Abstract
Aberrant expression of receptors tyrosine kinase of Eph gene in human cancers is extensively documented. We previously found that EphB1 subtype is down-regulated in gastric cancer and colorectal cancer. Fore the more, decreased expression of EphB1 is related to invasion and metastasis in cancers. There is no published data regarding the role of EphB1 in ovarian cancer, which is the focus of the present study. The expression of EphB1 protein was determined in tissues from 74 patients with serous ovarian carcinoma and 12 normal ovarian epithelial tissues. The expression level of EphB1 protein in serous ovarian carcinoma was analyzed with respect to clinicopathological parameters and survival. EphB1 protein was positively stained in 12 normal ovarian epithelial samples, and negatively stained in 32 out of 74 (43.2%) serous ovarian cancers. Loss of expression of EphB1 protein was associated with higher tumor grade (P = 0.006), metastasis (P = 0.049) and high proliferative index Ki67 expression (P = 0.022), but not with FIGO stage (P = 0.0937), age at diagnosis (P = 0.624), and diameter of carcinoma (P = 0.108). In addition, loss of EphB1 protein in serous ovarian carcinoma was associated with a significantly worse overall survival (P = 0.015). Our data indicate that loss of EphB1 protein is associated with metastasis and poorer survival in patients with serous ovarian cancer. EphB1 may be used as a prognostic marker and a therapeutic target in serous ovarian carcinoma.
Keywords: EphB1, serous ovarian carcinoma, metastasis, survival
Introduction
Ovarian carcinoma is the leading cause of death from gynecologic cancer and remains the most common cancer diagnosis in females. Ovarian carcinoma includes several histological types and serous ovarian carcinoma is the most common subtype of epithelial ovarian cancer that accounts for approximately 50-60% of all ovarian cancers [1-4]. Because of a lack of early warning signs and vague symptomatology, most ovarian cancer patients present with widespread metastatic disease. Although improvements in surgical management and advances in cytotoxic therapy have been accomplished in the past decades, the overall 5-year survival rate for women with advanced disease can be as low as 13%. Though a number of tumor markers have been evaluated for monitoring response to treatment and prognosis of ovarian cancer, these are still insufficient. Thus, there is an important need for additional diagnostic and prognostic markers for this disease [5-11].
The erythropoietin producing hepatocellular carcinoma (Eph) subfamily of genes encoding receptor tyrosine kinases are structurally characterized by the juxtaposition of a vestigial immunoglobin-like domain, a single cysteine-rich region, and two FN III domains in the extracellular region. The Eph receptors comprise eight EphA and six EphB receptors based on the similarity within each group of the extracellular domain sequences and on the affinity for binding ephrin-A and –B ligands. Eph receptors and their ligands of ephrin form a large family of receptor tyrosine kinases that involved in several physiological and pathological processes [12-16]. EphAs are typically bound to ephrinAs via a glycosylphosphatidylinositol anchor on the cell membrane. EphBs are typically bound to ephrinBs via a transmembrane domain. There is a great diversity of ligands and receptors that have tissue-specific expression patterns and overlapping ligand-receptor specificities. Because both the Eph receptors and ephrins localize to the cell surface, the signaling is restricted to the sites of direct cell-to cell contact. A key feature of these interactions is that their bi-directional signaling is triggered by ligand-receptor interactions and consequent receptor dimerization and the formation of higher-order clusters of activated receptors. The physiological role of Eph receptors is crucial in embryonic developmental processes, such as cell migration, vascular development, tissue border formation, axonal and synaptic network development.
Apart from development, emerging data also suggest the potential involvement of Eph receptors in tumorigenesis [17-21]. Eph-ephrin signaling has the multi-faceted functions as tumor promoters, tumor suppressors, angiogenic inducers and regulators of stem cell homeostasis. In our previous study on the Eph family, we have found that the reduced expression of EphB1 in colorectal cancer more often occurred in poorly differentiated and mucinous adenocarcinomas and showed more invasive power [22]. Interestingly, we also have found that underexpression of EphB1 protein is significantly associated with invasive, advanced stage and metastasis in gastric cancer [23]. To date, there have been no published reports evaluating the role of EphB1 expression in ovarian carcinomas, particularly with respect to clinical outcome. We examined the expression of EphB1 protein in a series of surgically treated serous carcinomas of ovary. The current study was undertaken with the aim to assess the expression of EphB1 protein and its correlation with clinicopathological variables and survival.
