Abstract
Argonaute 2 proteins (Ago2) have been demonstrated to be widely expressed and involved in post-transcriptional gene silencing and play key roles in carcinogenesis. However, its expression profile and prognostic value in urothelial carcinoma of the bladder (UCB) have not been investigated. Methods: Real-time quantitative PCR (qRT-PCR) and Western blot were used to explore Ago2 expression in UCBs and normal bladder tissues. Moreover immunohistochemistry (ICH) was used to detect the expression of Ago2 in UCBs. Spearman’s rank correlation, Kaplan-Meier plots and Cox proportional hazards regression model were used to analyze the data. Results: Up-regulated expression of Ago2 mRNA and protein was observed in the majority of UCBs by qRT-PCR and Western blot when compared with their paired normal bladder tissues. Clinic pathological analysis was showed a significant correlation existed between the higher expression of Ago2 protein with the Histological grade, lymph node metastasis and Distant metastasis (P<0.05); Survival analysis by Kaplan-Meier survival curve and log-rank test demonstrated that elevated Ago2 expression in cancer tissue predicted poorer overall survival (OS) compared with group in lower expression (62.2% VS 86.3%, P<0.05). Notably, multivariate analyses by Cox’s proportional hazard model revealed that expression of Ago2 was an independent prognostic factor in UCB. Conclusions: These results suggest that the aberrant expression of Ago2 in human UCB is possibly involved with tumorigenesis and development, and the Ago2 protein could act as a potential biomarker for prognosis assessment of bladder cancer. Further studies on the cellular functions of Ago2 need to address these issues.
Keywords: Urothelial carcinoma of bladder, Argonaute 2, immunohistochemical, prognosis
Introduction
Bladder cancer is the second most common urologic cancer with relatively high morbidity and mortality [1]. Urothelial carcinoma of the bladder (UCB) is the most common histological subtype of bladder cancer. Overall, 70% of bladder tumors present as noninvasive urothelial carcinoma, and the remainder present as muscle-invasive disease [2]. Early diagnosis and prompt intervention of bladder cancer is crucial important. Unfortunately, up to 40% of patients present with muscle-invasive disease, and approximately 25% will harbor lymph node metastases at time of cystectomy and the prognosis of bladder cancer patients with locally advanced or lymph node metastasis only have a median survival of approximately 12 months [3,4]. So it is clear that early diagnosis and medical intervention seems vital in decreasing mortality and promoting total quality of life, novel molecular markers about bladder cancer that can help individually evaluate risk of patient outcome and predict the prognosis are urgently required, as well as the prediction of therapy effect and advocating personalized treatment.
Argonaute protein is the core protein in RNA-induced silencing complex (RISC), tethers miRNA to the 3’UTR of the target mRNAs, which results in mRNA degradation or translational repression known as RNA interference (RNAi) [5]. These proteins bind different classes of small noncoding RNAs, including microRNAs (miRNAs), small interfering RNAs (siRNAs) and PIWI interacting RNAs (piRNAs), then small RNAs guide Argonaute proteins to their specific target through sequence complementarity, which typically leads to silencing of the target. Few reports indicated that these proteins can also partially responsible for a series of biological processes such as differentiation, cell proliferation and apoptosis [6,7]. Recent structural analysis of the Argonaute protein has revealed a third functionally important domain which located between the former two domains, called the MID domain which can bind the characteristic 5’ phosphates of small RNAs, then anchors these small RNAs onto the Argonaute protein [8]. The study demonstrated that the new domain may be have a key role in some protein-protein interactions [9]. Based on sequence similarities, the mammalian Argonaute protein family has been separated into the Ago and Piwi subfamilies, and the Ago subfamily consists of Ago1, Ago2, Ago3 and Ago4 [10]. But researches identified that Argonaute 2 protein (Ago2) is the only one with the slicer activity and is highly specialized member of the Ago family with a crucial function within RNA-induced silencing complex (RISC) in the regulation of miRNA homeostasis [11]. Recent data from several studies have showed that Ago2 is a potential factor related to tumorigenesis in liver cancer, gastric cancer and renal cancer study [12-14]. But there have been no reports about Ago2 in bladder cancer and the role of Ago2 in bladder cancer is still unknown. In the present study, we examined both Ago2 mRNA and protein expression by Real-time quantitative PCR (qRT-PCR) and Western blot and investigate the expression of the human Ago2 proteins by Immunohistochemistry (IHC) and identify their potential roles in tumor occurrence, development and prognosis for patients with bladder cancer.
