Abstract
Multiple intrahepatic portosystemic shunt (IPSS) without portal hypertension, now thought to be congenital in origin, is very rare. The presence of IPSS, unlike other congenital diseases, may not be recognized for several decades due to the time it takes to develop hepatic encephalopathy. In this article, we report an autopsy case of an 80-year-old Japanese woman with a one-month history of hyperammonemic encephalopathy. Radiological examination of the liver revealed some abnormal connections between the branches of the portal veins and the hepatic veins, but the cause of the aberrant blood flow was not found. The cause of death was extensive cerebral infarction due to thromboembolism. At postmortem examination, multiple anomalous blood vessels were identified histologically in both lobes of the non-cirrhotic liver. In comparison with the few similar cases existing in the literature, this case should be diagnosed as congenital IPSS. To our knowledge, this is the first detailed histological study of IPSS, as several autopsy case reports exist but their histological descriptions are poor. Unlike past reports, the shunt vessels were accompanied by clear elastic lamellae that were microscopically observed. In addition to shunt vessels, septal fibrosis, disorder of hepatic acinar structure, and sinusoidal dilatation and capillarization were observed in the liver. We suggest that these histological modifications observed in the circumference of the shunt vessels acted as secondary regenerative/hyperplastic changes based on blood-flow imbalance caused by the IPSS.
Keywords: Congenital intrahepatic portosystemic shunt, hepatic encephalopathy, autopsy
Introduction
Hepatic encephalopathy commonly results from critical liver damage caused by cirrhosis or fulminant hepatitis and the collateral circulation secondarily formed by liver damage or portal vein blockade in patients. In addition to these acquired portosystemic shunts, rare congenital abnormalities also occur. Congenital portosystemic shunts may be extrahepatic or intrahepatic; the former was first reported in 1793 by John Abernethy [1], known as “Abernethy malformation”. There are many examples of Abernethy malformation in the literature. Intrahepatic portosystemic shunt (IPSS) is rare and congenital in origin and was first reported in 1964 by Raskin et al. [2]. It is currently defined as a communication between the portal and the systemic venous circulation measuring more than 1 mm in diameter and at least partially located inside the liver [3,4]. Park et al. subclassified IPSS into four types based on the morphological varieties of the shunt vessels in the liver (Table 1) [5]. According to the classification, type 1 (a single large vessel connecting the right portal vein to the inferior vena cava) and type 2 (a localized peripheral shunt in which one hepatic segment has one or more communications between peripheral branches of the portal veins and the hepatic veins) are comparatively common. Some shunts spontaneously close during infancy. Most of these abnormalities, especially when symptomatic, are detectable radiologically and treated accordingly. In this article, we present the case of an aged woman with type 4 (multiple communications between the portal veins and the hepatic veins distributed in both lobes) IPSS and make histological observations regarding the etiology.
Table 1.
Classification of congenital intrahepatic shunt
| Type 1 | A single large vessel connecting the PV or its right branch to the IVC |
| Type 2 | Localized peripheral shunt between the peripheral branches of the PV and the hepatic veins in one hepatic segment |
| Type 3 | Peripheral portal and hepatic veins are connected through an aneurysm |
| Type 4 | Multiple communications between peripheral portal and hepatic veins distributed diffusely in both lobes |
Case report
An 80-year-old Japanese woman with a past history of cerebral infarction, atrial fibrillation, and unspecified jaundice for 20 years was admitted to our hospital due to repeated and progressive episodes of psychiatric disorders, perceptual disturbance, nystagmus, left side paresis, and aggravated voluntary hand and foot action for one month. At the time of admission, laboratory studies indicated hyperammonemia (300 mg/ml), and hepatic encephalopathy was suspected. However, she exhibited no clinical signs of either portal hypertension or liver cirrhosis. Other laboratory data revealed albumin 4.5 g/dl, glutamic oxaloacetic transaminase 37 IU/l, glutamic pyruvic transaminase 12 IU/l, total bilirubin 2.9 mg/dl, and alkaline phosphatase 330 IU/l. The patient was negative for both hepatitis B and hepatitis C virus infections. Contrast-enhanced computed tomography of the liver showed multiple shunts between the peripheral parts of the hepatic veins and portal veins (Figure 1), which were thought to be portosystemic-venous shunts of unknown cause. Extrahepatic portosystemic shunts and morphological aberration of the liver or spleen were not detected. Medical treatment with lactulose and branched-chain-amino-acid was administered for hepatic encephalopathy. Approximately one month after admission, she developed extensive cerebral infarction and died suddenly. A postmortem examination was performed.
