Abstract
Composite lymphoma (CL) refers to the presence of two or more distinct types of lymphomas in a single organ or tissue. CL is an infrequent finding and may be due to the existence of two genetically related neoplasms, i.e. transformation of a single lymphoma into another lymphoma, or be due to the presence of two clonally unrelated lymphomas. CL composed of more than two lymphomas is even rare. Herein we describe a case of diffuse large B-cell lymphoma (DLBCL) arising in a CL of follicular lymphoma (FL) and small lymphocytic lymphoma (SLL) in an inguinal lymph node of an 85 year old woman. The three lymphomas were morphologically and immunophenotypically distinct while flow cytometry detected two monoclonal B-cell populations. Karyotyping and Polymerase Chain Reaction (PCR) for B-cell clonality each detected a single monoclonal B-cell population. The morphology findings may suggest DLBCL being transformed from FL while Richter transformation from SLL appears to be less likely in our case. Due to the single clone by chromosome study and PCR study, the precise relationships of the three lymphomas are unknown.
Keywords: Composite lymphoma, bi-clonality, PCR, diffuse large B-cell lymphoma
Introduction
Composite lymphoma (CL) is defined as the co-occurrence of two or more distinct types of lymphoma at the same anatomic site. CL consisting of three morphologically and immunophenotypically separate lymphomas are a rare finding in the literature. We describe a CL composed of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and small lymphocytic lymphoma (SLL) in an inguinal lymph node of an 85 year old female.
Materials and methods
Case history
The patient was an 85 year old white female who presented with weight loss and growing masses in the groin and neck areas for several months. Her past medical history included breast cancer 12 years ago with a subsequent radical mastectomy. She did not receive chemotherapy as part of her breast cancer treatment. An excisional lymph node biopsy was performed in her left groin and revealed a 5.5 × 4.0 × 3.0 cm mass. After her surgery and final diagnosis, the patient was referred to the oncology service but expired before any intervention.
Pathology
This study used histologic evaluation by hematoxylin & eosin and immunohistochemical staining, multicolor flow cytometry analysis and Polymerase Chain Reaction (PCR) for immunoglobulin heavy (IgH) gene rearrangement using IgVH frame work 2 (FR2) as well as conventional karyotyping. PCR studies were performed at ARUP laboratories (Salt Lake City, UT, US) and chromosome analysis was performed at LabCorp laboratory (RTP, NC, US) while the remaining studies are performed at authors’ institution.
Results
Grossly, the cut surface of the lymph node was tan-yellow and smooth with focal areas of hemorrhage. Histologically, the lymph node architecture was completely effaced by a diffuse lymphoid infiltrate (Figure 1). The majority of the infiltrate showed sheets of large atypical lymphoid cells (Figure 2). The large atypical lymphoid cells had centroblastic morphology with abundant cytoplasm, vesicular nuclear chromatin pattern and one to several nucleoli. These large atypical lymphoid cells stained positive for CD20, CD10, and weak BCL-2 but not CD5 or cyclin D1. These features were consistent with a diagnosis of DLBCL. There was also a focal follicular pattern adjacent to the diffuse areas. These follicles were filled with sheets of large atypical lymphoid cells demonstrating similar immunohistochemistry profiles as that in diffuse areas. These follicular areas were consistent with a FL (grade IIIB). Additionally, there were areas composed of small lymphoid cells in interfollicular regions of residual lymphoid follicles. Small lymphoid cells had condensed nuclear chromatin and inconspicuous nucleoli. These cells were mostly B-cells expressing weak CD20. These B cells coexpressed with CD5 but not cyclin D1, with the features consistent with a SLL. The SLL cells were also focally present in the DLBCL areas.
Figure 1.

Low magnification of the lymphoid infiltrate (× 100).
Figure 2.
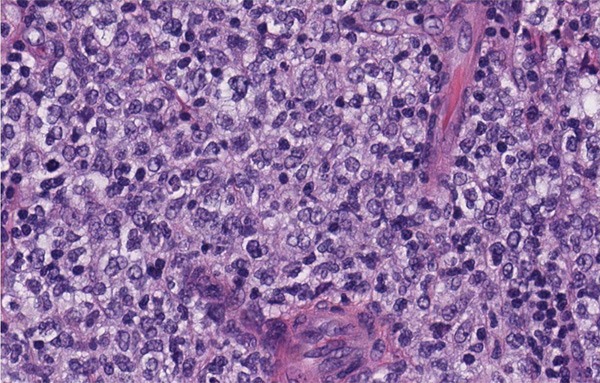
High magnification shows diffuse sheets of large atypical lymphoid cells with centroblast morphology (× 400).
