Abstract
We report a case of a 44-year-old woman with bilateral ovarian carcinoma that had metastasized from the colon and mimicked primary mucinous cystadenocarcinoma. Macroscopically, both ovarian tumors were large, multiloculated cystic masses with abundant mucinous content. Histologically, they were lined with mucinous epithelium with mild to moderate nuclear atypia and showed stromal invasion and surface involvement. At first, the tumors were diagnosed as bilateral primary ovarian mucinous cystadenocarcinomas. However, three months after surgery, a large villous tumor was discovered in the ascending colon by colonoscopic examination and was surgically resected. Histologically, the colonic tumor was a villous adenomatous tumor with invasive components of mucinous adenocarcinoma composed of well-differentiated adenocarcinoma and exhibited abundant extracellular mucin production. As a villous adenomatous component was present in the mucosal area, the colonic tumor was considered a primary tumor. Therefore, the original diagnosis of bilateral ovarian tumors was revised for consistent with metastasis from the colon carcinoma, in line with the findings of immunohistochemistry and loss of heterozygosity analysis. This case highlights the importance of considering the possibility of metastatic tumors from the gastrointestinal tract in the diagnosis of mucinous ovarian tumors.
Keywords: Krukenberg tumor, mucinous cystadenocarcinoma, metastatic ovarian carcinoma, mucinous adenocarcinoma
Introduction
The ovaries are a common site of metastasis in a variety of primary neoplasms and may also be a site of metastasis from unknown primary tumors. The colon and stomach are the most common primary cancer sites that metastasize to the ovary; however, tumors of the breast, lung, and pancreas have also been reported to metastasize in those sites [1-3]. In most cases, primary carcinoma is diagnosed prior to the detection of metastatic ovarian tumors; however, an ovarian mass can sometimes be the initial clinical manifestation of the primary disease. Such cases are often misdiagnosed as primary ovarian cancers [1,4-6]. It is of utmost importance to correctly classify ovarian tumors as either primary or metastatic, because the prognoses of these two conditions differ significantly and correct diagnosis has implications for the effective clinical management of patients [5,7].
Here, we report a case of metastatic ovarian adenocarcinoma initially diagnosed as primary adenocarcinoma in both ovaries. In this case, an accurate diagnosis of metastatic tumors was made following resection of the colonic adenocarcinoma accompanied by a loss of heterozygosity (LOH) analysis and immunohistochemistry.
Case report
A 44-year-old woman was admitted to our institute with bilateral ovarian masses. She had a history of epigastralgia for two years; however, an upper gastrointestinal examination revealed no significant findings. Physical examination revealed a large, firm, non-tender mass in the lower abdomen. Laboratory tests revealed elevated serum carcinoembryonic antigen levels (CEA) of 13.9 U and serum CA125 levels of 75.1 U. Abdominal ultrasonography revealed bilateral adnexal masses measuring 23.5 × 13.0 × 3.0 cm and 6.0 × 5.0 × 3.0 cm. Many ascites were also present. Abdominal computed tomography (CT) revealed that both ovarian tumors were multicystic tumors with thin intracystic septa and focal solid components, suggesting cystadenocarcinoma of the ovaries. No indications of liver metastases or lymph node metastases were found on CT. In light of these results, the ovarian tumors were diagnosed as primary ovarian carcinoma. Thereafter, radical hysterectomy and partial omentectomy were performed. Two liters of ascitic fluid and mucinous material were removed. Intraoperative findings included extremely large bilateral ovarian tumors and mucinous ascites, and the clinical diagnosis was “pseudomyxoma peritonei”.
Following surgery, the patient received platinum-based chemotherapy. Three months after surgery, the patient complained of hematochezia. Colonoscopic examination was performed and revealed a large villous tumor in the ascending colon. Biopsy samples taken from the villous tumor revealed tubule villous adenoma with moderate to severe atypia. Seven months after gynecological surgery, partial colectomy of the ascending colon was performed and a diagnosis of invasive mucinous adenocarcinoma was made. The patient eventually died of multiple liver metastases 34 months after her first admission.