Figure 1.
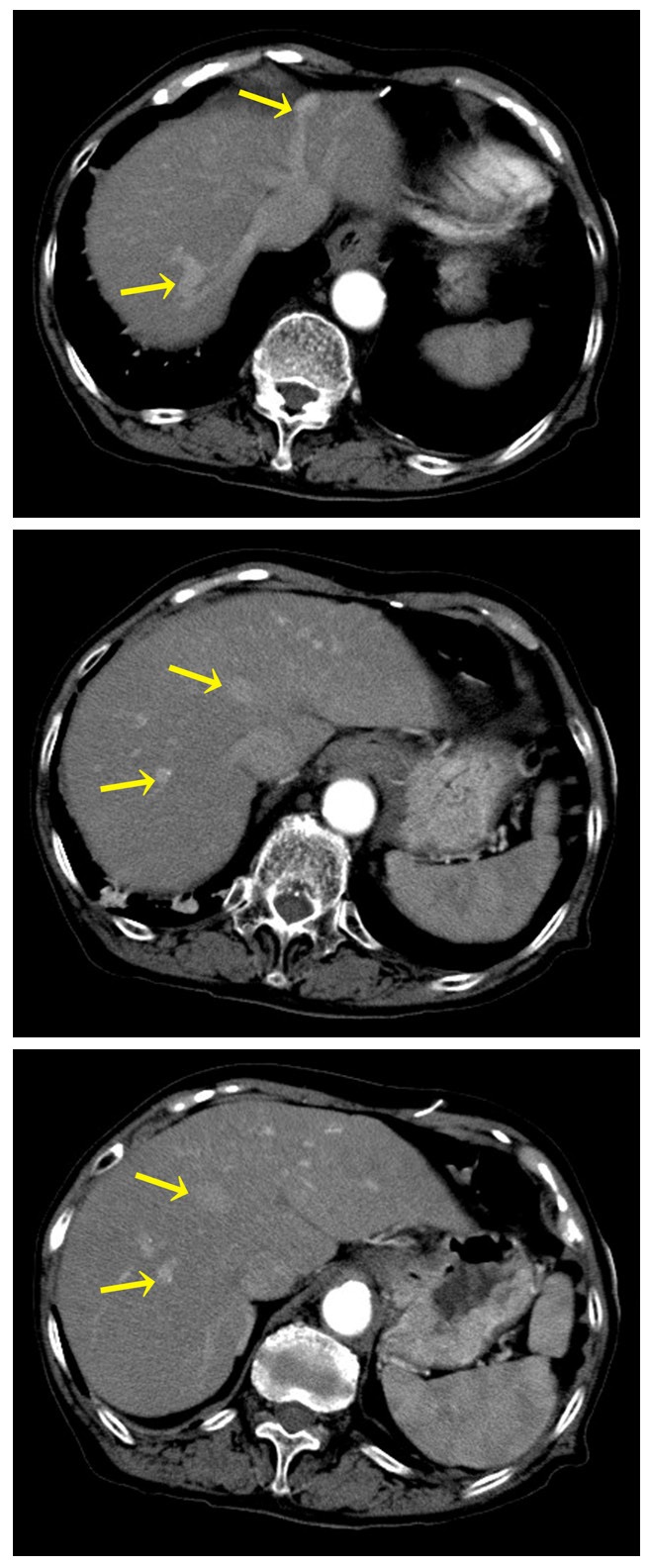
At contrast enhanced CT, Some unusual blood vessels connected hepatic veins and the portal branches (arrows) in both lobes of the liver.