Materials and methods
Patients and clinicopathological variables
Archival paraffin-embedded tissue blocks from 74 patients (range 22-79, mean 52 years) with serous cancer of ovary were obtained from Jinling Hospital, Nanjing, China, between the years 2001 and 2010. All hematoxylin-and eosin-stained slides were re-reviewed by a gynecological pathologist to verify the diagnosis, histological grade, and stages. Women with other histological subtypes were excluded from present study. Pathological stage and histological subtype were determined for each surgical specimen according to 2002 International Federation of Gynecology and Obstetrics (FIGO) criteria, and Pathology and Genetics Tumors of the Breast and Female Genital Organs (World Health Organization, WHO 2003). Patient data were obtained from hospital tumor registry and chart review. All cases of recurrence had radiographic evidence of disease or biopsy proven progression of disease. Ethical approval was obtained from Jinling Hospital Ethics Board. Follow-up ranged from 3-143 months. The records of patients who were alive at follow-up or who did not die of disease were considered to be censored.
Immunohistochemistry
Sections from surgical specimens had been fixed in 10% formalin and embedded in paraffin and they were used here for immunohistochemical staining according to a standard method. Briefly, each 4-μm tissue section was deparaffinized and rehydrated. After rehydration through a graded ethanol series, the sections were autoclaved in 10 mM citrate buffer (pH 6.0) at 120°C for 2 min for antigen retrieval, then cooled to 30°C and washed with phosphate-buffered saline (PBS, pH 7.3). After endogenous peroxidase had been quenched with aqueous 3% H2O2 for 10 minutes and washed with PBS, the sections were incubated at 4°C overnight with an EphB1 polyclonal antibody (Abgent, San Diego, CA, USA) at a 1:100 dilution in antibody diluent solution (Zymed, Invitrogen) and then washed with PBS. Next, the sections were incubated with secondary antibody (Dako REAL EnVision Detection System, Dako, UK) for 30 min at room temperature. Color development was performed with 3, 3′-diaminobenzidine (DAB). Nuclei were lightly counterstained with hematoxylin. Two pathologists independently assessed the immunostained slides. Any difference in immunohistochemical scores was resolved by a consensus. Immunohistochemical staining of cancer cells was semi-quantitatively assessed according to the staining intensity and percentage of positive cells. EphB1 expression was assessed for intensity (0 = no staining, 1 = weak, 2 = moderate, 3 = strong) and the percentage of positive cells (0 = 0%, 1≤10%, 2 = 10% to 50%, 3 = 51% to 80%, 4≥80% positive cells) as defined previously. The scores for intensity and percentage were multiplied and a cut-off of 6 was used. Here, we used colorectal cancer and normal mucosa tissues that showed negative and positive expression of EphB1 as controls. The specificity of EphB1 antibody was investigated by using blocking peptide as we previously reported [22]. The percentage of tumor cells positive for Ki-67 was considered low, moderate and high when <10%, 10-60, and >60% of tumor cells, respectively, showed positive staining.
Statistical analysis
The statistical significance of intergroup differences was evaluated by a chi-square test. Kaplan-Meier survival curve was calculated using breast cancer-related death (overall survival) as the endpoints. All statistical analyses were performed using SPSS software (SPSS 16.0, Chicago, IL). A two-sided P value of less than 0.05 was considered statistically significant.
Results
Clinicopathological characterization
The mean patients age at the time of diagnosis was 52 years (range 22-79 years). Lymph node sampling or dissection was performed for 46 patients (62%) with 30 patients (65%) demonstrating lymph node metastasis. Eight (11%) and 6 (8%) patients were diagnosed in FIGO stage I and II, respectively, while 49 (66%) patients had FIGO stage III and 11 patients (15%) presented with metastatic disease (FIGO IV). Histological classification was performed according to the World Health Organization system in well-differentiated (G1; n = 10), moderately differentiated (G2; n = 11), and poorly differentiated (G3; n = 53).
EphB1 protein expression in normal ovarian epithelium and in serous ovarian carcinoma
The EphB1 protein was localized in the cytoplasm of the epithelial cells. The cytoplasm of normal ovarian surface epithelium and the normal serous epithelium of fallopian tube cells stained strongly for EphB1 in 12 samples (Figure 1A). Expression of EphB1 was immunostained in 74 patients with serious ovarian carcinoma. The staining level of EphB1 protein was varied among serous ovarian carcinoma samples, showing as negative, weak, moderate and strong staining of EphB1 (Figure 1B-D). The scores for intensity and percentage of carcinoma cells with positive staining of EphB1 were multiplied and a cut-off of 6 was used. The EphB1 protein was negatively stained in 32 out of 74 (43%) serous ovarian cancers.