Pathological findings
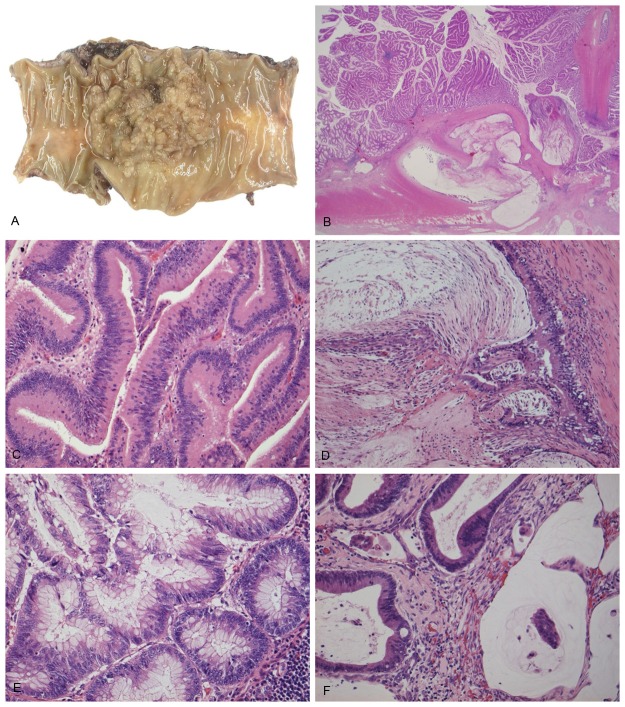
Grossly, the ovarian tumors were large and multicystic with thin cyst walls and septa, containing mucinous fluid (Figure 1A). The tumor surfaces were coarse. Microscopically, the cyst wall was lined with tall columnar mucinous-type epithelium showing various degrees of atypia with areas that appeared cytologically and histologically benign, areas that appeared borderline malignancy, and areas that appeared to be malignant (Figure 1B, 1C). Benign-appearing epithelial cells were composed of tall, columnar mucinous-type epithelia occurring in a single layer, with basally situated oval to mildly elongated nuclei (Figure 1D). The borderline-appearing areas showed finger-like projections of loose stroma covered by a single layer of cells, or complex papillary epithelial growth (Figure 1E). The epithelial cells had definitely enlarged nuclei that showed pseudostratification. The malignant-appearing areas had labyrinthine spaces with a loss of polarity (Figure 1F, 1G). The tumor showed stromal and surface invasion with extracellular mucinous fluid (Figure 1H). The pathological diagnosis was mucinous cystadenocarcinoma of both ovaries.
A.
The ovarian tumor is a largely multiloculated cyst with mural nodules and mimics a primary mucinous cystic tumor; (B) The cyst is lined in part by tall columnar cells, composed of cytologically benign-appearing cells in a single layer (arrow) or borderline cells with papillary architecture (arrow head); (C) The carcinoma area has a more complex papillary structure (arrow). (D-H) Benign area (D), borderline area (E), carcinoma area (F, G). Tumor cells were invading into the stroma with a mucin pool (H).