Complete autopsy, including brain examination, was performed 16 h after death. The woman was 143.5 cm in height and weighed 37.5 kg (body mass index: 18.2 kg/m2). Gross hepatopathic findings, such as jaundice, blood spot, spider angioma and dilated epigastric veins), were not observed. On internal examination, effusions (pleural, pericardial, and peritoneal) and splenomegaly were not present. Gastric and esophageal varices and angiectopia in the abdomen were not detected. The liver was brown-colored, and the exterior features were normal in shape (910 g in weight). On cut sections of the liver, irregular congestion and a slight granular appearance were noted. Unusual blood vessels that were detected radiologically were not expressed on the cut surface. No palpable nodular mass, hemorrhage, necrosis, or infarction was found (Figure 2A). Upon cranial examination, expanded brain stem necrosis and large (over 4 cm in diameter) blood clots were found; thus, the cause of death was compatible with brainstem hemorrhagic infarction due to thromboembolism.
Figure 2.
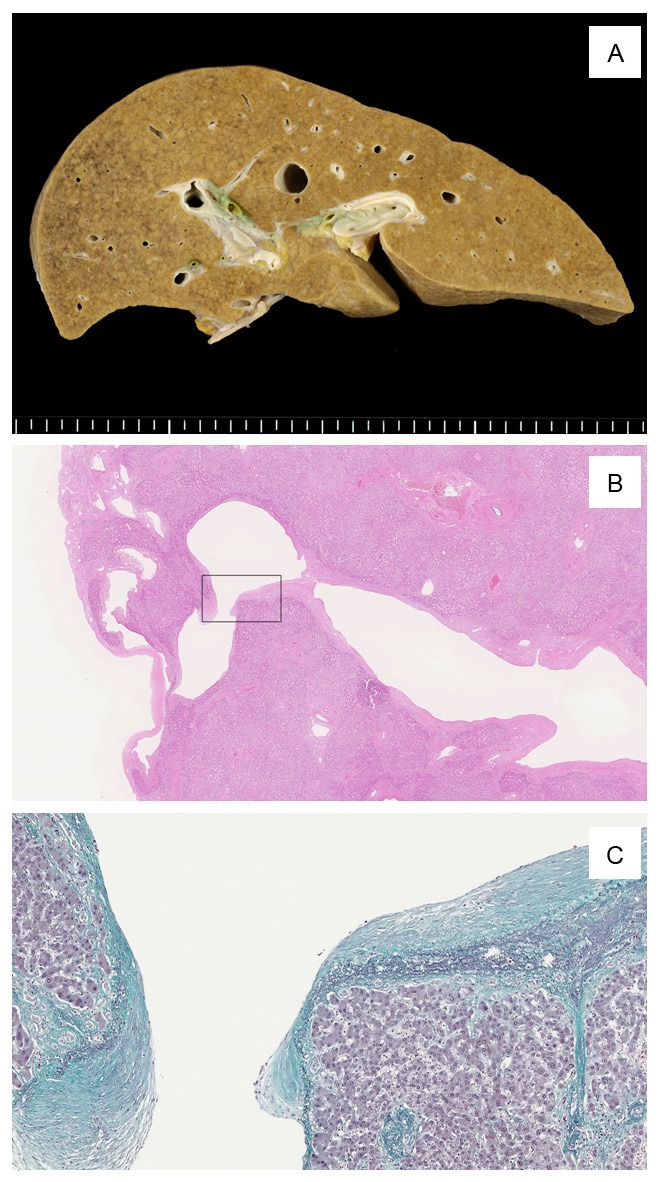
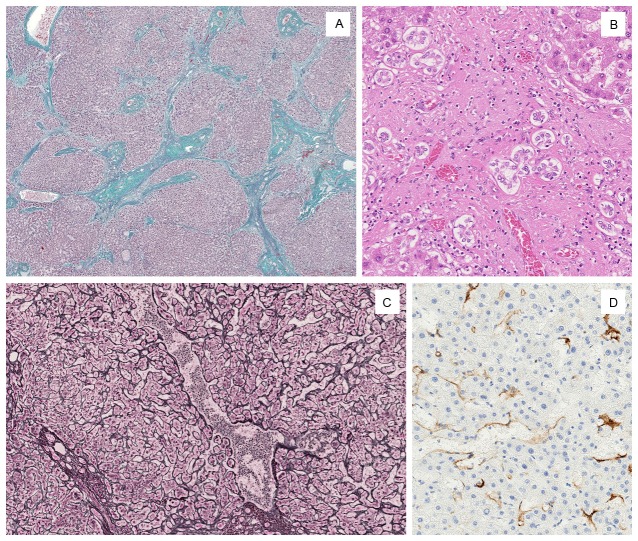
A: Liver shows a little irregular congestion and slight granular appearance macroscopically. B: Some distended shunt vessels were identified histologically in both lobes of the liver. This image shows one of those vessels. This vessel was located directly under the serosa aside from venous ligament. Box indicates the site of image C. C: Shunt vessel was accompanied by incrassate elastic lamellae (Elastica-Masson stain).