Figure 1.
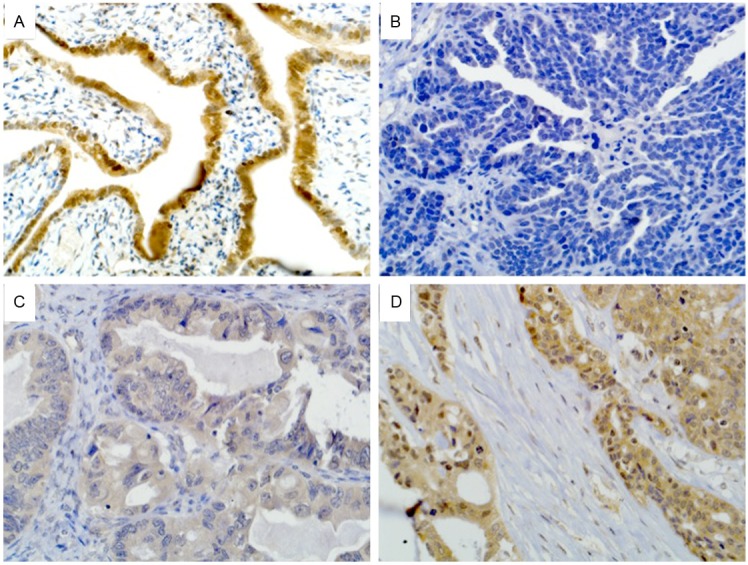
Expression of EphB1 in normal ovarian epithelial and serous ovarian carcinoma. A: Positive staining of EphB1 in normal serous epithelium of fallopian tube cells. B: Negative staining of EphB1 in serous ovarian carcinoma. C: Weak staining of EphB1 in serous ovarian carcinoma. D: Strong staining of EphB1 in serou ovarian carcinoma.
Association between EphB1 expression and clinicopathological parameters
Table 1 shows the association between EphB1 expression and clinicopathologic parameters. Loss of expression of EphB1 is more often detected in serious ovarian carcinomas with high grade (P = 0.006) and metastasis (P = 0.049). No relationship was found between expression of EphB1 and tumor stage (P = 0.0937), diameter (P = 0.108), age (P = 0.624), and recurrence (P = 0.815).
Table 1.
Correlation of clinicopathological variables with EphB1 expression in patients with serous ovarian cancer
| Variable | EphB1 expression | P Value | |
|---|---|---|---|
|
| |||
| Positive (n = 42) | Negative (n = 32) | ||
| Stage | |||
| I | 4 | 4 | 0.093 |
| II | 4 | 2 | |
| III | 28 | 21 | |
| IV | 6 | 5 | |
| Grade | |||
| 1 | 8 | 2 | 0.006 |
| 2 | 10 | 1 | |
| 3 | 24 | 29 | |
| Low | 34 | 31 | 0.069 |
| High | 8 | 1 | |
| Diemeter (cm) | |||
| ≤5 | 19 | 11 | 0.108 |
| 5-10 | 10 | 15 | |
| ≥10 | 13 | 6 | |
| Age (years) | |||
| <50 | 13 | 12 | 0.624 |
| ≥50 | 29 | 20 | |
| Recurrence | |||
| Yes | 17 | 14 | 0.815 |
| No | 25 | 18 | |
| Metastasis | |||
| Yes | 17 | 13 | 0.049 |
| No | 14 | 2 | |
| Not available | 11 | 17 | |
| Ki-67 | |||
| ≤10% | 8 | 6 | 0.022 |
| 10%-60% | 20 | 6 | |
| ≥60% | 14 | 20 | |
Decreased expression of EphB1 is related high cell proliferation index of Ki-67
Ki-67, a nuclear marker of cell proliferation, is associated with worse outcomes in certain carcinomas. In the present study, we analyzed the relation between expression of EphB1 and Ki-67 protein. Ki-67 immunopositivity was observed in tumor cell nuclei. The median percentage of Ki-67-positive tumor cell nuclei was 45.5% (range, 1% to 90%). High proliferation (>60%) was observed in 33.3% of tumor samples with positive expression of EphB1, while in 62.5% of tumor samples with negative expression of EphB1 (Table 1). Loss expression of EphB1 is positively related to high cell proliferation index of Ki-67 (P = 0.022).