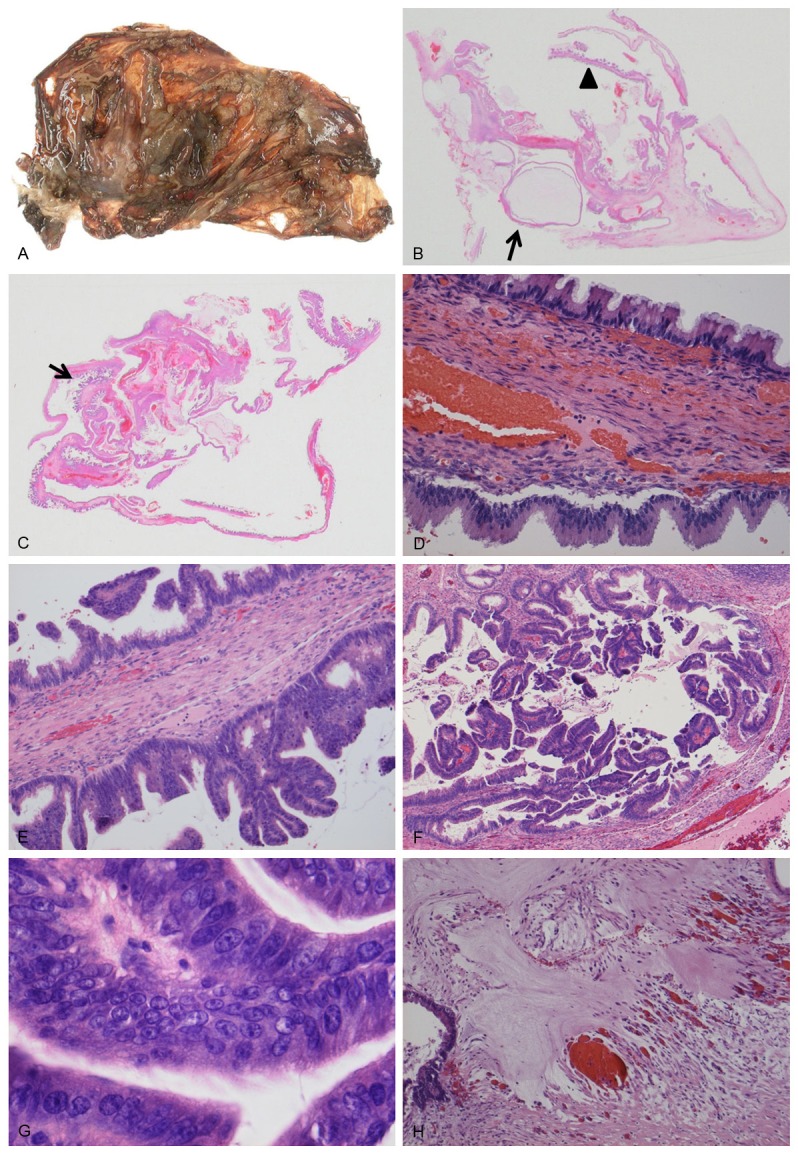
The colonic tumor measured 6.5 × 4.0 × 3.9 cm in size and showed a polypoid mass with a villous structure on the surface (Figure 2A). Microscopically, the tumor was composed of a tubule villous adenomatous component on the surface that extended to the middle portion of the tumor, and a mucinous carcinoma component in the deeply invading area extending from the muscularis propria to the subserosal adipose tissue (Figure 2B-F). Lymphovascular invasion was noted; however, no lymph node metastasis was identified. In light of these results, the colonic tumor was considered to be the primary mucinous adenocarcinoma that had arisen from the villous adenoma.
A.
A polypoid mass with a villous structure is seen on the surface of the ascending colon. Tumor size: 6.5 × 4.0 × 3.9 cm; (B) Ascending colon tumor directly invading the muscular tissue with pools of extracellular mucin; (C-F) Villous (C), mucinous (D), and tubular (E) components of the colonic tumor. The disseminated tumor of the omentum was composed of mucinous components (F).
Immunohistochemical findings
Representative sections from both the ovarian and colonic tumors were stained immunohistochemically with cytokeratin 7 (CK7) (1:100, OV-TL-12-30, DAKO, Glostrup, Denmark), CK20 (1:40, Ks 20.8, DAKO, Denmark), CEA (1:175, DAKO, Glostrup, Denmark), MUC2 (1:200, Ccp58, Novocastra, Newcastle upon Tyne, UK), MUC5AC (1:100, CLH2, Novocastra, Newcastle upon Tyne, UK).