Materials and methods
Patients and specimens
For qRT-PCR and Western blot analysis, we collected 10 paired fresh UCBs and normal tissue samples from patient’s who underwent surgery between Januray 2012 and December 2012. In addition, a cohort of 106 formalin fixed, paraffin embedded tissues of UCBs diagnosed between January 2007 and December 2009 at the Department of Urology, Shanghai Tenth People’s Hospital of Tongji University (Shanghai, China) was retrieved. The cases selected were based on distinctive pathologic diagnosis of UCBs, undergoing transurethral resection, partial cystectomy and radical cystectomy without preoperative chemotherapy or radiotherapy. The disease stage of each patient was classified or reclassified according to the 2002 AJCC staging system [15]. The patients were totally 66 men and 40 women, whose age range from 32 to 79 years (median: 61 years). Clinical-pathological characteristics in our study are presented in Table 1. All patients were followed up until September 2012 with a median observation time of 36 months.
Table 1.
Correlation between Argonaute 2 expression and clinicopathological characteristics of urothelial carcinoma of the bladder patients
| Parameters | Group | Total | Argonaute 2 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Gender | Male | 66 | 32 (48.5) | 24 (51.5) | 0.351 |
| Female | 40 | 19 (47.5) | 21 (52.5) | ||
| Age (years) | <62 | 55 | 26 (47.3) | 29 (52.7) | 0.822 |
| ≥62 | 51 | 23 (45.1) | 28 (54.9) | ||
| Histological grade | Low grade | 39 | 25 (64.1) | 14 (35.6) | 0.005 |
| High grade | 67 | 24 (35.8) | 43 (64.2) | ||
| Tumor size (cm) | <3.5 cm | 62 | 34 (54.8) | 28 (45.2) | 0.470 |
| ≥3.5 cm | 44 | 21 (47.7) | 23 (52.3) | ||
| Tumor stage | <T1 | 65 | 35 (53.9) | 30 (46.2) | 0.319 |
| ≥T1 | 41 | 18 (43.9) | 23 (56.1) | ||
| Lymph nodes metastasis | No | 90 | 43 (47.8) | 47 (52.2) | 0.009 |
| Yes | 16 | 2 (12.5) | 14 (87.5) | ||
| Distant metastasis | No | 95 | 44 (46.3) | 51 (53.7) | 0.018 |
| Yes | 11 | 1 (0.91) | 10 (90.1) | ||
| Tumor multiplicity | Unifocal | 51 | 24 (47.1) | 27 (52.9) | 0.869 |
| Multifocal | 55 | 25 (45.4) | 30 (54.5) | ||
Patients were only included in the study if they had provided written consent to participate in the study after receiving oral and written information regarding its course and purpose. Approval for the study was received from the Ethics Committee of the host institution.
Real-time quantitative PCR
Total RNA was isolated tissue using TRIZOL reagent according to the manufacturer’s protocol (Invitrogen). RNA was reverse transcribed using SuperScript First Strand cDNA System (Invitrogen) according to the manufacturer’s instructions. The Ago2 sense primer was 5’-AAGGCTGCTCTAACCCTCTTG-3’, and the antisense primer was 5’-ACGCTGTTGCTGACACA-TC-3’. For the GAPDH gene, the sense primer was 5’-TGCACCACCAACTGCTTAGC-3’, and the antisense primer was 5’-GGCATGGACTGTGGTCATGAG-3’. The PCR amplification were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, on a Applied Biosystems 7900HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (Takara). Data was collected and analyzed by SDS2.3 Software (Applied Biosystems). The expression level of each candidate gene was internally normalized against that of the GAPDH. The relative quantitative value was expressed by the 2-ΔΔCt method. Each experiment was performed in triplicates and repeated three times.
