Abstract
Integrins are cell surface adhesion molecules (CAM) that regulate via intercellular and cell-matrix signaling various cellular processes including wound healing, cell differentiation, division, growth, migration and metastatic dissemination. Although a correlation between carcinogenesis and changes in integrin expression, especially β1 integrin, has been reported, its role in colorectal liver metastases remains unclear. This study aimed to evaluate the expression of β1 integrin in colorectal liver metastases and to correlate the pattern of expression with clinicopathological features and to investigate the putative role of β1 integrin expression on survival of these patients. Methods: Formalin-fixed, paraffin-embedded (FFPE) tumor samples of 81 patients who were operated because of colorectal liver metastases without any neoadjuvant therapy were obtained and stained with hematoxylin and eosin (H & E). An immunohistochemical examination was performed using Dako, Peroxidase/DAB kit and a primary monoclonal β1 integrin (CD29, fibronectin receptor subunit beta; ab3167, Abcam plc). β1 integrin expression was evaluated according to the immunoreactive score of Remmele and Stegner and was related with clinicopathological features of prognostic significance and with disease-free and overall survival as well. Statistical analysis was performed using SPSS version 21.0. Results: β1 integrin was overexpressed in tumor cells in 37 (48%) patients and in stromal cell in 27 (33%) patients. The β1 expression was not statistically correlated with clinicopathological features of the primary tumors but it was statistically correlated (p=0.03) with the histological grading of liver metastases. Kaplan-Meier survival analysis showed that there is a tendency but no statistically significant correlation in disease-free and overall survival. Conclusion: Considering that expression of β1 integrin in colorectal liver metastases remains controversial, specially its relation with survival of patients, we showed that the β1 expression represents a reliable prognostic factor regarding the grading of liver metastases of CRC and our findings imply that β1 integrin expression profiles may have further potential in identifying the stage of colorectal liver metastases and being a marker of prognosis in these patients.
Keywords: Colorectal liver metastases, beta1, β1 integrin, expression, prognosis
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer incidence and cancer-associated mortality in both males and females in Western society [1-3]. The prognosis of CRC patients is mainly determined by the metastatic spread of the tumor [1,4]. Thus, understanding the mechanisms that contribute to metastasis is of fundamental importance for designing better therapeutic strategies for treating this disease.
The liver is the most important and common metastatic site of CRC [2,5]. It is a unique feature, since the sinusoidal endothelial layer is characterised by an incomplete cover of micro-vessel structures, which leaves extracellular matrix (ECM) components directly accessible to circulating cells [6,7]. Indeed, the metastatic cascade is a dynamic process consisting of a series of sequentially linked, interrelated steps [8,9]. During these steps, tumor cells progress from cell-cell interactions to cell-ECM interactions mainly involving cell surface adhesion molecules (CAM), including integrins, selectins, immunoglobulins, cadherins and CD44 [10,11]. These interactions appear to be crucial for the formation of hepatic metastases [12].
Amongst CAM, integrins are a versatile family which consists of heterodimer cell surface receptors composed of α and β transmem-brane subunits; each of them includes a large extracellular, transmembrane and short cytoplasmic domain [13]. In mammals, 19 α and 8 β subunits combine with each other to form a family of 25 cell adhesion molecules, however splice variants have been identified for some subunits [14,15]. Their ligands include components of the extracellular matrix such as fibronectin, vitronectin, collagen, laminin and IgSFCAMs [14,16,17]. Functionally integrins contribute to intercellular adhesion and contact, anchorage-dependent cell survival and regulate via outside-in and inside-out signalling various cellular processes including wound healing, cell differentiation, division, growth and migration [18,19].
Integrins expressed by tumor cells and host cells can directly contribute to the control and progress of metastatic dissemination. During tumor development, changes in integrin expression, intracellular control of integrin functions and signals perceived from integrin ligand binding influence the ability of tumor cells to interact with their environment. This enables metastatic cells to convert from a sessile, stationary to a migratory and invasive phenotype [20]. Therefore integrin expression can profoundly influence formation of metastasis [21-23]. Alterations of integrin expression and their receptors have been observed in various cancers including colorectal cancer [23,24]. However, the mechanisms by which integrins participate in the steps of the metastasis formation in vivo are only partially understood [20].
According to their β subunits, integrins are divided into four subfamilies and integrins with β1-subunits are called β1 integrins. The β1 integrin subfamily (or Very Late Antigens, VLA) is characterised by a β1 subunit associated with at least nine a subunits (termed a1-a9, CD49a-i) constituting the largest subfamily of the integrins [25,26] and can bind to collagen, laminin and fibronectin [26]. β1 integrin is the mainly expressed integrin in normal and tumor cells and controls developmental processes including angiogenesis, tumor progression and metastasis [27]. This integrin typically mediates adhesion of epithelial cells to the basement membrane and may also contribute to cell survival of tumor cells by interacting with others molecules. For example, it usually activates cytokine receptors or growth factors receptors [28]. Based on these observations, β1 integrin has become a target of interest for immunotherapy in several types of cancers, including breast cancer [29-34], gastric cancer [35], lung ad enocarcinoma [36-38], glioblastoma [39], prostate cancer [40], pancreatic adenocarcinoma [41] and CRC [42-45]. Immunohis-tochemical studies of expression of β1 integrin in CRC have shown that the expression of this integrin has considerable heterogeneity within the primary tumor [42-45] and is correlated with cellular differentiation [46], indicating that alteration of expression of β1 integrin in colorectal cancer might contribute to an alteration in adhesiveness and invasiveness of cancer cells. But there are no studies which investigate the prognostic value of the expression of β1 integrin within liver metastases of CRC.
The aim of this study is to assess the immunohistochemical β1 integrin expression in colorectal liver metastases and its relationship with clinicopathological features of prognostic significance. Our additional hypothesis is that the enhanced expression of β1 integrin in liver metastases could affect the progress of metastasis and may therefore be of prognostic relevance influencing disease-free and overall survival.