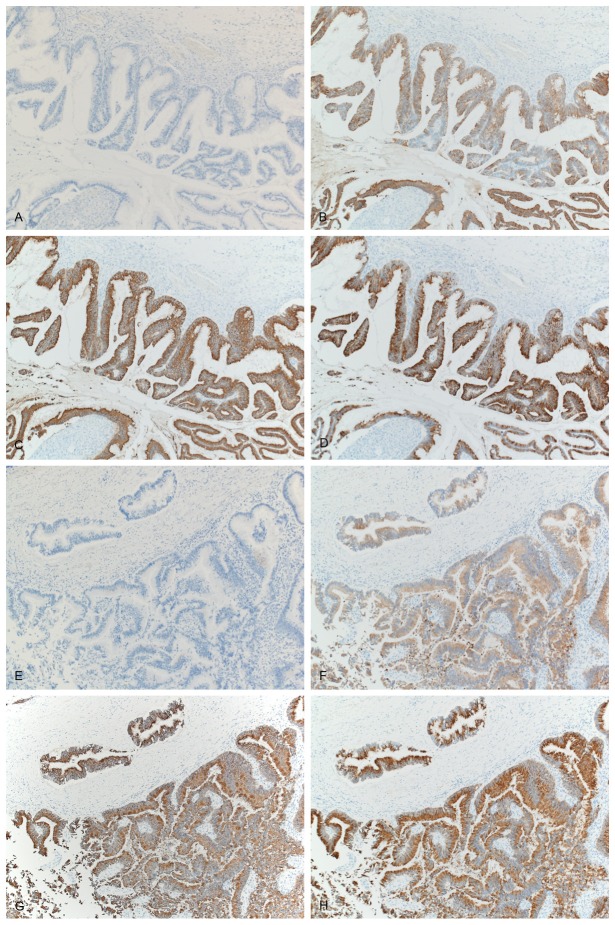
The results of the immunohistochemical stains were the same in both the ovarian and colonic tumors (Figure 3A-H). Mucinous tumor cells showed positive immunoreactivity for CK20, MUC2, and MUC5AC, but negative staining for CK7, leading to a final diagnosis of primary colonic mucinous adenocarcinoma and metastatic ovarian tumor.
Figure 3.
Immunohistochemical findings of ovarian (A-D) and ascending colon (E-H) tumors. Both tumors showed negative staining for CK7 (A, E) but positive staining for CK20 (B, F), MUC2 (C, G), and MUC5AC (D, H).
Loss of heterozygosity analysis
LOH analysis was conducted from three different foci in the ovarian tumor: the benign-appearing area (OT1), the borderline-appearing area (OT2), and the malignant-appearing area (OT3) (Figure 4). Similar analyses were conducted on samples of the colon tumor: the villous area (CT1), the invasive mucinous carcinoma area (CT2), the adenomatous area (CT3), and the omentum nodule (CT4). Normal control tissue was obtained from adjacent non-malignant colonic tissues (N) and microdissection and DNA extraction were performed as described previously [8]. All polymerase chain reaction primers for the microsatellite markers were purchased from Research Genetics (Huntsville, AL, USA). The following primers were used: 1p (D1S228); 3p (D2S1234, D3S1286, D3S1293); 5q (D5S644, D5S346, D5S1956, D5S2072); 6q (D6S473); 8p (D8S133); and 22q (D22S- 584).
Figure 4.
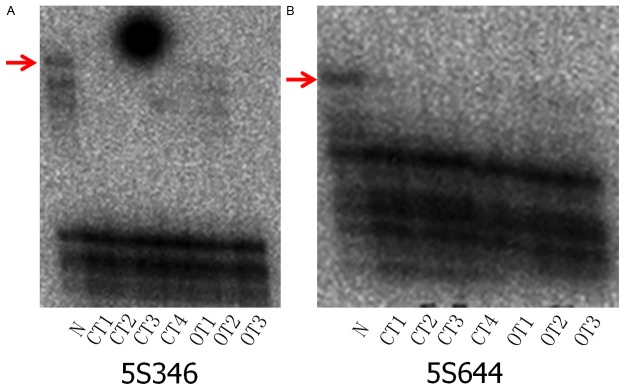
Representative gels showing loss of heterozygosity (LOH). Gels for each sample were run in duplicate wells. N: Normal control DNA, CT1: villous component from colon carcinoma, CT2: mucinous component from colon carcinoma, CT3: tubular component from colon carcinoma, CT4: dissemination of the omentum. OT1: Adenomatous area of the ovarian tumor, OT2: borderline area from ovarian tumor, OT3: Adenocarcinoma from ovarian tumor, 5S346 (A) and D5S644 (B).
Analyses revealed identical allelic loss of the chromosomal locus 5q (D5S644, D5S346) in all of the tumor components from both tumors (OT1-3, CT1-4) and further confirmed the diagnosis in this case (Figure 4A, 4B).