Upon microscopic examination, more than two distended blood vessels (up to 6 mm in diameter) were identified in both lobes of the liver. One of the distended vessels was directly under the serosa and represented the vessel detected by antemortem radiological study (Figures 1, 2B); this vessel was assumed to be the shunt vessel. Elastica-Masson staining revealed the presence of elastic lamellae in the wall of these shunt vessels (Figure 2C). The normal acinar structure was retained in most areas of the liver. Irregular slight fibrosis, size differences in the hepatic acinar structure, and extended sinusoids were observed in the circumference of the shunt vessels (Figure 3A, 3C). These nodules of hepatocytes were focally surrounded by fibrous septa that contained artery branches, portal vein branches and bile ductules. Several portal vein branches were markedly dilated and congested. Some degree of bile ductule proliferation was observed (Figure 3B). Inflammatory cell infiltration was absent or scarce. Hepatocytes were normal or exhibited slight swelling. Immunohistochemically, the extended sinusoids were positive for anti-CD34 (Dako, dilution 1:50) (Figure 3D).
Figure 3.
Surrounding shunt vessels. A: Normal hepatic acinar structure was disordered and remodeled by septal fibrosis (Elastica-Masson stain). B: Bile ductule proliferation and slight inflammatory cell infiltration were observed in the fibrous septa. C: Sinusoidal dilatation was observed in hepatocyte nodules (reticulin-silver stain). D: Anti-CD34 immunostain confirmed sinusoidal capillarization around shunt vessels.
Severe arteriosclerosis was observed, especially in the aortic wall. The heart was enlarged to 380 g, and the mitral valve was replaced with an artificial one. The final cause of death was acute hemorrhagic brain stem necrosis due to thromboembolism. No thromboses were found in other organs.
Discussion
Currently, hepatic encephalopathy caused by a congenital portosystemic shunt is commonly believed to develop after middle age regardless of its extrahepatic or intrahepatic subtype, and most patients live uneventfully while young. Whether the patients exhibit symptoms of hepatic encephalopathy depends on the shunt ratio and the sensitivity of the brain to ammonia as well as the liver function. Although many young patients do not develop hepatic encephalopathy when the shunt rate is low, the cerebral tolerance and/or liver function for hepatotoxic substances decrease gradually with age and increase the patient’s risk for hepatic encephalopathy, particularly in adult life [6-12]. Small intrahepatic shunts sometimes disappear spontaneously during the first year of life [6,7,13]. The large intrahepatic shunt as well as extrahepatic shunts persist throughout life and carry risks of hepatic encephalopathy. It is likely that our patient had hepatic encephalopathy prior to admission to our hospital, but the clinical symptom was masked and mixed by cerebral infarction conditions, such as psychiatric disorders, perceptual disturbance, nystagmus and left side paresis; therefore, it is unkn-own when the hepatic encephalopathy developed. Although there was no detailed clinical information concerning her 20-year past history of jaundice, she may have had hepatic encephalopathy during those 20 years.