Loss of expression of EphB1 protein is correlated to a poor overall survival
Seventy-four cases stained for EphB1 had follow-up data for survival and were available for assessment. At the end of the follow-up period, 34 (45.9%) patients were dead of their ovarian carcinoma. Decreased expression of EphB1 was significantly associated with poor overall survival (Figure 2, P = 0.015).
Figure 2.
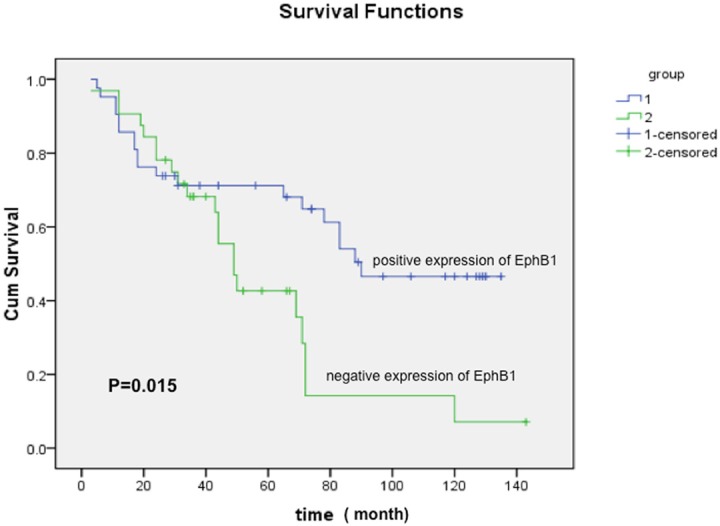
Survival curve of EphB1 immunoreactivity for serous ovarian carcinoma patients. Patients with carcinoma loss of expression of EphB1 showing poor survival, P = 0.015.
Discussion
The receptor tyrosine kinases (RTKs) have well-established roles in both normal physiology and oncogenesis. Eph receptors, the largest subfamily of receptor tyrosine kinase, were primarily considered as a classical oncogene. The overexpression of EphA2 receptor is well documented in many human cancers including prostate cancer [24], colorectal cancer [25], gastric cancer [26], and ovarian cancer [27]. Elevated EphA2 expression is correlated with disease stage, increased tumor metastasis, and poor patient survival. EphA2 plays a critical role in cancer progression. EphA2 is frequently overexpressed in non-small cell lung cancer. High level of EphA2 in the primary tumor predicts brain metastasis [28]. Huang et al found that overexpression of EphA2 in gastric cancer cells resulted in the upregulation of the epithelial-mesenchymal transition (EMT) molecular marker N-cadherin and Snail, as well as the Wnt/beta-catenin targets TCF4, CyclinD1, and c-Myc. These demonstrate that EphA2 upregulation is a common event in gastric cancer specimens that is closely correlated with cancer metastasis and that EphA2 promotes EMT of gastric cancer cells through activation of Wnt/beta-catenin signaling [29]. EphB2 is overexpressed in gastric cancer and colorectal cancer, and could be used as a prognostic factor and a therapeutic antibody drug target for the cancer treatment [30-32].
However, the role of Eph receptors and Ephrin ligands in oncogenesis is apparently complex and remains ill-defined. There are increasing conflicted data regarding Eph receptors in different cancer types, especially on its putative function as an oncogene or a tumor suppressor gene. Expression of EphA3 lost in human T-cell line HPB-ALL is associated with the hypermethylation of 5’ upstream CpG island [33]. Recently, research results from the EphB2 and EphB4 in colorectal cancer have strengthened that Eph receptor can be a tumor suppressor gene [30,34]. Our group previously reported that down-regulation of the EphA7 receptor in colon cancer cell lines and colorectal cancer samples are secondary to hypermethylation of the 5’CpG island, and down-regulation of the EphA7 is related to differentiation of colorectal cancers [35]. The expression of EphA7 in gastric carcinomas showed that EphA7 was decreased in all tested gastric cancer cell lines, but exhibited marked variability in gastric carcinoma specimens. Overexpression of EphA7 was more frequently observed in younger patients and in patients with advanced stage, and there is no significant relation between the expression of EphA7 and differentiation in gastric carcinoma [36]. These data indicated that Eph receptors carry unique functions depending on the organ or cell lineage.