Western blot assay
Total proteins from tissues were lysed in lysis buffer containing protease inhibitor. Protein concentration was determined using a Bio-Rad protein assay system (Bio-Rad). Equivalent amounts of proteins were separated by SDS-PAGE, and then transferred to polyvinylidene difluoride membranes (Bio-Rad). After being blocked in Tris buffered saline (TBS) containing 5% non-fat milk, the membranes were incubated with specific primary antibodies (Abcam) at 4°C for 12 hours and then with horseradish peroxidase conjugated anti-Rabbit antibody for 2 hours at room temperature. ECL detection reagent (Amersham LifeScience, Piscataway, NJ) was used to demonstrate the results.
Immunohistochemistry staining
All samples were fixed in 10% formaldehyde solution, embedded in paraffin blocks, cut in 4 μm thick sections, and mounted on glass slides. Each slide was dewaxed in xylene and rehydrated in grade alcohol, followed by boiling in 10 mmol/L of citrate buffer (PH 6.0) for antigen retrieval. After inhibition of endogenous peroxidase activities for 30 minutes with methanol containing 0.3% H2O2, the sections were blocked with 2% bovine serum albumin for 30 minutes and incubated overnight at 4°C with primary Rabbit monoclonal anti-Ago2 antibody (Abcam). After washing thrice with PBS, the slides were incubated with horseradish peroxidase-conjugated mouse-anti-rabbit IgG for 30 minutes, followed by reaction with diaminobenzidine and counterstaining with Mayer/hematoxylin. Negative control was done by omission of the primary antibody and substituting it with nonspecific rabbit IgG.
Evaluation of immunohistochemical staining
The evaluation of the immunohistochemical staining was performed independently by two authors without knowledge of the clinicopathological information. The immunoreactive scores besides Ago2 were determined by the sum of extension and intensity as literature reported previously [16]. The intensity of the staining was scored using the following scale: 0, no staining of the tumor cells; +, mild staining; ++, moderate staining and +++, marked staining. The area of staining was evaluated and recorded as a percentage: 0, less than 5%; +, 5%-25%; ++, 26%-50%; 3+, 51%-75% and 4+, more than 75%. The combined scores were recorded and graded as follows: -, 0; +, 1-2; ++, 3-5; +++, 6-7. Additionally, for statistical analysis, the - and 1+ cases were pooled into the low-expression group, and the 2+ and 3+ cases were pooled into the high-expression group.
Statistical analysis
Computerized statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0. The t-test was used to analyze the data from qRT-PCR and Western blot in the tissues. Clinical and histopathologic information and the results from the ICH studies were entered into a database. The significance of Ago2 expression for tumor was analyzed by the Kaplan-Meier method, and the differences were evaluated by the log-rank test. Multivariable recurrence-free survival analyses were performed with the Cox proportional hazards model. Differences were considered significant if the P-value from a two-tailed test was <0.05.
Results
Expression of Ago2 mRNA by qRT-PCR and Ago2 protein expression by Western blot in paired bladder tissues
We examined Ago2 mRNA expression in 10 paired fresh urothelial carcinoma of bladder and normal tissue samples by qRT-PCR. It showed that the increasing Ago2 mRNA expression could be detected in bladder cancer samples in comparison with the normal bladder samples (P<0.05, Figure 1A). To investigate whether Ago2 was also elevated at the protein level, Western blot was performed. We found that the protein level of Ago2 in tumor samples was significantly higher than that in normal bladder samples (Figure 1B).