Material and methods
Patients
Our study included patients with colorectal liver metastases who underwent curative hepatic resection for the first event of the metastases from 1995 to 2010 in the Department of Surgery at the University Hospital Erlangen. Patients upon whom a neoadjuvant therapy of the primary tumor or of the liver metastases was performed, patients with synchronous metastases in other organs or patients who died postoperatively were excluded. The clinical and histopathological data of the patients were retrieved from medical files of our cancer registration system and included age, gender, clinicopathological parameters of primary tumor and liver metastases such as location, differentiation, tumor size, regional lymph nodes, venous and lymphatic invasion and stage of the primary tumor, time interval between primary and metastatic tumor and location, number, diameter and differentiation’s grade of the liver metastases. Tumors were classified at the institute of pathology according to the 7th TNM cancer staging system of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) [47] and were graded as well, moderately or poorly differentiated depending on their behavior according to the recommendations of the World Health Organization (WHO) [48]. Disease-free survival and overall survival were defined as time from the first diagnosis of liver metastasis to local recurrence in the liver and to cancer-related death, respectively.
The subsequent 81 patients of our study comprised of 45 males (56%) and 36 females (44%) with a median age of 62 years (mean: 62, range 43-81 years) at the diagnosis of liver metastases. The clinicopathological characteristics of the primary tumors are illustrated in Table 1A. The liver metastases occurred synchronously in 18 cases (22%) and metachronously in 63 cases (78%) with a median time interval of 19 months (range 4-163 months). The characteristics of the hepatic metastases and the type of the performed hepatic resection are demonstrated in Table 1B. The mean follow-up for all patients was 65 months (median follow-up: 55 months), with a range between 1 and 188 months. During the follow-up, 20 patients (25%) received adjuvant chemotherapy. Twenty-seven patients (33.3%) developed metachronous recurrent hepatic metastases, 10 of them in other organs as well (peritoneum, lymph nodes, lung, pleura, brain). These events occurred in a median period of 16 months (range, 4-60) after the curative resection (R0) of the first event of liver metastases.
Table 1A.
Characteristics of the primary colorectal tumors (n=81)
| characteristics | n | % |
|---|---|---|
| location | ||
| colon | 43 | 53 |
| rectum | 38 | 47 |
| differentiation | ||
| well | 1 | 1 |
| moderately | 68 | 84 |
| poorly | 12 | 15 |
| pT category | ||
| T1 | 2 | 2.5 |
| T2 | 14 | 17.5 |
| T3 | 58 | 71.5 |
| T4 | 7 | 8.5 |
| pN category | ||
| N0 | 42 | 52 |
| N1 | 22 | 27 |
| N2 | 17 | 21 |
| venous invasion | ||
| V0 | 57 | 70.5 |
| V1 | 10 | 12.5 |
| lymphatic invasion | ||
| L0 | 26 | 32 |
| L1 | 41 | 50.5 |
| stage | ||
| I | 10 | 12.5 |
| II | 23 | 28.5 |
| III | 30 | 37 |
| IV | 18 | 22 |
Table 1B.
Characteristics of hepatic metastases (n=81)
| characteristics | ||
|---|---|---|
|
| ||
| n | % | |
| liver metastasis in relation to the primary tumor | ||
| synchronous | 18 | 22 |
| metachronous | 63 | 78 |
| median time internal: 19 months (range, 4-163) | ||
| distribution of liver metastases | ||
| unilobular | 66 | 81.5 |
| bilobar | 15 | 18.5 |
| number of liver metastases | ||
| 1 | 54 | 67 |
| 2 | 17 | 21 |
| 3 | 4 | 5 |
| >3 | 6 | 7 |
| type of hepatic resection | ||
| right lobectomy | 23 | 28 |
| extended right lobectomy | 3 | 4 |
| left lobectomy | 9 | 11 |
| trisegmentectomy | 3 | 4 |
| bisegmentectomy | 11 | 14 |
| non-anatomic bisegmentectomy | 7 | 8 |
| segmentectomy | 15 | 18 |
| non-anatomic segmentectomy | 7 | 9 |
| mesohepatectomy | 3 | 4 |
| differentiation grade | ||
| moderate | 70 | 87 |
| poor | 9 | 11 |
| unknown | 2 | 2 |
| hepatic tumor size | mm | |
| median | 47 | |
| range | 6-270 | |
Tumor samples and immunochemistry
Tumor blocks (samples) were taken from the diagnostic archives of the Institute of Pathology of the University Hospital Erlangen and the use of materials was positively stated by the local Ethical Committee. Tumor samples were previously fixed in 4% buffered formalin and embedded in paraffin (FFPE). One section of FFPE tissue of each sample was stained with hematoxylin and eosin to confirm the presence of the tumor by light microscopy. All stained slides were reviewed by an experienced pathologist. Sections (4 μm) were obtained from the paraffin blocks and immunohistochemical reactions were performed with the Dako REALTM EnVisionTM Detection System, Peroxidase/DAB kit (Dako Denmark A/S, Denmark). The kit was employed in a two-step procedure. The first step was incubation of the tissue with an optimally diluted primary antibody and the second step was incubation with Dako REALTM EnVisionTM/HRP reagent of the kit. This reagent was a peroxidase-conjugated polymer, which also carries antibodies to immunoglobulins. The reaction was visualized by Dako REALTM DAB+ Chromogen. Primary monoclonal β1 integrin (CD29, fibronectin receptor subunit beta) antibody [4Β7R] (ab3167) was obtained from Abcam plc, Cambridge, UK (dilution 1:35). Negative controls were prepared omitting the primary antibody and using non-immune IgG instead of primary antibodies showed no staining.