EphB1 is a member of the Eph receptor tyrosine kinase family shown to have very important roles in nervous and vascular system development. It is a marker expressed in venous endothelial cells throughout embryonic development to adulthood. The forward signaling by EphB1/EphB2 interacting with ephin-B ligands at the optic chiasm is required to form the ipsilateral projection [37,38], and EphB1 forward signaling in the spinal cord plays critical roles in the development of bone cancer pain and morphine tolerance in treating bone cancer pain [39,40]. In contrast to these more established roles, EphB1’s function in cancer is much less clear. Previously, we detected EphB1 transcript and protein in colorectal cancer and gastric cancer [41,42]. EphB1 transcript and protein were differentially expressed in gastric and colorectal cancer cells among specimens. Reduced expression of EphB1 in colorectal cancers more often occurred in poorly differentiated and mucinous adenocarcinomas than in well- and moderately differentiated adenocarcinomas. Colorectal cancer cells with a low level of EphB1 protein showed more invasive power. In gastric cancer, we found that loss of expression of EphB1 protein is significantly associated with invasion, advanced stage and metastasis. In the present study, we show that EphB1 expression is associated with patient outcome in a series of consecutive cases of serous ovarian cancer specimens (n = 74). Loss of EphB1 protein was shown in 43.2% serous ovarian cancers. To our knowledge EphB1 expression has not been previously studied in malignant ovarian tissue. Loss of expression of EphB1 was associated with higher tumor grade (P = 0.006) and metastasis (P = 0.049), but not with stage (P = 0.0937), age (P = 0.624), and diameter (P = 0.108). In addition, loss expression of EphB1 is positively related to high cell proliferation index of Ki-67 (P = 0.022), and is associated with a significantly worse survival (P = 0.015).
Our data indicate that loss of EphB1 protein is associated with metastasis and poorer survival in patients with serous ovarian cancer. EphB1 may be used as a prognostic marker and a therapeutic target in serous ovarian carcinoma.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81371611, 81171391, 81372743) and the National Basic Research Priorities Program 973 Project (2014CB744504) from the Ministry of Science and Technology of China. We thank Prof. Fred Biddle for his help in preparation of the manuscript.
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Varga D, Deniz M, Schwentner L, Wiesmuller L. Ovarian cancer: in search of better marker systems based on DNA repair defects. Int J Mol Sci. 2013;14:640–73. doi: 10.3390/ijms14010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tognon G, Carnazza M, Ragnoli M, Calza S, Ferrari F, Gambino A, Zizioli V, Notaro S, Sostegni B, Sartori E. Prognostic factors in early-stage ovarian cancer. Ecancermedicalscience. 2013;7:325. doi: 10.3332/ecancer.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–5. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 4.Smolle E, Taucher V, Pichler M, Petru E, Lax S, Haybaeck J. Targeting signaling pathways in epithelial ovarian cancer. Int J Mol Sci. 2013;14:9536–55. doi: 10.3390/ijms14059536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Zhou Z, Di W, Li N. Correlation of CD44v6 expression with ovarian cancer progression and recurrence. BMC Cancer. 2013;13:182. doi: 10.1186/1471-2407-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC, Köbel M PRACTICAL Consortium; Ziogas A, Zheng W, Yang HP, Wu AH, Wozniak EL, Woo YL, Winterhoff B, Wik E, Whittemore AS, Wentzensen N, Weber RP, Vitonis AF, Vincent D, Vierkant RA, Vergote I, Van Den Berg D, Van Altena AM, Tworoger SS, Thompson PJ, Tessier DC, Terry KL, Teo SH, Templeman C, Stram DO, Southey MC, Sieh W, Siddiqui N, Shvetsov YB, Shu XO, Shridhar V, Wang-Gohrke S, Severi G, Schwaab I, Salvesen HB, Rzepecka IK, Runnebaum IB, Rossing MA, Rodriguez-Rodriguez L, Risch HA, Renner SP, Poole EM, Pike MC, Phelan CM, Pelttari LM, Pejovic T, Paul J, Orlow