Figure 1.
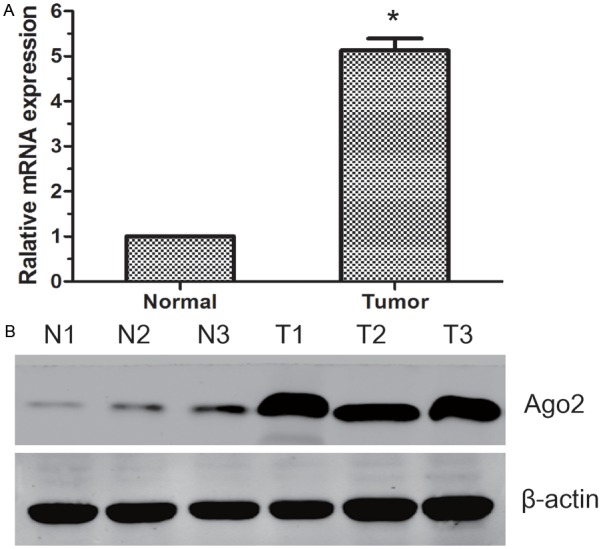
The expression of Ago2 mRNA and protein in the human UCB surgical specimens. A: The relative mRNA expression of Ago2 was higher in UCB tissues than in matched normal bladder tissues (P<0.05); B: The Ago2 protein expression was higher in the UCB tissues than in matched normal bladder tissues. N: normal bladder tissues; T: UCB tissues.
Expression of Ago2 in UCBs as determined by ICH analysis
By using of ICH we investigated the protein expression of Ago2 in UCBs specimens and specimens of normal bladder. The tumorous or nontumorous staining was semiquantitatively scored by the intensity and the percentage of positive staining. ICH staining showed that the Ago2 protein was mainly accumulated in the cytoplasm of malignant cells. And the expression of Ago2 in UCBs was significantly higher than in normal bladder tissues (Figure 2A).
Figure 2.
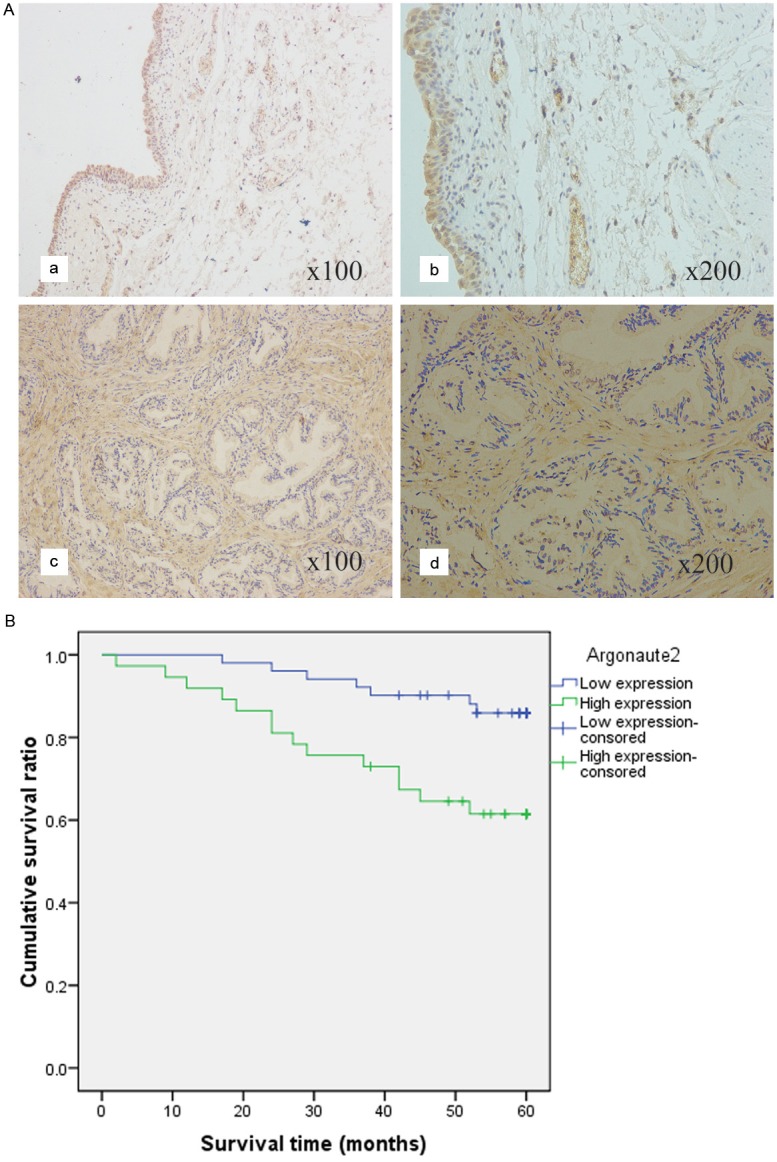
Ago2 protein expression in UBC surgical specimens and patient survival. A: ICH analysis of Ago2 protein expression in 106 cases of UCB tissues. a: ICH expression of Ago2 in normal bladder tissues (×100). b: ICH expression of Ago2 in normal bladder tissues (×200). c: ICH expression of Ago2 in UCB tissues (×100). d: ICH expression of Ago2 in UCB tissues (×200). B: The survival analysis of Ago2. Patients with higher Ago2 expression in tumor tissue were closely correlated with poorer overall survival than patients with tumor with lower expression (p<0.05, respectively).
Relationship between Ago2 expression and UCB patients’ clinicopathologic variables
In our UCB cohort, the relationship between the expression of Ago2 and patient clinical characteristics was shown in Table 1. High expression of Ago2 was found to significantly correlate with higher histological grade (P=0.005), lymph node metastasis (P=0.009) and distant metastasis (P=0.018). No significant difference in Ago2 expression was observed with gender, age, tumor size, tumor stage and tumor multiplicity (P>0.05).
Relationship between clinicopathologic features, Ago2 expression, and UCB patients’ survival: univariate survival analysis