Discussion
It is sometimes extremely challenging to distinguish between metastatic mucinous carcinomas and primary mucinous carcinomas of the ovary [4,6,9], due to the fact that both present as cystic tumors on gross examination. In general, macroscopically, mainly cystic and/or multicystic ovarian tumors with thin septa are considered primary ovarian tumors. Many authors have reported that colorectal adenocarcinomas that have metastasized to the ovary may mimic primary ovarian mucinous neoplasms, as they may be cystic and non-necrotic [4-6,10]. Lee et al. [6] suggested the following findings as being strongly suggestive of a metastatic ovarian tumor: (1) bilaterality, (2) microscopic surface involvement by epithelial cells (surface implants), and (3) an infiltrative pattern of stromal invasion. On the other hand, the findings strongly suggestive of a primary ovarian cancer are (1) an “expansile” pattern of invasion and (2) a complex papillary pattern. Findings that are often common to both and make it difficult to distinguish between the two are (1) a cystic gross appearance, (2) gross solid or papillary necrotic or hemorrhagic areas, (3) nature of the cyst contents, (4) stromal mucin, (5) cribriform, villous, or solid growth patterns, (6) focal areas resembling typical colonic carcinoma, (7) presence of goblet cells, and (8) presence of various grades of tumor. In this case, the bilateral ovarian tumors and presence of surface implants may have more strongly indicated toward metastatic carcinoma, although it has also been reported that 75% of metastatic ovarian carcinomas are unilateral and that all metastatic ovarian carcinomas from primary tumors of the large intestine show solid and cystic components, with a smooth surface [11].
As was seen in the current case, the so-called “maturation phenomenon”, which refers to the presence of benign-appearing and borderline-appearing areas, occurs in approximately one-third of metastatic mucinous carcinomas [6]. The presence of regions resembling ovarian mucinous neoplasms of borderline tumors was notable, a finding that has been reported in previous studies of mucinous ovarian neoplasms [5,6,12] and represents a significant problem for the correct diagnosis of metastatic lesions.
Immunohistochemistry can be very helpful in distinguishing primary ovarian tumors from metastatic tumors [5], although immunohistochemistry was not initially performed for the ovarian tumor in this case. In the majority of cases, primary ovarian neoplasms show positive staining for CK7 and negative staining for CK20, whereas colorectal tumors are most frequently negative for CK7 and positive for CK20 [13,14]. Examination of the expression of mucin gene products for MUC5AC and MUC2 may also be of diagnostic value [3,10]. Constance et al. reported that all but one of 10 metastatic ovarian tumors from colon adenocarcinomas expressed MUC2, whereas none expressed MUC5AC, and that all 32 primary mucinous ovarian tumors in their study expressed MUC5AC; the percentages of MUC2-positive immunostaining for cystadenomas, borderline tumors, and cystadenocarcinomas were 0 of 10, 5 of 10, and 7 of 10, respectively [15]. MUC5AC has been shown to be typically expressed in ovarian mucinous neoplasms, but not in colorectal carcinomas [10]. A retrospective review of the immunohistochemical profile in this case strongly suggested that the ovarian tumor metastasized from colonic invasive mucinous adenocarcinoma, even though positive staining for MUC5AC seemed to be inconsistent with a colonic origin.
It has previously been shown that the LOH in the 5q region is relatively frequent in ovarian neoplasms such as serous and undifferentiated adenocarcinomas and endometrioid carcinomas, though it is rare in mucinous adenocarcinomas [16]. On the other hand, in colorectal carcinoma, frequency of LOH in the 5q region has been reported to be rare [17]. Considering the lower frequency of LOH in the 5q region in ovarian mucinous adenocarcinoma and adenocarcinoma of colorectal origin in this patient, the pattern of LOH in the case reported here provides strong evidence that both tumors are the consequent tumors of the same origin.
In this case, the absence of previously diagnosed or concurrent carcinoma with similar morphology allowed us to make an original diagnosis of primary mucinous cystadenocarcinoma of the bilateral ovaries. It is a well-known phenomenon that the metastatic lesion sometimes grows rapidly after resection of the primary tumor [18,19], although the inverse scenario is not well described in the literature. It is not clear whether the mucinous adenocarcinoma of the ascending colon was already relatively large in size at the time of first admission or whether it grew rapidly after resection of the metastatic ovarian tumors, because endoscopic examination of the colon was not performed.
We report a case of bilateral ovarian carcinoma that had metastasized from the colon and mimicked primary mucinous cystadenocarcinoma, especially with respect to MUC5AC expression. This case also provides a clinical learning that systemic examination is necessary even if the large ovarian tumors suspicious of primary malignancy were noticed.