I, Omar SZ, Olson SH, Odunsi K, Nickels S, Nevanlinna H, Ness RB, Narod SA, Nakanishi T, Moysich KB, Monteiro AN, Moes-Sosnowska J, Modugno F, Menon U, McLaughlin JR, McGuire V, Matsuo K, Adenan NA, Massuger LF, Lurie G, Lundvall L, Lubiński J, Lissowska J, Levine DA, Leminen A, Lee AW, Le ND, Lambrechts S, Lambrechts D, Kupryjanczyk J, Krakstad C, Konecny GE, Kjaer SK, Kiemeney LA, Kelemen LE, Keeney GL, Karlan BY, Karevan R, Kalli KR, Kajiyama H, Ji BT, Jensen A, Jakubowska A, Iversen E, Hosono S, Høgdall CK, Høgdall E, Hoatlin M, Hillemanns P, Heitz F, Hein R, Harter P, Halle MK, Hall P, Gronwald J, Gore M, Goodman MT, Giles GG, Gentry-Maharaj A, Garcia-Closas M, Flanagan JM, Fasching PA, Ekici AB, Edwards R, Eccles D, Easton DF, Dürst M, du Bois A, Dörk T, Doherty JA, Despierre E, Dansonka-Mieszkowska A, Cybulski C, Cramer DW, Cook LS, Chen X, Charbonneau B, Chang-Claude J, Campbell I, Butzow R, Bunker CH, Brueggmann D, Brown R, Brooks-Wilson A, Brinton LA, Bogdanova N, Block MS, Benjamin E, Beesley J, Beckmann MW, Bandera EV, Baglietto L, Bacot F, Armasu SM, Antonenkova N, Anton-Culver H, Aben KK, Liang D, Wu X, Lu K, Hildebrandt MA Australian Ovarian Cancer Study Group; Australian Cancer Study. Schildkraut JM, Sellers TA, Huntsman D, Berchuck A, Chenevix-Trench G, Gayther SA, Pharoah PD, Laird PW, Goode EL, Pearce CL. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nat Commun. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwede M, Spentzos D, Bentink S, Hofmann O, Haibe-Kains B, Harrington D, Quackenbush J, Culhane AC. Stem cell-like gene expression in ovarian cancer predicts type II subtype and prognosis. PLoS One. 2013;8:e57799. doi: 10.1371/journal.pone.0057799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GG, Skinner HG. Prospective studies of total and ionized serum calcium in relation to incident and fatal ovarian cancer. Gynecol Oncol. 2013;129:169–72. doi: 10.1016/j.ygyno.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, Rodrigues DN, Renwick A, Seal S, Ramsay E, Duarte Sdel V, Rivas MA, Warren-Perry M, Zachariou A, Campion-Flora A, Hanks S, Murray A, Ansari Pour N, Douglas J, Gregory L, Rimmer A, Walker NM, Yang TP, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Eccles D, Evans DG, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, Walker L, Gore M, Houlston R, Brown MA, Caufield MJ, Deloukas P, McCarthy MI, Todd JA Breast Ovarian Cancer Susceptibility Collaboration; Wellcome Trust Case Control. Turnbull C, Reis-Filho JS, Ashworth A, Antoniou AC, Lord CJ, Donnelly P, Rahman N. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–10. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pils D, Horak P, Vanhara P, Anees M, Petz M, Alfanz A, Gugerell A, Wittinger M, Gleiss A, Auner V, Tong D, Zeillinger R, Braicu EI, Sehouli J, Krainer M. Methylation status of TUSC3 is a prognostic factor in ovarian cancer. Cancer. 2013;119:946–54. doi: 10.1002/cncr.27850. [DOI] [PubMed] [Google Scholar]
- 11.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, Buckley M, Fridley BL, Tyrer JP, Shen H, Weber R, Karevan R, Larson MC, Song H, Tessier DC, Bacot F, Vincent D, Cunningham JM, Dennis J, Dicks E Australian Cancer Study; Australian Ovarian Cancer Study Group. Aben KK, Anton-Culver H, Antonenkova N, Armasu SM, Baglietto L, Bandera EV, Beckmann MW, Birrer MJ, Bloom G, Bogdanova N, Brenton JD, Brinton LA, Brooks-Wilson A, Brown R, Butzow R, Campbell I, Carney ME, Carvalho RS, Chang-Claude J, Chen YA, Chen Z, Chow WH, Cicek MS, Coetzee G, Cook LS, Cramer DW, Cybulski C, Dansonka-Mieszkowska A, Despierre E, Doherty JA, Dörk T, du Bois A, Dürst M, Eccles D, Edwards R, Ekici AB, Fasching PA, Fenstermacher D, Flanagan J, Gao YT, Garcia-Closas M, Gentry-Maharaj A, Giles G, Gjyshi A, Gore M, Gronwald J, Guo Q, Halle MK, Harter P, Hein A, Heitz F, Hillemanns P, Hoatlin M, Høgdall E, Høgdall CK, Hosono S, Jakubowska A, Jensen A, Kalli KR, Karlan BY, Kelemen LE, Kiemeney LA, Kjaer SK, Konecny GE, Krakstad C, Kupryjanczyk J, Lambrechts D, Lambrechts S, Le ND, Lee N, Lee J, Leminen A, Lim BK, Lissowska J, Lubiński