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. Differences in survival times were assessed using the log-rank test. First, to confirm the representativeness of the UCBs in our study, we analyzed established prognostic predictors of patient survival. Kaplan-Meier analysis demonstrated a significant impact of well-known clinical pathological prognostic parameters, such as histological grade, lymph node status and distant metastasis status on patient survival (P<0.05, Table 2). Assessment of survival in UCBs patients revealed that higher expression of Ago2 was correlated with adverse survival of UCB patients (P=0.009, Table 2, Figure 2B).
Table 2.
Prognostic factors in Cox proportional hazards model
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | 0.917 | 0.642-1.693 | 0.742 | |||
| Male vs Female | ||||||
| Age (years) | 1.478 | 0.789-2.254 | 0.273 | |||
| ≥62 vs <62 | ||||||
| Tumor multiplicity | 0.946 | 0.627-1.751 | 0.633 | |||
| Unifocal vs Multifocal | ||||||
| Tumor stage | 3.212 | 2.671-4.754 | 0.146 | |||
| ≥T1 vs <T1 | ||||||
| Histological grade | 4.371 | 2.773-7.159 | <0.001 | 2.841 | 1.944-4.396 | <0.001 |
| Low vs High | ||||||
| Lymph node | 5.384 | 4.129-8.479 | <0.001 | 2.463 | 1.609-4.175 | 0.018 |
| N1-2 vs N0 | ||||||
| Distant metastasis | 9.272 | 3.778-21.314 | <0.001 | 4.114 | 2.238-7.116 | 0.023 |
| M1 vs M0 | ||||||
| Argonaute 2 | 3.887 | 2.765-6.429 | 0.009 | 3.273 | 2.364-5.877 | <0.001 |
| high vs low | ||||||
Independent prognostic factors for UCB: multivariate cox regression analysis
Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of Ago2 and those clinicopathological parameters that were significant in univariate analysis (histological grade, lymph node status and distant metastasis status) were further examined in multivariate analysis. The results showed that the expression of Ago2 was an independent prognostic factor for overall patient survival (relative risk: 3.273, CI: 2.364-5.877, P<0.001, Table 2). With regard to other parameters, histological grade, lymph node status and distant metastasis status were also shown to be an independent prognostic factor for overall survival (P<0.05, Table 2).