Immunohistochemical assessment
Tumorous immunoreactivity was graded depending on the percentage of cancer cells stained: Score 0 means negative; score 1 means 1 to 10% of tumor cells positive; score 2 means 11 to 50% of tumor cells positive; score 3 means 51-80% of tumor cells positive and score 4, >80% of tumor cells positive within the tumors. The immunostaining intensity was evaluated by light microscopy and scored on the following scale: negative (0), weak (1), moderate (2) and strong (3). We took into account both the proportion of positive cells and the intensity to give a semiquantitative estimate levels of antigen in the tumor and in the neighboring stroma. To identify the integrin’s expression, a combination factor of immunoreactivity and intensity according to the Remmele-Scoring system was performed [49]. The theoretical limits of this immunoreactive score range from 0 (0% of cells stained) to 12 (>80% of the cells stained at 3+ intensity). The interpretation was negativity from 0 to 2 and positivity from 3 to 12 according to the Remmele-score recommendations [50]. Evalu-ation was performed independently and blinded by two of the authors.
Statistical analysis
Data analysis was performed with SPSS v21.0 (SPSS Inc., Woking, UK). Relation between β1 integrin and the clinicopathologic features were analysed by Fisher exact test or Chi-square tests and all tests were two-tailed. Univariate survival analysis concerning disease-free survival and overall- survival were made with the log rank test [50]. All results were displayed in the Kaplan-Meier performance [51]. The Cox regression [52] has been used to model the effect of various factors on survival and to observe the independent prognostic value of immunoexpression of β1 integrin. P-values <0.05 were considered to be statistically significant.
Results
Staining pattern of β1 integrin in colorectal liver metastases
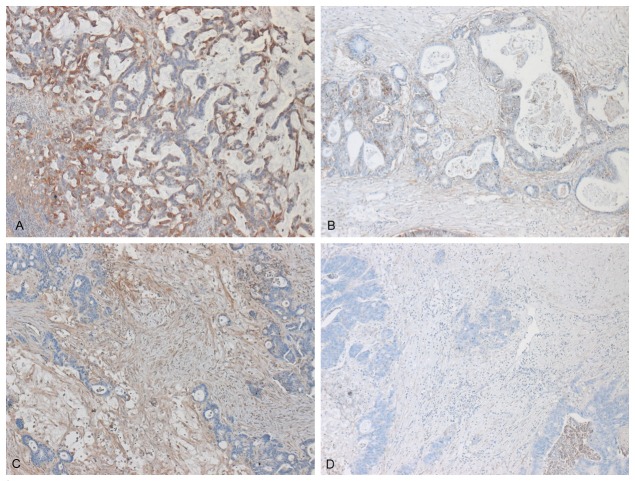
β1 integrin expression was observed throughout the colorectal hepatic metastases. An example of strong immunoreactivity of β1 expression in tumor cells is shown in Figure 1A and, in contrast, a tumor sample negative for β1 expression in Figure 1B. Within metastases β1 integrin was expressed by tumor stroma as well but in less amounts compared to the tumor cells. Samples positive and negative for β1 expression in stromal cells are presented in Figure 1C, 1D, correspondingly. Specifically, expression of the β1 integrin was increased in tumors in 37 (48%) patients, 24 of whom had a negative expression in stroma, whereas the β1 integrin was increasingly expressed in 27 patients (33%), 14 of whom had a negative expression in tumor.
Figure 1.
Immunohistochemical presentation of the expression of β1 integrin in tumor cells of colorectal liver metastases, poorly differentiated, with positive (A) and negative immunoreactive score (B) and in stromal cells of moderately differentiated metastases with positive (C) and negative (D) immunoreactive score.
Correlation of β1 integrin expression in liver metastases with clinicopathological features of the primary colorectal cancer
The expression of β1 integrin was related to clinicopathological background of the primary CRC. There was no significant relation between the expression of β1 integrin in liver metastases with patient’s sex or age, location and histopathological features of the primary tumors. These results are shown in Table 2. The β1 integrin’s expression in liver metastases stroma was also not statistically significant correlated with the clinicopathological features of the primary tumors (Table 3).
Table 2.
Correlation of β1 expression in tumor cells of liver metastases with clinicopathological findings of primary tumors
| Characteristics | Patients with positive (≥3) Remmele score (n=37) (%) | Patients with negative (0-2) Remmele score (n=44) (%) | p-value |
|---|---|---|---|
| sex | |||
| male | 19 (51.5) | 26 (59.0) | 0.485 |
| female | 18 (48.5) | 18 (41.0) | |
| age | |||
| ≤60 | 18 (48.5) | 17 (38.5) | 0.365 |
| >60 | 19 (51.5) | 27 (61.5) | |
| location of primary tumor | |||
| colon | 20 (54.0) | 23 (52.5) | 0.873 |
| rectum | 17 (46.0) | 21 (47.5) | |
| differentiation of primary tumor | |||
| well | 0 (0.0) | 1 (2.5) | 0.062 |
| moderately | 28 (75.5) | 40 (91.0) | |
| poorly | 9 (24.5) | 3 (6.5) | |
| tumor size of primary tumor | |||
| T1 | 2 (5.5) | 0 (0.0) | 0.061 |
| T2 | 3 (8.0) | 11 (25.0) | |
| T3 | 30 (81.0) | 28 (63.5) | |
| T4 | 2 (5.5) | 5 (11.5) | |
| lymph node metastasis of primary tumor | |||
| 0 | 17 (46.0) | 24 (54.5) | 0.365 |
| 1 | 13 (35.0) | 9 (20.5) | |
| 2 | 7 (19.0) | 11 (25.0) | |
| venous invasion of primary tumor | |||
| absent | 9 (24.5) | 17 (38.5) | 0.343 |
| present | 19 (51.5) | 22 (50.0) | |
| lymphatic invasion of primary tumor | |||
| absent | 25 (67.5) | 32 (79.5) | 0.412 |
| present | 3 (4.5) | 7 (16.0) | |
| stadium | |||
| I | 4 (10.5) | 6 (13.5) | 0.314 |
| II | 12 (32.5) | 11 (25.0) | |
| III | 16 (43.5) | 14 (32.0) | |
| IV | 5 (13.5) | 13 (29.5) |
Table 3.