J, Lundvall L, Lurie G, Massuger LF, Matsuo K, McGuire V, McLaughlin JR, Menon U, Modugno F, Moysich KB, Nakanishi T, Narod SA, Ness RB, Nevanlinna H, Nickels S, Noushmehr H, Odunsi K, Olson S, Orlow I, Paul J, Pejovic T, Pelttari LM, Permuth-Wey J, Pike MC, Poole EM, Qu X, Risch HA, Rodriguez-Rodriguez L, Rossing MA, Rudolph A, Runnebaum I, Rzepecka IK, Salvesen HB, Schwaab I, Severi G, Shen H, Shridhar V, Shu XO, Sieh W, Southey MC, Spellman P, Tajima K, Teo SH, Terry KL, Thompson PJ, Timorek A, Tworoger SS, van Altena AM, van den Berg D, Vergote I, Vierkant RA, Vitonis AF, Wang-Gohrke S, Wentzensen N, Whittemore AS, Wik E, Winterhoff B, Woo YL, Wu AH, Yang HP, Zheng W, Ziogas A, Zulkifli F, Goodman MT, Hall P, Easton DF, Pearce CL, Berchuck A, Chenevix-Trench G, Iversen E, Monteiro AN, Gayther SA, Schildkraut JM, Sellers TA. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet. 2013;45:362–70. 70e1–2. doi: 10.1038/ng.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Mukouyama YS. Tissue-specific venous expression of the Eph family receptor EphB1 in the skin vasculature. Dev Dyn. 2013;242:976–88. doi: 10.1002/dvdy.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieden LA, Townsend TA, Vaught DB, Delaughter DM, Hwang Y, Barnett JV, Chen J. Regulation of heart valve morphogenesis by Eph receptor ligand, ephrin-A1. Dev Dyn. 2010;239:3226–34. doi: 10.1002/dvdy.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puschmann TB, Turnley AM. Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J Neurochem. 2010;113:881–94. doi: 10.1111/j.1471-4159.2010.06655.x. [DOI] [PubMed] [Google Scholar]
- 15.Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19:275–83. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Hogerheyde TA, Stephenson SA, Harkin DG, Bray LJ, Madden PW, Woolf MI, Richardson NA. Evaluation of Eph receptor and ephrin expression within the human cornea and limbus. Exp Eye Res. 2013;107:110–20. doi: 10.1016/j.exer.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt F, Nguyen PH, Gupta N, Mayer D. Eph receptor B4 is a regulator of estrogen receptor alpha in breast cancer cells. J Recept Signal Transduct Res. 2013;33:244–8. doi: 10.3109/10799893.2013.795971. [DOI] [PubMed] [Google Scholar]
- 18.Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, Liu X, Bruno J, Heguy A, Olshen AB, Socci ND, Teruya-Feldstein J, Weis-Garcia F, Tam W, Shaknovich R, Melnick A, Himanen JP, Chaganti RS, Wendel HG. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–64. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herath NI, Spanevello MD, Doecke JD, Smith FM, Pouponnot C, Boyd AW. Complex expression patterns of Eph receptor tyrosine kinases and their ephrin ligands in colorectal carcinogenesis. Eur J Cancer. 2012;48:753–62. doi: 10.1016/j.ejca.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Fox BP, Kandpal RP. A paradigm shift in EPH receptor interaction: biological relevance of EPHB6 interaction with EPHA2 and EPHB2 in breast carcinoma cell lines. Cancer Genomics Proteomics. 2011;8:185–93. [PubMed] [Google Scholar]
- 21.Kuang SQ, Bai H, Fang ZH, Lopez G, Yang H, Tong W, Wang ZZ, Garcia-Manero G. Aberrant DNA methylation and epigenetic inactivation of Eph receptor tyrosine kinases and ephrin ligands in acute lymphoblastic leukemia. Blood. 2010;115:2412–9. doi: 10.1182/blood-2009-05-222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng Z, Wang J, Dong Y, Ma H, Zhou H, Sugimura H, Lu G, Zhou X. EphB1 is underexpressed in poorly differentiated colorectal cancers. Pathobiology. 2008;75:274–80. doi: 10.1159/000151707. [DOI] [PubMed] [Google Scholar]
- 23.Wang JD, Dong YC, Sheng Z, Ma HH, Li GL, Wang XL, Lu GM, Sugimura H, Jin J, Zhou XJ. Loss of expression of EphB1 protein in gastric carcinoma associated with invasion and metastasis. Oncology. 2007;73:238–45. doi: 10.1159/000127421. [DOI] [PubMed] [Google Scholar]