Discussion
MicroRNAs (miRNAs) are single-stranded RNA molecules of 20-23 nucleotide length which could regulate global gene expression often act synergistically to repress target genes, and their dysregulation can contribute to the initiation and progression of a variety of cancers as either a tumor suppressor or a tumor promoter. The mature miRNA directs a RNA-induced silencing complex (RISC) to the complementary target genes and causes inhibition of translation and degradation of the messenger RNA [17,18]. Argonaute proteins are the essential components of RISC that directly recruit miRNAs and function as the interface between miRNAs and their mRNA targets. Mammals have four Argonauts (Ago1-4) that are involved in the miRNA pathway and among them, Ago2 is unique, with the slicer activity that mediates the cleavage of perfectly matched targets for miRNAs and siRNAs [19,20]. Previous studies have reported the altered expression of Ago2 in kinds of cancers such as gastric carcinoma and hepatocellular carcinoma and renal carcinoma [12-14].
Bladder carcinogenesis is characterized by distinct morphological, genetic and cellular events. Development and progression of bladder cancer to metastasis and lethal state are believed to be driven by multiple genetic alterations, the nature of which has remained poorly understood. In the present study, we sought to determine whether there was any difference in Ago2 expression between UCBs and normal tissue samples which had not been studied previously. This study demonstrated that Ago2 proteins were up-regulated in the UCBs, and explored available evidence of close correlation of Ago2 expression and the total patients’ survival during a five-year follow-up survey.
To directly address the potential roles for Ago2 protein in the occurrence and development of bladder cancer, an elaborate experiment was conducted and a rigorous analysis was performed of human Ago2 mRNA and proteins on a bladder cancer samples. Our results revealed that the Ago2 expression in bladder cancer tissues was remarkably higher than that in normal bladder tissues (P<0.05). Zhang’s study also indicated that the abnormal expression of Argonaute 2 might be correlated with gastric oncogenic event [13].
In the present study, we found the expression level of Ago2 in cytoplasm was significantly associated with histological grade (p=0.005), lymph node metastasis (p=0.009) and distant metastasis (p=0.018). It is suggested that Ago2 are associated with tumor development and progression and may promote tumor invasion. We further imagined that the Ago2 proteins may interfere with the activation of cellular signal transduction pathway, cell division cycle and tumor angiogenesis to influence biological behavior of tumor, and this had just been unraveled in the latest relevant researches. Recent study showed that Argonaute 1 can promote angiogenesis, In the study, the author combined bioinformatics in conjunction with lab experimental validation and revealed that let-7 and miR-103/107 are robustly induced in vascular endothelial cells and consequently target Argonaute 1. Subsequently, a translational desuppression pathway up-regulates VEGF and enhances angiogenesis in vitro and in vivo. The findings suggest a novel role of microRNAs in gene expression and the consequent angiogenesis via targeting Argonaute protein [21].
Ultimately, A total of 106 patients histologically proven bladder cancer with follow-up information were conducted a systematically analysis to confirm the relationship of the Ago2 proteins and outcome of patient initially. Our finding demonstrated that patients with lower expression of Argonaute 2 in tumor tissue had a better overall survival than patients with higher expression (P=0.009, respectively), providing an evidence that elevated expression of Ago2 in bladder cancer might facilitate an increased malignant and worse prognostic phenotype. It is noteworthy that by multivariate Cox analysis combining expression of Ago2 proteins with other parameters, Ago2 was found as an independent prognostic factor for patient survival (p<0.001). The aberrant expression of Ago2 protein linked to a poor prognosis of patients has never been investigated in bladder cancer before.