IPSS was classified into type 1 to type 4 by Parks et al. based on morphological observations; although rare, a persistent patent ductus venosus could be considered type 5. The most common type consists of a single large shunt that connects the right portal vein to the inferior vena cava. Our case should likely be classified as type 4 because multiple shunt vessels were found in both lobes of the liver. Due to the rarity of this type, the histological appearance could not be investigated in detail. To our knowledge, this is the second autopsy case report of multiple IPSS. In previous pathological reports [14,15], the shunt vessels lacked both the muscular layers and elastic lamellae in the wall. Thus, the shunts looked like extended sinusoids. In this case, we observed the incrassate elastic lamellae clearly in the shunt wall in addition to dilatation of the adjacent sinusoids. These extended sinusoids were positive for anti-CD34 immunohistochemistry, which designates capillarization of the sinusoids.
Surrounding the shunt vessels, irregular septal fibrosis and ductular proliferation were observed around the hepatocyte nodules and terminal veins. Hepatic acinar structures were remodeled to some degree, and those proliferated ductules appeared to arise from the limiting plate regions. Therefore, the histologic features reported here structurally mimicked or resembled cirrhosis. However, such changes were restricted, and there was only minimal inflammation and no histological evidence of liver cell damage. The cellular morphology of all of the components of the liver was almost normal, and we observed no fat degeneration, cholestasis, Mallory hyalines, biliary aberration, or thrombosis. We believe that the dilatation and capillarization of the sinusoids, fibrosis, and disordered and rearranged hepatic acinar structures in the background are all secondary hyperplastic/regenerative changes based on blood-flow imbalance caused by the IPSS circulation. These secondary changes partially resemble the peripheral part of focal nodular hyperplasia.
Congenital portosystemic shunts are increasingly recognized using ultrasound, Doppler, CT or MRI. The etiology of this disease is not yet elucidated. We identified some interesting case reports in which congenital IPSSs were found in patients with Down syndrome [16], LEOPARD syndrome [17], and Rendu-Osler-Weber disease [18]. Mori et al. reported congenital IPSS associated with multiple coronary artery fistulas [19]. Komaba et al. described congenital IPSS coexisting with atrial septal defect and persistent primitive hypoglossal artery [20]. Yamashita et al. described the familial prevalence of congenital intrahepatic and extrahepatic portosystemic shunts [21]. These reports suggest the possibility that some patients with congenital IPSS have genetic or developmental abnormalities. Furthermore, in the field of veterinary medicine, congenital portosystemic shunts are diseases that are comparatively frequent in dogs and are relatively well investigated. Evidence of inheritance and aberrant gene expression in these diseases has been reported [22,23]. Tivers et al. described that the intrahepatic level of vascular endothelial growth factor (VEGF) of a congenital portosystemic shunt in dogs is greater than in normal dogs [24]. This significant difference of intrahepatic VEGF levels in dogs may reflect the sinusoidal capillarization in the human that we observed here. Consistent with this hypothesis, there are some reports that mention the effect of VEGF in reducing hepatic sinusoidal capillarization in mice or rats [25,26].
Clinical studies of congenital IPSSs are scarce, and pathological examinations are almost non-exist. Therefore, elderly patients with this disease, including our patient, are sometimes misdiagnosed with psychiatric disorders or irrational liver damage, and to make matters worse, ineffective therapies such as medications and/or dietary restrictions are given or advised [9,27,28]. However, this disease can be cured completely under suitable medical treatment by interventional radiological techniques [29,30]. Therefore, accurate diagnosis of IPSS and an awareness of this disease are important. In this article, we described the histopathological findings of IPSS in detail for the first time, made some observations regarding the etiology, and suggested some hypotheses of congenital intrahepatic portosystemic shunts.
Disclosure of conflict of interest
None of the authors have any conflicts of interest associated with this study.