Correlation of β1 expression in stromal cells of liver metastases with clinicopathological findings of primary tumors
| Characteristics | Patients with positive (≥3) Remmele score (n=27) (%) | Patients with negative (0-2) Remmele score (n=54) (%) | p-value |
|---|---|---|---|
| sex | |||
| male | 13 (48.0) | 32 (59.0) | 0.343 |
| female | 14 (52.0) | 22 (41.0) | |
| age | |||
| ≤60 | 12 (44.5) | 23 (42.5) | 0.874 |
| >60 | 15 (55.5) | 31 (57.5) | |
| location of primary tumor | |||
| colon | 17 (63.0) | 26 (48.0) | 0.208 |
| rectum | 10 (37.0) | 28 (52.0) | |
| differentiation of primary tumor | |||
| well | 0 (0.0) | 1 (2.0) | 0.776 |
| moderately | 23 (85.0) | 45 (83.0) | |
| poorly | 4 (15.0) | 8 (15.0) | |
| tumor size of primary tumor | |||
| T1 | 1 (3.5) | 1 (2.0) | 0.626 |
| T2 | 4 (15.0) | 10 (18.5) | |
| T3 | 21 (78.0) | 37 (68.5) | |
| T4 | 1 (3.5) | 6 (11.0) | |
| lymph node metastasis of primary tumor | |||
| 0 | 14 (52.0) | 27 (50.0) | 0.972 |
| 1 | 7 (26.0) | 15 (27.5) | |
| 2 | 6 (22.0) | 12 (22.5) | |
| venous invasion of primary tumor | |||
| absent | 7 (26.0) | 19 (35.0) | 0.816 |
| present | 10 (37.0) | 31 (57.5) | |
| lymphatic invasion of primary tumor | |||
| absent | 14 (52.0) | 43 (79.5) | 0.715 |
| present | 3 (11.0) | 7 (13.0) | |
| stadium | |||
| I | 4 (15.0) | 6 (11.0) | 0.542 |
| II | 9 (33.0) | 14 (26.0) | |
| III | 7 (26.0) | 23 (42.5) | |
| IV | 7 (26.0) | 11 (20.5) |
Correlation of β1 integrin expression with histological grading of liver metastases
The pattern of β1 integrin expression within the tumor cells of metastases was strong in poor differentiated metastases and weak in moderate differentiated metastases, as showed in Figure 1, where the expression of β1 integrin in poorly differentiated metastases (Figure 1A) is really stronger than this one in moderated differentiated metastases (Figure 1C). These differences were statistically significant (p=0.03). In contrast, the expression of β1 integrin in tumor stromal cells shows no correlation between histopathological grading and β1 integrin expression (p=1.12) (Table 4).
Table 4.
Correlation of β1-integrin immunolabeling with the differentiation grade of liver metastases
| tumor (p=0.03) | Remmele score in tumor cells | |||
|
| ||||
| - | + | total | ||
|
| ||||
| histological grade | G2 | 41 | 29 | 70 |
| G3 | 2 | 7 | 9 | |
| total | 43 | 36 | 79 | |
|
| ||||
| stroma (p=0.11) | Remmele score in stromal cells | |||
|
| ||||
| - | + | total | ||
|
| ||||
| histological grade | G2 | 44 | 26 | 70 |
| G3 | 8 | 1 | 9 | |
| total | 52 | 27 | 79 | |
Prognostic value of integrin β1 expression
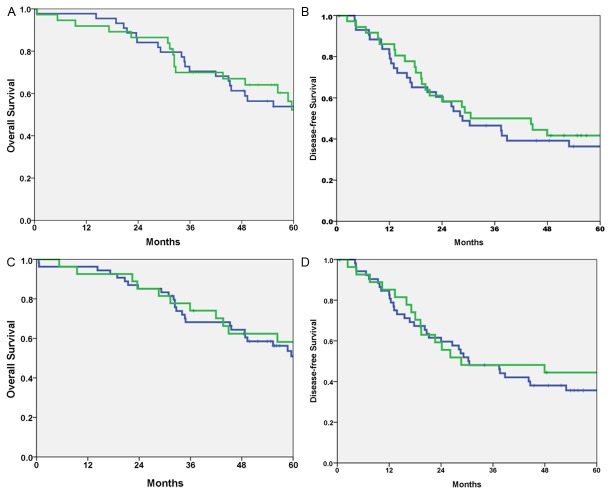
We examined whether there was an association between β1 expression and patient survival. We analyzed the data by considering only disease-related death as an event, censoring deaths unrelated to disease and the patients who were alive when they were last seen. To determine differences in the disease-free and overall survival among patients who differed in β1 expression, the log-rank test was performed and the corresponding Kaplan-Meier plot is shown in Figure 2. The log-rank test indicated there was a tendency but no significant difference in disease-free and overall survival between patients with score 0-2 and patients with score 3-12 in tumor cells (p=0.198 and p=0.340 respectively). Similarly, comparing the survival of the 81 patients according to the integrin β1 expression in stroma, the disease-free and overall survival rate for patients who had positive immunoreactive score of β1 integrin in stromal cells was not significantly better compared to individuals who had negative immunolabeling (p=0.890 and p=0.740 respectively).
Figure 2.
A. Kaplan-Meier analysis of overall survival of patients with colorectal liver metastases correlated with immunoreactive score of β1-integrin in tumor cells (positive score: green - negative score: blue, p=0.34); B. Kaplan-Meier analysis of disease free survival of patients with colorectal liver metastases correlated with immunoreactive score of β1-integrin in tumor cells (positive score: green - negative score: blue, p=0.198); C. Kaplan-Meier analysis of overall survival of patients with colorectal liver metastases correlated with immunoreactive score of β1-integrin in stromal cells (positive score: green - negative score: blue, p=0.740); D. Kaplan-Meier analysis of disease free survival of patients with colorectal liver metastases correlated with immunoreactive score of β1-integrin in stromal cells (positive score: green - negative score: blue, p=0.890).