Conclusion
In this study, we reported for the first time that Ago2 expression was upregulated in clinical UCB tissues, and high expression of Ago2 was associated closely with a more malignant clinical feature and poor prognosis of UCB patients. Our results suggest that Ago2 over-expression might be useful as a prognostic factor for UCB patients. Apparently, a further understanding of the molecular mechanism by Ago2 in human UCB would help in the discovery of novel targeted agents and might also lead to the development of new approaches for effective therapy of human UCB.
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81000311 and No. 81270831).
Disclosure of conflict of interest
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- 1.Liou LS. Urothelial cancer biomarkers for detection and surveillance. Urology. 2006;67:25–33. doi: 10.1016/j.urology.2006.01.034. discussion 33-24. [DOI] [PubMed] [Google Scholar]
- 2.Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RL Jr. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology. 2008;72:613–616. doi: 10.1016/j.urology.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 4.Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486–492. doi: 10.1016/j.juro.2006.03.038. discussion 491-482. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47:163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Kim WC, Lee CH. The role of mammalian ribonucleases (RNases) in cancer. Biochim Biophys Acta. 2009;1796:99–113. doi: 10.1016/j.bbcan.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zha X, Xia Q, Adam Yuan Y. Structural insights into small RNA sorting and mRNA target binding by Arabidopsis Argonaute Mid domains. FEBS Lett. 2012;586:3200–7. doi: 10.1016/j.febslet.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Juvvuna PK, Khandelia P, Lee LM, Makeyev EV. Argonaute identity defines the length of mature mammalian microRNAs. Nucleic Acids Res. 2012;40:6808–6820. doi: 10.1093/nar/gks293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 11.O’Carroll D, Mecklenbrauker I, Das PP, Santana A, Koenig U, Enright AJ, Miska EA, Tarakhovsky A. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng N, Li Y, Han ZG. Argonaute2 promotes tumor metastasis by way of up-regulating focal adhesion kinase expression in hepatocellular carcinoma. Hepatology. 2013;57:1906–1918. doi: 10.1002/hep.26202. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Fan XS, Wang CX, Liu B, Li Q, Zhou XJ. Up-regulation of Ago2 expression in gastric carcinoma. Med Oncol. 2013;30:628. doi: 10.1007/s12032-013-0628-2. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Liu M, Feng Y, Xu YF, Che JP, Wang GC, Zheng JH, Gao HJ. Evaluation of Argonaute protein as a predictive marker for human clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2013;6:1086–94. [PMC free article] [PubMed] [Google Scholar]
- 15.Greene FL, Page DL, Fleming ID, April F, Balch CM, Haller DG, Monica M. American Joint Committee on Cancer (AJCC) staging manual. 6th edition. Philadelphia: Springer; 2002. [Google Scholar]
- 16.Lynch HT, Smyrk TC. Identifying hereditary nonpolyposis colorectal cancer. N Engl J Med. 1998;338:1537–1538. doi: 10.1056/NEJM199805213382109. [DOI] [PubMed] [Google Scholar]
- 17.Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, Ferro V, McMillan NA, Swarbrick A, Mittal V, Mellick AS. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–352. doi: 10.1158/0008-5472.CAN-12-0271. [DOI] [PubMed] [Google Scholar]
- 18.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNA Cancer Regulation. Springer; 2013. MicroRNAs in Human Cancer; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Chahar HS, Abraham S, Wu H, Pierson TC, Wang XA, Manjunath N. Ago-2-mediated slicer activity is essential for anti-flaviviral efficacy of RNAi. PLoS One. 2011;6:e27551. doi: 10.1371/journal.pone.0027551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, Shyy JY. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057–1067. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]