Cytogenetics analysis revealed one hyperdiploid clone with a karyotyping of 47-48, XX, add (2) (p23), del (6) (q15), + 11, + mar ([12] / 46, XX [8]. Flow cytometry detected two populations of monoclonal B-cells (Figure 3). One expressed CD10 without CD5, while the other expressed CD5 but was negative for CD10. Both B-cell populations were lambda light chain restricted. PCR for B-cell clonality detected a single monoclonal immunoglobulin heavy chain gene rearrangement in the framework 2 region (Figure 4).
Figure 3.

Histogram of the flow cytometry study demonstrated two populations of monoclonal B-cells. The gated events in red are lambda light chain restricted B-cells expressing CD19, CD20, and CD10 while gated events in green are lambda light chain restricted B-cells expressing CD19, CD20, CD5, CD43 but negative for CD10.
Figure 4.

Capillary electrophoresis based PCR study showed one restricted monoclonal band.
Discussion
The term CL refers to the presence of two or more morphologically and immunophenotypically distinct lymphomas in a single anatomic site [1]. They can occur as multiple B-cell lymphomas, multiple T-cell lymphomas or a combination of a B-cell and T-cell lymphoma. CL is a rare finding in the literature. The actual incidence of CL maybe underestimated due to the lack of advanced techniques in many parts of the world that are needed to diagnose them [2]. CL consisting of three different lymphomas is even rarer. Steinhoff et al reported a case of CL composed of three lymphomas in one patient but in different anatomic sites. The lymphomas were primary cutaneous marginal zone B-cell lymphoma, nodal Epstein-Barr virus associated classic Hodgkin’s lymphoma of B-cell type, and peripheral T-cell lymphoma coexisting in the skin and cervical lymph node [3]. In their case, molecular studies proved that the three lymphomas were clonally unrelated. In our case, three lymphomas were found in a single lymph node. These lymphomas were one low grade lymphoma (SLL) and two high grade lymphomas (FL IIIB and DLBCL). Flow cytometry findings of two monoclonal B-cell populations support the CL. However, PCR and cytogenetics analyses each reported a single monoclonal B-cell population.
CL consisting of two low grade B-cell lymphomas has been reported to be biclonal. Fend et al presented three different cases of CL that consisted of two distinct low grade B-cell lymphomas [4]. The molecular studies failed to show biclonality based on DNA extracted from the whole tissue block. The use of laser microdissection might help to establish the presence of the two clonal populations. A more recent study by Boiocchi et al reported that CL composed of CLL and FL was biclonal and pathogenetically different from a CL of a low grade B-cell lymphoma and an aggressive B-cell lymphoma [5]. They used laser microdissection to isolate the appropriate tissue samples and sequenced IgH rearrangements to prove the presence of two separate B-cell clones.
Unique to our case is the presence of a high grade lymphoma in addition to SLL. The DLBCL component characterized the majority of the atypical lymphoid infiltrate seen in the lymph node. Of note, there was a focal follicular pattern adjacent to the DLBCL. This may indicate a large cell transformation from an underlying follicular lymphoma. FL and transformed DLBCL have been shown to be clonally related [6,7]. One suggested mechanism of the transformation of FL to DLBCL is via increased expression of genes involved in cellular proliferation [8]. Our cytogenetics findings included the amplification and deletion of different chromosomal segments. Deleted segments or whole chromosome loss usually involves the loss of known or putative tumor suppressor genes while the amplified segments typically correspond to regions which are thought to provide clonal selective proliferative advantage. The combination of the chromosomal loss and amplification could have led to a net increase in cellular proliferation gene activity and been the inciting factor of the transformation from FL to DLBCL. Interestingly, neoplastic SLL cells are focally present and intersperse in the DLBCL areas. However, there were no obvious transitional zones as seen in the FL/DLBCL areas. It does not appear to be a Richter transformation from CLL in our case. The Richter transformation describes the development of an aggressive non-Hodgkin’s lymphoma or Hodgkin’s lymphoma from SLL or chronic lymphocytic leukemia (CLL) [9]. Richter’s transformation occurs in about 3-11% of patients with CLL [10,11]. Trisomy 12 and chromosome 11 abnormalities, as well as multiple genetic defects, have been described in patients with Richter’s transformation [12]. Because of the heterogeneity of the genetic lesions seen, it is likely that there are different Richter’s transformation subsets, possibly due to different transforming mechanisms [13]. The clinical outcome of Richter’s transformation is generally poor. Numerous therapies can induce a response, but patients typically die within a few months after Richter’s transformation [14].
In summary, DLBCL arising from a CL including indolent lymphomas is rare. Although the morphology may impose diagnostic challenges for the SLL component in a CL, the biclonality by flow cytometry should provide a clue for CL. The karyotyping results of one clone and one single monoclonal band by PCR might suggest the three lymphomas are clonally related, but further study, such as microdissection, may needed to clarify this.
Disclosure of conflict of interest
None.