We also examined if there was a prognostic relevance of integrin β1 expression in patients with positive β1 expression in tumor cells and negative expression in stromal cells in comparison with the patients with negative β1 expression in tumor cells and positive expression in stromal cells. A statistical significance was not confirmed neither for the disease-free survival (p=0.890) nor for the overall survival (p=0.511).
Multivariate regression analysis using the cox proportional hazards model was performed to observe the independent prognostic value of β1 integrin expression in tumor cells with several prognostic factors such sex and age of the patient, clinicopathological parameters of primary tumor (location, differentiation, tumor size, nodal status, venous and lymphatic invasion), differentiation of liver metastases and time interval between primary tumor and liver metastases, but no correlation was found (Supplementary Table 1).
Discussion
The development of liver metastasis is an ominous event in the natural history and progression of CRC. The fact that not all disseminated CRC cells develop into macrometastases indicates that subpopulations of malignant cells evolve a genetic advantage to adhere, migrate, and invade through the ECM, and survive in the new liver environment [53]. This advantage is associated with the up-regulation of various CAM, and in particular integrins [53,54], which are the prime example of bidirectional receptor signalling.
Analysing cell adhesion, current research attempts to clarify the numerous cell interactions which are evident within the liver sinusoids and thus control the outcome of the whole metastatic process [55,56]. During the last years a number of reports and multiple research studies have investigated different integrins (αvβ3, αvβ5, αvβ1, αvβ6, αvβ8, α2) in CRC and its metastases, showing that blockade of them in cell lines of primary or metastatic cancer caused a significant reduction of tumour cell proliferation and prevention of increase of colorectal hepatic metastases [57-62]. There are also immunohistochemical studies in colorectal cancer samples showing that the expression of integrins is remarkably altered in CRC [42-45,63,64], since patients with the highest levels of e.g. αvβ6 or αvβ3 protein expression on immunostaining had the poorest survival [63,65,66] and moreover the expression‘s level of these integrins in colorectal cancer samples were correlated with the development of liver metastases [65].
Concerning the CRC, it is known that blockade of β1 integrin-mediated cell adhesion reduced adhesive properties of CRC cells within the hepatic microcirculation [67]. In vitro studies on human colorectal cancer cell lines revealed that β1 integrin regulates differentiation toward cancer progression and invasion [67-69]. Furthermore immunohistochemical studies in colon or colorectal cancer specimens have consistently revealed altered β1 integrin expression in the development and progression of this disease [42,44] which was also correlated with lymph node metastasis or depth of invasion [27]. A few reports have shown correlations between integrin expression and tumor prognosis or clinical stage too [70-78].
But the prognostic significance of integrins› deposition around liver metastases remains unclear since there are only a few studies in metastatic cancer samples consisting of a very small number of patients which examine the expression of β1 integrin in hepatic metastatic cancer samples [43,79-81]; however without any investigation of the clinicopathological parameters and without any correlation with tumor prognosis. This is the first study which examines the expression of β1 integrin in liver metastases samples in a large group of patients and correlates it with different parameters.
For the evaluation of the β1 integrin’s expression we choose the score proposed by Remmele and Stegner [49] based on the level of signal and percentage of tumor cells expressing the signal. It is the first study which evaluates integrins’ expression in CRC with this score. We found increased cell surface expression of the integrin β1 protein in tumor and stromal cells in hepatic metastases compared to normal liver tissue. Although a number of studies have implicated integrin expression in the metastatic process, including liver metastases, the findings presented here are the first to directly show a role for integrin β1 in selective liver metastasis since our results suggest that β1 in human colorectal cancer mediates the potential of liver colonization and liver metastasis, because of its high expression in metastatic tissues of liver and supports the idea that β1 integrin in CRC correlates with disease progression and poorer clinical outcome.
We found that the stroma, which is characterized with a dense vascular network and a loose connective tissue, was also intensely positive for β1 but in low levels in comparison with tumor. This difference in β1-expression in tumor and stromal cells of the metastatic liver could be due to the fact that the β1 integrin receptor, fibronectin, is found in the surrounding tissue around the tumor but β1 integrin are anchored to the membrane of tumor cells, leading to a stronger expression [82,83]. Gulubova and Vlaykova [81] showed that the intensity of immune reaction of α5β1 and α9β1 integrins in liver metastases tended to be lower than that in primary tumor. Similar findings were observed by Dueck too [84]. This could be due to the fact that stroma-forming cells in the liver are far fewer in number than those in the connective tissue stroma of the primary tumor [77], but till now there is no explanation about the difference of β1 integrin’s expression in tumor and in stromal cells within liver metastases. Furthermore, it has already been showed that the formation of a fibrotic capsule around the hepatic lesions in CRC liver metastases is associated with better prognosis, since they showed that α5β1 and α9β1 integrins were significantly increased in cases without a fibrotic capsule and linked with shorter survival [80].
An additional aim of our study was to investigate the relationship between the levels of immunoreactivity (expression) of β1-integrin with the clinicopathological characteristics of the primary tumors, but there was no correlation between them. Interesting finding in this respect was the observed association between the staining levels of β1 integrin and the grade of differentiation in liver metastases, since the alteration of β1 integrin expression in hepatic tumor cells was statistically significant correlated with the grading of liver metastases. This indicates a significant role of β1 integrin in the differentiation of the liver metastases in it and implies that the poorer is the differentiation grade of the metastatic liver, the higher is the expression of β1 integrin in it.
We found that β1 integrin expression in colorectal liver metastases does not represent any reliable prognostic factor neither for other histopathological features except grading nor for patients’ survival. This can be explained by the fact that patients who were treated with a neoadjuvant (radio)-chemotherapy either for the primary or for the metastatic disease or suffered from synchronous metastases in other organs were excluded from our group; meaning that we had a high selective group of patients. An interesting finding was also the different expression of β1 integrin in tumor and stromal cells; this difference is of biological implication and needs further investigation.