- 24.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K, Nakamura T, Arai H, Kajimura M, Hanai H, Tanaka M, Sugimura H. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–41. doi: 10.1111/j.1349-7006.2004.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Konno H, Shinmura K, Tanaka M, Sugimura H. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–7. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, Fletcher MS, Urbauer DL, Kinch MS, Sood AK. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–40. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 28.Kinch MS, Moore MB, Harpole DH Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–8. [PubMed] [Google Scholar]
- 29.Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, Hou F, Ge J, Zhong M, Tang Y, Xia X, Chen Z. EphA2 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer cells. Oncogene. 2013 doi: 10.1038/onc.2013.238. doi: 10.1038/onc.2013.238. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Jubb AM, Zhong F, Bheddah S, Grabsch HI, Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P, Koeppen H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181–7. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- 31.Mao W, Luis E, Ross S, Silva J, Tan C, Crowley C, Chui C, Franz G, Senter P, Koeppen H, Polakis P. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781–8. doi: 10.1158/0008-5472.can-03-1047. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka H, Tanaka M, Kanamori M, Yoshii S, Ihara M, Wang YJ, Song JP, Li ZY, Arai H, Otsuki Y, Kobayashi T, Konno H, Hanai H, Sugimura H. Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human gastric cancer. J Cancer Res Clin Oncol. 2002;128:343–8. doi: 10.1007/s00432-002-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dottori M, Down M, Huttmann A, Fitzpatrick DR, Boyd AW. Cloning and characterization of EphA3 (Hek) gene promoter: DNA methylation regulates expression in hematopoietic tumor cells. Blood. 1999;94:2477–86. [PubMed] [Google Scholar]
- 34.Davalos V, Dopeso H, Castaño J, Wilson AJ, Vilardell F, Romero-Gimenez J, Espín E, Armengol M, Capella G, Mariadason JM, Aaltonen LA, Schwartz S Jr, Arango D. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Kataoka H, Suzuki M, Sato N, Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M, Tanaka M, Kanamori M, Nakamura T, Shinmura K, Sugimura H. Downregu-lation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637–47. doi: 10.1038/sj.onc.1208720. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Li G, Ma H, Bao Y, Wang X, Zhou H, Sheng Z, Sugimura H, Jin J, Zhou X. Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum Pathol. 2007;38:1649–56. doi: 10.1016/j.humpath.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Petros TJ, Shrestha BR, Mason C. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. J Neurosci. 2009;29:3463–74. doi: 10.1523/JNEUROSCI.5655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chenaux G, Henkemeyer M. Forward signaling by EphB1/EphB2 interacting with ephrin-B ligands at the optic chiasm is required to form the ipsilateral projection. Eur J Neurosci. 2011;34:1620–33. doi: 10.1111/j.1460-9568.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Zhang J, Cheng W, Chang DY, Huang J, Wang X, Jia L, Rosen DG, Zhang W, Yang D, Gershenson DM, Sood AK, Bast RC Jr, Liu J. CA-125 Level as a Prognostic Indicator in Type I and Type II Epithelial Ovarian Cancer. Int J Gynecol Cancer. 2013;23:815–22. doi: 10.1097/IGC.0b013e31828f7a24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Y, Mao-Ying QL, Chen JW, Yang CJ, Wang YQ, Tan ZM. Involvement of EphB1 receptor/ephrinB1 ligand in bone cancer pain. Neurosci Lett. 2011;496:163–7. doi: 10.1016/j.neulet.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Dong Y, Wang J, Sheng Z, Li G, Ma H, Wang X, Zhang R, Lu G, Hu Q, Sugimura H, Zhou X. Downregulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis. Mod Pathol. 2009;22:151–60. doi: 10.1038/modpathol.2008.188. [DOI] [PubMed] [Google Scholar]
- 42.Richards AB, Scheel TA, Wang K, Henkemeyer M, Kromer LF. EphB1 null mice exhibit neuronal loss in substantia nigra pars reticulata and spontaneous locomotor hyperactivity. Eur J Neurosci. 2007;25:2619–28. doi: 10.1111/j.1460-9568.2007.05523.x. [DOI] [PubMed] [Google Scholar]