References
- 1.Sanchez S, Holmes H, Katabi N, Newman J, Domiatti-Saad R, Stone M, Netto G. Composite lymphocyte-rich Hodgkin’s lymphoma and peripheral T-cell lymphoma associated with Epstein Barr virus: A case report and review of the literature. Arch Pathol Lab Med. 2006;130:107–112. doi: 10.5858/2006-130-107-CLHLAP. [DOI] [PubMed] [Google Scholar]
- 2.Mokhtar NM. Composite Lymphoma. J Egyptian Nat Cancer Inst. 2007;19:171–175. [PubMed] [Google Scholar]
- 3.Steinhoff M, Assaf C, Anagnostopoulos I, Geilen CC, Stein H, Hummel M. Three coexisting lymphomas in one patient: genetically related or only a coincidence? J Clin Pathol. 2006;59:1312–1315. doi: 10.1136/jcp.2005.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fend F, Quintanilla-Martinez L, Kumar S, Beaty MW, Blum L, Sorbara L, Jaffe ES, Raffeld M. Composite Low Grade B-Cell Lymphomas with Two Immunophenotypically Distinct Cell Populations Are True Biclonal Lymphomas. A Molecular Analysis Using Laser Capture Microdissection. Am J Pathol. 1999;154:1857–1866. doi: 10.1016/S0002-9440(10)65443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boiocchi L, Witter RE, He B, Subramaniyam S, Mathew S, Nie K, Cerutti A, Coleman M, Knowles DM, Orazi A, Tam W. Composite Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma and Follicular Lymphoma Are Biclonal Lymphomas. A report of Two Cases. Am J Clin Pathol. 2012;137:647–659. doi: 10.1309/AJCPHXO5UGW2OELA. [DOI] [PubMed] [Google Scholar]
- 6.Matolcsy A, Schattner EJ, Knowles DM, Casali P. Clonal evolution of B cells in transformation from low- to high-grade lymphoma. Eur J Immunol. 1999;29:1253–1264. doi: 10.1002/(SICI)1521-4141(199904)29:04<1253::AID-IMMU1253>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelenetz AD, Chen TT, Levy R. Histologic transformation of follicular lymphoma to diffuse lymphoma represents tumor progression by a single malignant B cell. J Exp Med. 1991;173:197–207. doi: 10.1084/jem.173.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AJ, Rosenwald A, Wright G, Lee A, Last KW, Weisenburger DD, Chan WC, Delabie J, Braziel RM, Campo E, Gascoyne RD, Jaffe ES, Muller-Hermelink HK, Ott G, Calaminici M, Norton AJ, Goff LK, Fitzgibbon J, Staudt LM, Lister TA. Transformation of follicular lymphoma to diffuse large B-cell lymphoma proceeds by distinct oncogenic mechanisms. Br J Haematol. 2007;136:286–293. doi: 10.1111/j.1365-2141.2006.06439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giardino AA, O’Regan K, Jagannathan JP, Elco C, Ramaiya N, Lacasce A. Richter’s Transformation of Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2011;29:e274–276. doi: 10.1200/JCO.2010.32.6579. [DOI] [PubMed] [Google Scholar]
- 10.Rossi D, Gaidano G. Richter syndrome: Molecular insights and clinical perspectives. Hematol Oncol. 2009;27:1–10. doi: 10.1002/hon.880. [DOI] [PubMed] [Google Scholar]
- 11.Omoti CE, Omoti AE. Richter syndrome: A review of clinical, ocular, neurological and other manifestations. Br J Haematol. 2008;142:709–716. doi: 10.1111/j.1365-2141.2008.07248.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsimberidou AM, Keating MJ. Richter’s Transformation in Chronic Lymphocytic Leukemia. Semin Oncol. 2006;33:250–256. doi: 10.1053/j.seminoncol.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Scandurra M, Rossi D, Deambrogi C, Rancoita P, Chigrinova E, Mian M, Cerri M, Rasi S, Sozzi E, Forconi F, Ponzoni M, Moreno SM, Piris MA, Inghirami M, Zucca E, Gattei V, Rinaldi A, Kwee I, Gaidano G, Bertoni F. Genomic profiling of Richter’s syndrome: recurrent lesions and differences with de novo diffuse large B-cell lymphoma. Hematol Oncol. 2010;28:62–67. doi: 10.1002/hon.932. [DOI] [PubMed] [Google Scholar]
- 14.Tsimberidou AM, O’Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, Wen S, Do KA, Smith SC, Lerner S, Freireich EJ, Keating MJ. Clinical Outcomes and Prognostic Factors in Patients With Richter’s Syndrome Treated With Chemotherapy or Chemoimmunotherapy With or Without Stem-Cell Transplantation. J. Clin. Oncol. 2006;24:2343–2350. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]