It bears no question that integrin biology is interwoven with each and every step of tumorigenesis, but critically affects cell cycle, apoptosis and aggressiveness. Treatments targeting all these processes have to take into account the role of integrins in CRC. It is more likely that combinatorial therapeutics which considers redundant pathways may be more efficacious that single-agent treatments. Therefore further research in the integrin field may ultimately suggest more rational treatment combinations [23].
Nowadays, therapies directed at influencing integrin cell expression and function are being explored for inhibition of tumor growth, metastasis and angiogenesis. Such therapeutic strategies include anti-integrin monoclonal antibodies, peptidic inhibitors (cyclic and linear), calcium-binding protein antagonists, proline analogs, apoptosis promotors, and antisense oligonucleotides. Moreover, platelet aggregation induced by tumor cells, which facilitates metastatic spread, can be inhibited by the disintegrins, a family of viper venom-like peptides. Therefore, adhesion molecules from the integrin family and components of angiogenesis might be useful as tumor progression markers for diagnostic and prognostic purposes. Already, β1 integrin appear to be promising target for drug discovery, and currently, and currently, anti-integrin antibodies [85], disintegrins and synthetic peptides [86,87] have been reported to be effective antimetastatic agents and inhibited invasion and metastasis in in vitro and in vivo models. Surely, integrin inhibition alone and with other targeted therapeutic approaches should be further investigated in clinical trials in patients with CRC in order to identify new molecular markers and signalling pathways that are characteristic of aggressive colorectal carcinomas and, in turn, provide novel therapeutic candidates.
Conclusion
Although there was no statistically significant prognostic association between β1 integrin expression with the different clinicopathological parameters, the relationship in CRC liver metastases needs to be better understood, and further studies are needed to clarify the molecular basis involved in this process. However, the finding that the alteration of expression of β1 integrin was statistically collated with the differentiation grade of liver metastases indicate that development of integrin cell expression profiles for individual tumors may have further potential in identifying a cell surface signature for a specific tumor type and/or stage. Thus, recent advances in elucidating the structure, function, ECM binding, and signalling pathways of the β1 integrin can be led to new and exciting modalities for colorectal metastatic cancer therapeutics and diagnoses.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Rothbarth J, van de Velde CJ. Treatment of liver metastases of colorectal cancer. Ann Oncol. 2005;16(Suppl 2):ii144–149. doi: 10.1093/annonc/mdi702. [DOI] [PubMed] [Google Scholar]
- 4.McLoughlin JM, Jensen EH, Malafa M. Resection of colorectal liver metastases: current perspectives. Cancer Control. 2006;13:32–41. doi: 10.1177/107327480601300105. [DOI] [PubMed] [Google Scholar]
- 5.Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: a review. J Surg Oncol. 2006;94:68–80. doi: 10.1002/jso.20558. [DOI] [PubMed] [Google Scholar]
- 6.Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21:63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1–11. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 8.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G. H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 9.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed’ and ‘soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 10.Nicolson GL. Cancer metastasis: tumor cell and host properties important in colonization of specific secondary sites. Biochim Biophys Acta. 1988;948:175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- 11.Weiss L. Biomechanical interactions of cancer cells with the microvasculature during hematogenous metastasis. Cancer Metastasis Rev. 1992;11:227–235. doi: 10.1007/BF01307179. [DOI] [PubMed] [Google Scholar]
- 12.Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT-29 colon carcinoma cells with extracellular matrix components: role of integrin expression and cytoskeletal components. Br J Cancer. 1999;80:1867–1874. doi: 10.1038/sj.bjc.6690614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 15.Gilcrease MZ. Integrin signaling in epithelial cells. Cancer Lett. 2007;247:1–25. doi: 10.1016/j.canlet.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 16.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 17.Danen EH. Integrins: regulators of tissue function and cancer progression. Curr Pharm Des. 2005;11:881–891. doi: 10.2174/1381612053381756. [DOI] [PubMed] [Google Scholar]
- 18.Chung J, Kim TH. Integrin-dependent translational control: Implication in cancer progression. Microsc Res Tech. 2008;71:380–386. doi: 10.1002/jemt.20566. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150:89–96. doi: 10.1083/jcb.150.2.f89. [DOI] [PubMed] [Google Scholar]
- 20.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 21.Gulubova MV. Expression of cell adhesion molecules, their ligands and tumour necrosis factor alpha in the liver of patients with metastatic gastrointestinal carcinomas. Histochem J. 2002;34:67–77. doi: 10.1023/a:1021304227369. [DOI] [PubMed] [Google Scholar]
- 22.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM. Integrins and cancer. Oncology (Williston Park) 2007;21:13–20. [PubMed] [Google Scholar]
- 24.Le Tourneau C, Faivre S, Raymond E. The role of integrins in colorectal cancer. Oncology (Williston Park) 2007;21:21–24. [PubMed] [Google Scholar]
- 25.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita S, Watanabe M, Kubota T, Teramoto T, Kitajima M. Alteration of expression in integrin beta1-subunit correlates with invasion and metastasis in colorectal cancer. Cancer Lett. 1995;91:145–149. doi: 10.1016/0304-3835(95)03735-f. [DOI] [PubMed] [Google Scholar]