Acknowledgements
This study was conducted with the approval of the Ethics Committee of Juntendo University School of Medicine. This work was supported in part by a grant-in-aid for General Scientific Research from the Ministry of Education, Science, Sports and Culture (#23590434 to Tsuyoshi Saito), Tokyo, Japan.
Disclosure of conflict of interest
None.
References
- 1.Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract: analysis of 325 cases. Cancer. 1984;53:1978–1984. doi: 10.1002/1097-0142(19840501)53:9<1978::aid-cncr2820530929>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff JD, Novak ER. The Krukenberg tumor: study of 48 cases from the ovarian tumor registry. Obstet Gynecol. 1960;15:351–360. [PubMed] [Google Scholar]
- 3.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: A clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277–299. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 4.Ha HK, Baek SY, Kim SH, Kim HH, Chung EC, Yeon KM. Krukenberg’s tumor of the ovary: MR imaging features. AJR Am J Roentgenol. 1995;164:1435–1439. doi: 10.2214/ajr.164.6.7754887. [DOI] [PubMed] [Google Scholar]
- 5.Lewis MR, Deavers MT, Silva EG, Malpica A. Ovarian involvement by metastatic colorectal adenocarcinoma: still a diagnostic challenge. Am J Surg Pathol. 2006;30:177–184. doi: 10.1097/01.pas.0000176436.26821.8a. [DOI] [PubMed] [Google Scholar]
- 6.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 8.Fujii H, Inagaki M, Kasai S, Miyokawa N, Tokusashi Y, Gabrielson E, Hruban RH. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151:1447–1454. [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KC, Gold BM. Computed tomography of Krukenberg tumor. AJR Am J Roentgenol. 1985;145:285–288. doi: 10.2214/ajr.145.2.285. [DOI] [PubMed] [Google Scholar]
- 10.McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231–247. doi: 10.1111/j.1365-2559.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 11.Daya D, Nazerali L, Frank GL. Metastatic ovarian carcinoma of large intestinal origin simulating primary ovarian carcinoma: A clinicopathologic study of 25 cases. Am J Clin Pathol. 1992;97:751–758. doi: 10.1093/ajcp/97.6.751. [DOI] [PubMed] [Google Scholar]
- 12.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol. 1999;23:617–635. doi: 10.1097/00000478-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Berezowski K, Stastny JF, Kornstein MJ. Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol. 1996;9:426–429. [PubMed] [Google Scholar]
- 14.Wauters CC, Smedts F, Gerrits LG, Bosman FT, Ramaekers FC. Keratins 7 and 20 as diagnostic markers of carcinomas metastatic to the ovary. Hum Pathol. 1995;26:852–855. doi: 10.1016/0046-8177(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 15.Albarracin CT, Jafri J, Montag AG, Hart J, Kuan SF. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 2000;31:672–677. doi: 10.1053/hupa.2000.6799. [DOI] [PubMed] [Google Scholar]
- 16.Tavassoli M, Steingrimsdottir H, Pierce E, Jiang X, Alagoz M, Farzaneh F, Campbell IG. Loss of heterozygosity on chromosome 5q in ovarian cancer is frequently accompanied by TP53 mutation and identifies a tumour suppressor gene locus at 5q13.1-21. Br J Cancer. 1996;74:115–119. doi: 10.1038/bjc.1996.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold CN, Goel A, Niedzwiecki D, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. APC promoter hypermethylation contributes to the loss of APC expression in colorectal carcinoma with allelic loss on 5q. Cancer Biol Ther. 2004;3:960–964. doi: 10.4161/cbt.3.10.1113. [DOI] [PubMed] [Google Scholar]
- 18.Simpson-Herren L, Sanford AH, Holmquist JP. Effects of surgery on the cell kinetics of residual tumor. Cancer Treat Rep. 1976;12:1749–1760. [PubMed] [Google Scholar]
- 19.Simpson-Herren L, Sanford AH, Holmquist JP. Cell population kinetics of transplanted and metastatic Lewis lung carcinoma. Cell Tissue Kinet. 1974;7:349–361. doi: 10.1111/j.1365-2184.1974.tb00417.x. [DOI] [PubMed] [Google Scholar]