References
- 1.Abernethy J. Account of two instances of uncommon formation in the viscera of the human body. Philos Trans R Soc Lond. 1793;17:292–99. [Google Scholar]
- 2.Raskin NH, Price JB, Fisher man RA. Portal-systemic encephalopathy due to congenital intrahepatic shunts. N Engl J Med. 1964;270:225–9. doi: 10.1056/NEJM196401302700503. [DOI] [PubMed] [Google Scholar]
- 3.Pocha C, Maliakkal B. Spontaneous intrahepatic portal-systemic venous shunt in the adult: case report and review of the literature. Dig Dis Sci. 2004;49:1201–6. doi: 10.1023/b:ddas.0000037813.24605.d5. [DOI] [PubMed] [Google Scholar]
- 4.Witters P, Maleux G, George C, Delcroix M, Hoffman I, Gewillig M, Verslype C, Monbaliu D, Aerts R, Pirenne J, Van Steenbergen W, Nevens F, Fevery J, Cassiman D. Congenital veno-venous malformations of the liver: widely variable clinical presentations. J Gastroenterol Hepatol. 2008;23:e390–4. doi: 10.1111/j.1440-1746.2007.05156.x. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Cha SH, Han JK, Han MC. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990;155:527–8. doi: 10.2214/ajr.155.3.2117349. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Gamarra E, Parron M, Perez A, Prieto C, Hierro L, Lopez-Santamaria M. Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics. 2011;31:707–22. doi: 10.1148/rg.313105070. [DOI] [PubMed] [Google Scholar]
- 7.Uchino T, Endo F, Ikeda S, Shiraki K, Sera Y, Matsuda I. Threebrothers with progressive hepatic dysfunction and severe hepatic steatosis due to a patent ductus venosus. Gastroenterology. 1996;110:1964–8. doi: 10.1053/gast.1996.v110.pm8964424. [DOI] [PubMed] [Google Scholar]
- 8.Crespin J, Nemcek A, Rehkemper G, Blei AT. Intrahepatic portal-hepatic venous anastomosis: a portal-systemic shunt with neurological repercussions. Am J Gastroenterol. 2000;95:1568–71. doi: 10.1111/j.1572-0241.2000.02096.x. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969–79. doi: 10.1046/j.1440-1746.2000.02283.x. [DOI] [PubMed] [Google Scholar]
- 10.Namekata K, Fujiwara N, Sugo H, Yoshimoto J, Okuyama K, Ohashi K, Kojima K, Fukasawa M, Beppu T, Futagawa S, Wakashima M, Miyake K, Yamanaka M. A resected case of intrahepatic porto-venous shunt in noncirrhotic liver. Nihon Shokakibyo Gakkai Zasshi. 1996;93:851–6. [PubMed] [Google Scholar]
- 11.Uchino T, Matsuda I, Endo F. The long-term prognosis of congenital portosystemic venous shunt. J Pediatr. 1999;135:254–6. doi: 10.1016/s0022-3476(99)70031-4. [DOI] [PubMed] [Google Scholar]
- 12.Caturelli E, Ghittoni G, Niro GA, Clemente R, Accadia L, Nardella M, Andriulli A, Anti M. Multiple intrahepatic vascular shunts causing hyperammoniaemic encephalopathy in a patient without liver cirrhosis. Dig Liver Dis. 2006;38:347–51. doi: 10.1016/j.dld.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E, Bernard O. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–30. doi: 10.1097/MPG.0b013e3181d9cb92. doi: 10.1097/MPG.0b013e3181-d9cb92. [DOI] [PubMed] [Google Scholar]
- 14.Mori H, Hayashi K, Fukuda T, Matsunaga N, Futagawa S, Nagasaki M, Mutsukura M. Intrahepatic portosystemic venous shunt: occurrence in patients with and without liver cirrhosis. AJR Am J Roentgenol. 1987;149:711–4. doi: 10.2214/ajr.149.4.711. [DOI] [PubMed] [Google Scholar]
- 15.Kozuka S, Sassa R, Kakumu S. An enormous intrahepatic shunt between portal vein and hepatic one. Angiology. 1975;26:365–71. doi: 10.1177/000331977502600409. [DOI] [PubMed] [Google Scholar]