- 28.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.dos Santos PB, Zanetti JS, Ribeiro-Silva A, Beltrao EL. Beta 1 integrin predicts survival in breast cancer: a clinicopathological and immunohistochemical study. Diagn Pathol. 2012;7:104. doi: 10.1186/1746-1596-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased β1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez MA, Pinder SE, Wencyk PM, Bell JA, Elston CW, Nicholson RI, Robertson JF, Blamey RW, Ellis IO. An immunohistochemical examination of the expression of E-cadherin, alpha- and beta/gamma-catenins, and alpha2- and beta1- integrins in invasive breast cancer. J Pathol. 1999;187:523–529. doi: 10.1002/(SICI)1096-9896(199904)187:5<523::AID-PATH296>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Lanzafame S, Emmanuele C, Torrisi A. Correlation of alpha 2 beta 1 integrin expression with histological type and hormonal receptor status in breast carcinomas. Pathol Res Pract. 1996;192:1031–1038. doi: 10.1016/s0344-0338(96)80045-8. [DOI] [PubMed] [Google Scholar]
- 33.Berry MG, Gui GP, Wells CA, Carpenter R. Integrin expression and survival in human breast cancer. Eur J Surg Oncol. 2004;30:484–489. doi: 10.1016/j.ejso.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Petricevic B, Vrbanec D, Jakic-Razumovic J, Brcic I, Rabic D, Badovinac T, Ozimec E, Bali V. Expression of Toll-like receptor 4 and beta 1 integrin in breast cancer. Med Oncol. 2012;29:486–494. doi: 10.1007/s12032-011-9885-0. [DOI] [PubMed] [Google Scholar]
- 35.Shen Z, Ye Y, Kauttu T. Seppänen H, Vainionpää S, Wang S, Mustonen H, Puolakkainen P. Novel focal adhesion protein kindling-2 promotes the invasion of gastric cancer cells through phosphorylation of integrin β1 and β3. J Surg Oncol. 2013;108:106–112. doi: 10.1002/jso.23353. [DOI] [PubMed] [Google Scholar]
- 36.Zhang PF, Zeng GQ, Yi RZ, Liu JP, Wan XX, Qu JQ, Li JH, Li C, Tang CE, Hu R, Ye X, Chen Y, Chen ZC, Xiao ZQ. Identification of integrin β1 as a prognostic biomarker for human lung adenocarcinoma using 2D-LC-MS/MS combined with iTRAQ technology. Oncol Rep. 2013;30:341–349. doi: 10.3892/or.2013.2477. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Zhang F, Cai Y, Liu T. Expression profiling of integrins in lung cancer cells. Pathol Res Pract. 2009;205:847–853. doi: 10.1016/j.prp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Dingemans AM, van den Boogaart V, Vosse BA, van Suylen RJ, Griffioen AW, Thijssen VL. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol Cancer. 2010;9:1–9. doi: 10.1186/1476-4598-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carbonell WS, DeLay M, Jahangiri A, Park CC, Anhi MK. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth ofbevacizumab-resistant glioblastoma. Cancer Res. 2013;73:3145–3154. doi: 10.1158/0008-5472.CAN-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pontes-Junior J, Reis ST, Bernardes FS, Oliveira LC, de Barros EA, Dall’Oqlio MF, Timosczuk LM, Ribeiro-Filho LA, Srouqi M, Leite KR. Correlation between β1 integrin expression and prognosis in clinically localized prostate cancer. Int Braz J Urol. 2013;39:335–343. doi: 10.1590/S1677-5538.IBJU.2013.03.06. [DOI] [PubMed] [Google Scholar]
- 41.Bottger TC, Maschek H, Lobo M, Gottwohl RG, Brenner W, Junginger T. Prognostic value of immunohistochemical expression of beta-1 integrin in pancreatic carcinoma. Oncology. 1999;56:308–313. doi: 10.1159/000011984. [DOI] [PubMed] [Google Scholar]
- 42.Pignatelli M. Smith ME, Bodmer WF. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990;61:636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koretz K, Schlag P, Boumsell L, Möller P. Expression of VLA-alpha 2, VLA-alpha 6 and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastasis. Am J Pathol. 1991;138:741–750. [PMC free article] [PubMed] [Google Scholar]
- 44.Stallmach A, von Lampe B, Matthes H, Bornhöft G, Riecken EO. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992;33:342–346. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam AK, Savage FJ, Boulos PB, Stamp GW, Liu D, Pignatelli M. Loss of cell-cell and cell-matrix adhesion molecules in colorectal cancer. Br J Cancer. 1993;68:507–514. doi: 10.1038/bjc.1993.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, Wilding G. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14:7924–7929. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 48.Jass JR, Sobin LH, Watanabe H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::aid-cncr2820661020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 49.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 50.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 51.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SW, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analyses and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 53.Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- 54.Ngan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, Hemmi H, Takemasa I, Ikeda M, Sekimoto M, Monden M. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. 2007;95:652–662. doi: 10.1002/jso.20638. [DOI] [PubMed] [Google Scholar]
- 55.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 56.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 57.Conti JA, Kendall TJ, Bateman A, Armstrong TA, Papa-Adams A, Xu Q, Packham G, Primrose JN, Benyon RC, Iredale JP. The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumor growth and surgical via alphav integrin ligation. Clin Cancer Res. 2008;14:6405–6413. doi: 10.1158/1078-0432.CCR-08-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Bij GJ, Oosterling SJ, Bögels M, Bhoelan F, Fluitsma DM, Beelen RH, Meijer S, van Egmond M. Blocking alpha2 integrins on rat CC531s colon carcinoma cells prevents operation-induced augmentation of liver metastases outgrowth. Hepatology. 2008;47:532–543. doi: 10.1002/hep.22013. [DOI] [PubMed] [Google Scholar]
- 59.Enns A, Korb T, Schlüter K, Gassmann P, Spiegel HU, Senninger N, Mitjans F, Haier J. Alphavbeta5-integrins mediate early steps of metastasis formation. Eur J Cancer. 2005;41:1065–1072. doi: 10.1016/j.ejca.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 60.Kikkawa H, Kaihou M, Horaguchi N, Uchida T, Imafuku H, Takiquchi A, Yamazaki Y, Koike C, Kuruto R, Kakiuchi T, Tsukada H, Takada Y, Matsuura N, Oku N. Role of integrin alpha(v)beta3 in the early phase of liver metastasis: PET and IVM analyses. Clin Exp Metastasis. 2002;19:717–725. doi: 10.1023/a:1021356019563. [DOI] [PubMed] [Google Scholar]
