance of preoperative serum CA-125 levels in the prediction of lymph node metastasis in epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1136–1142. doi: 10.1080/00016340802478158. [DOI] [PubMed] [Google Scholar]
- 6.Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Loijens JC, Martin KH, Karginov AV, Parsons JT. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda A, Wada I, Miura K, Okawa K, Kadoya T, Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, Nishitani C, Matsuno K, Ishino M, Machesky LM, Fujita H, Randazzo P. CrkL directs ASAP1 to peripheral focal adhesions. J Biol Chem. 2003;278:6456–6460. doi: 10.1074/jbc.M210817200. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31:4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo R, Jacques K, Ahvazi B, Stauffer S, Premont RT, Randazzo PA. Mutational analysis of the Arf1*GTP/Arf GAP interface reveals an Arf1 mutant that selectively affects the Arf GAP ASAP1. Curr Biol. 2005;15:2164–2169. doi: 10.1016/j.cub.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, Sabe H. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Worley L, Onken MD, Harbour JW. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin Cancer Res. 2005;11:3609–3613. doi: 10.1158/1078-0432.CCR-04-1941. [DOI] [PubMed] [Google Scholar]
- 14.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Shiba Y, Randazzo PA. GEFH1 binds ASAP1 and regulates podosome formation. Biochem Biophys Res Commun. 2011;406:574–579. doi: 10.1016/j.bbrc.2011.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Yerushalmi GM, Grigera PR, Parsons JT. Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J Biol Chem. 2005;280:8884–8892. doi: 10.1074/jbc.M412200200. [DOI] [PubMed] [Google Scholar]
- 17.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Kelly WG, Logsdon JM Jr, Schurko AM, Harfe BD, Hill-Harfe KL, Kahn RA. Functional genomic analysis of the ADP-ribosylation factor family of GTPases: phylogeny among diverse eukaryotes and function in C. elegans. FASEB J. 2004;18:1834–1850. doi: 10.1096/fj.04-2273com. [DOI] [PubMed] [Google Scholar]
- 20.Furman C, Short SM, Subramanian RR, Zetter BR, Roberts TM. DEF-1/ASAP1 is a GTPase-activating protein (GAP) for ARF1 that enhances cell motility through a GAP-dependent mechanism. J Biol Chem. 2002;277:7962–7969. doi: 10.1074/jbc.M109149200. [DOI] [PubMed] [Google Scholar]
- 21.Kruljac-Letunic A, Moelleken J, Kallin A, Wieland F, Blaukat A. The tyrosine kinase Pyk2 regulates Arf1 activity by phosphorylation and inhibition of the Arf-GTPase-activating protein ASAP1. J Biol Chem. 2003;278:29560–29570. doi: 10.1074/jbc.M302278200. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Watahiki A, Bayani J, Zhang F, Liu L, Ling V, Sadar MD, English J, Fazli L, So A, Gout PW, Gleave M, Squire JA, Wang YZ. ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res. 2008;68:4352–4359. doi: 10.1158/0008-5472.CAN-07-5237. [DOI] [PubMed] [Google Scholar]
- 23.Muller T, Stein U, Poletti A, Garzia L, Rothley M, Plaumann D, Thiele W, Bauer M, Galasso A, Schlag P, Pankratz M, Zollo M, Sleeman JP. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene. 2010;29:2393–2403. doi: 10.1038/onc.2010.6. [DOI] [PubMed] [Google Scholar]
- 24.Kowanetz K, Husnjak K, Holler D, Kowanetz M, Soubeyran P, Hirsch D, Schmidt MH, Pavelic K, De Camilli P, Randazzo PA, Dikic I. CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol Biol Cell. 2004;15:3155–3166. doi: 10.1091/mbc.E03-09-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron AT, Wilken JA, Haggstrom DE, Goodrich ST, Maihle NJ. Clinical implementation of soluble EGFR (sEGFR) as a theragnostic serum biomarker of breast, lung and ovarian cancer. IDrugs. 2009;12:302–308. [PubMed] [Google Scholar]
- 26.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 27.Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang YW, Chen C, Xu MM, Li JD, Xiao J, Zhu XF. Expression of c-Src and phospho-Src in epithelial ovarian carcinoma. Mol Cell Biochem. 2013;376:73–79. doi: 10.1007/s11010-012-1550-1. [DOI] [PubMed] [Google Scholar]