- 61.Wai PY, Mi Z, Guo H, Sarraf-Yazdi S, Gao C, Wei J, Marroquin CE, Clary B, Kuo PC. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005;26:741–751. doi: 10.1093/carcin/bgi027. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimura K, Meckel KF, Laird LS, Chia CY, Park JJ, Olino KL, Tsunedomi R, Harada T, Iizuka N, Hazama S, Kato Y, Keller JW, Thompson JM, Chang F, Romer LH, Jain A, Iacobuzio-Donahue C, Oka M, Pardoll DM, Schulick RD. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009;69:7320–7328. doi: 10.1158/0008-5472.CAN-09-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Trascriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarty JH. Alphav integrins lead the way for colorectal metastases. Clin Cancer Res. 2008;14:6351–6353. doi: 10.1158/1078-0432.CCR-08-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vonlaufen A, Wiedle G, Borisch B, Birrer S, Luder P, Imhof BA. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod Pathol. 2001;14:1126–1132. doi: 10.1038/modpathol.3880447. [DOI] [PubMed] [Google Scholar]
- 66.Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, Chen R, Niu J. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879–887. doi: 10.1111/j.1349-7006.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enns A, Gassmann P, Schlüter K, Korb T, Spiegel HU, Senninger N, Haier J. Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg. 2004;8:1049–1059. doi: 10.1016/j.gassur.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Kirkland SC, Ying H. Alpha2β1 integrin regulated lineage commitment in multipotent human colorectal cancer cells. J Biol Chem. 2008;283:27612–27619. doi: 10.1074/jbc.M802932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthnes cell-matrix adhension and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 70.Takazawa H. Association between expression of integrin (VLA-3, VLA-5) and malignancy in human colon-cancer. Nippon Rinsho. 1995;53:1672–7. [PubMed] [Google Scholar]
- 71.Lindmark G, Gerdin B, Pahlman L, Glimelius B, Gehlsen K, Rubin K. Interconnection of integrins alpha 2 and alpha 3 and structure of the basal membrane in colorectal cancer: relation to survival. Eur J Surg Oncol. 1993;19:50–60. [PubMed] [Google Scholar]
- 72.Agrez MV, Bates RC. Colorectal cancer and the integrin family of cell adhesion receptors: Current status and future directions. Eur J Cancer. 1994;14:2166–2170. doi: 10.1016/0959-8049(94)00473-i. [DOI] [PubMed] [Google Scholar]
- 73.Streit M, Schmidr R, Hilgenfeld RU, Thiel E, Kreuse ED. Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. Recent Results Cancer Res. 1996;142:19–50. doi: 10.1007/978-3-642-80035-1_3. [DOI] [PubMed] [Google Scholar]
- 74.Haier J, Nasralla M, Nicolson GL. Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg. 2000;231:11–24. doi: 10.1097/00000658-200001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT-29 colon carcinoma cells with extracellular matrix components: Role of integrin expression and cytoskeletal components. Br J Cancer. 1999;80:1867–1874. doi: 10.1038/sj.bjc.6690614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haier J, Nasralla MY, Nicolson GL. Beta1-integrin-mediated dynamic adhesion of colon carcinoma cells to extracellular matrix under laminar flow. Clin Exp Metastasis. 1999;17:377–387. doi: 10.1023/a:1006658414040. [DOI] [PubMed] [Google Scholar]
- 77.Hanamura N, Yoshida T, Matsumoto E, Kawarada Y, Sakakura T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer. 1997;73:10–15. doi: 10.1002/(sici)1097-0215(19970926)73:1<10::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 78.Gulubova MV, Vlaykova T. Tenascin immunoreactivity in the large bowel and the liver in patients with colorectal cancer. Histochem J. 2001;33:111–120. doi: 10.1023/a:1017952331618. [DOI] [PubMed] [Google Scholar]
- 79.Koretz K, Fietz T, Laque M, Bruderlein S, Henne C, Moller P. Fibronectin and fibronection-receptors of the integrin type in normal colon mucosa, adenomas and carcinoma. Int J Oncol. 1994;5:1315–1323. doi: 10.3892/ijo.5.6.1315. [DOI] [PubMed] [Google Scholar]
- 80.Gulubova MV, Vlaykova TI. Significance of tenascin-C, fibronectin, laminin, collagen IV, alpha5beta1 and alpha9β1 integrins and fibrotic capsule formation around liver metastases originating from cancers of the digestive tract. Neoplasma. 2006;53:372–383. [PubMed] [Google Scholar]
- 81.Gulubova M, Vlaykova T. Immunohistochemical assessment of fibronectin and tenascin and their integrin receptors alpha5beta1 and alpha9beta1 in gastric and colorectal cancers with lymph node and liver metastases. Acta Histochem. 2006;108:25–35. doi: 10.1016/j.acthis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 82.De Wever O, Marrel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 83.Albrecht M, Renneberg H, Wennemuth G, Möschler O, Janssen M, Aumüller G, Konrad L. Fibronectin in human prostatic cells in vivo and in vitro: expression, distribution and pathological significance. Histochem Cell Biol. 1999;112:51–61. doi: 10.1007/s004180050391. [DOI] [PubMed] [Google Scholar]
- 84.Dueck M, Riedl S, Hinz U, Tandara A, Möller P, Herfarth C, Faissner A. Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int J Cancer. 1999;82:477–483. doi: 10.1002/(sici)1097-0215(19990812)82:4<477::aid-ijc2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 85.Fujita S, Suzuki H, Kinoshita M, Hirohashi S. Inhibition of cell attachment, invasion and metastasis of human carcinoma cells by anti-integrin beta 1 subunit antibody. Jpn J Cancer Res. 1992;83:1317–1326. doi: 10.1111/j.1349-7006.1992.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saiki I, Murata J, Matsuno K, Ogawa R, Nishi N, Tokura S, Azuma I. Anti-metastatic and anti-invasive effects of polymeric Arg-Gly-Asp (RGD) peptide, poly(RGD), and its analogues. Jpn J Cancer Res. 1990;81:660–667. doi: 10.1111/j.1349-7006.1990.tb02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Humphries MJ, Olden K, Yamada K. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.