- 16.Saxena AK, Sodhi KS, Arora J, Thapa BR, Suri S. Congenital intrahepatic portosystemic venous shunt in an infant with down syndrome. AJR Am J Roentgenol. 2004;183:1783–4. doi: 10.2214/ajr.183.6.01831783. [DOI] [PubMed] [Google Scholar]
- 17.Digilio MC, Capolino R, Marino B, Sarkozy A, Dallapiccola B. Congenital intrahepatic portosystemic venous shunt: an unusual feature in LEOPARD syndrome and in neurofibromatosis type 1. Am J Med Genet A. 2005;134:457–8. doi: 10.1002/ajmg.a.30582. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Mori H, Yamada Y, Hayashida T, Hori Y, Kiyosue H. Intrahepatic porto-hepatic venous shunts in Rendu-Osler-Weber disease: imaging demonstration. Eur Radiol. 2004;14:592–6. doi: 10.1007/s00330-003-2063-9. [DOI] [PubMed] [Google Scholar]
- 19.Mori K, Dohi T, Yamamoto H, Kamada M. An enormous shunt between the portal and hepatic veins associated with multiple coronary artery fistulas. Pediatr Radiol. 1990;21:66–8. doi: 10.1007/BF02010820. [DOI] [PubMed] [Google Scholar]
- 20.Komaba Y, Nomoto T, Hiraide T, Kitamura S, Terashi A. Persistent primitive hypoglossal artery complicated by atrial septal defect and congenital intrahepatic shunts. Intern Med. 1998;37:60–4. doi: 10.2169/internalmedicine.37.60. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita S, Nakata K, Muro T, Furukawa R, Kusumoto Y, Munehisa T, Miyake S, Nagataki S, Ishii N, Koji T. A case of hepatic encephalopathy due to diffuse intrahepatic porto-systemic shunts. Nihon Naika Gakkai Zasshi. 1982;71:844–50. [PubMed] [Google Scholar]
- 22.van Steenbeek FG, Van den Bossche L, Grinwis GC, Kummeling A, van Gils IH, Koerkamp MJ, van Leenen D, Holstege FC, Penning LC, Rothuizen J, Leegwater PA, Spee B. Aberrant gene expression in dogs with portosystemic shunts. PLoS One. 2013;8:e57662. doi: 10.1371/journal.pone.0057662. doi: 10.1371/journal.pone.0057662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Steenbeek FG, Leegwater PA, van Sluijs FJ, Heuven HC, Rothuizen J. Evidence of inheritance of intrahepatic portosystemic shunts in Irish Wolfhounds. J Vet Intern Med. 2009;23:950–2. doi: 10.1111/j.1939-1676.2009.0319.x. doi: 10.1111/j.1939-1676.2009.0319.x. [DOI] [PubMed] [Google Scholar]
- 24.Tivers MS, Lipscomb VJ, Scase TJ, Priestnall SL, House AK, Gates H, Wheeler-Jones CP, Smith KC. Vascular endothelial growth factor (VEGF) and VEGF receptor expression in biopsy samples of liver from dogs with congenital portosystemic shunts. J Comp Pathol. 2012;147:55–61. doi: 10.1016/j.jcpa.2011.09.001. doi: 10.1016/j.jcpa.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 25.May D, Djonov V, Zamir G, Bala M, Safadi R, Sklair-Levy M, Keshet E. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PLoS One. 2011;6:e21478. doi: 10.1371/journal.pone.0021478. doi: 10.1371/journal.pone.0021478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Shi BM, Lu XF, Liang F, Jin X, Wu TH, Xu J. Vascular endothelial growth factor attenuates hepatic sinusoidal capillarization in thioacetamide-induced cirrhotic rats. World J Gastroenterol. 2008;14:2349–57. doi: 10.3748/wjg.14.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatama M. Hepatic encephalopathy in aged patients and its differential diagnosis from dementia. Med Tribune. 1989:21. [Google Scholar]
- 28.Matsuura B, Akamatsu K, Kitai K, Kimura H, Ohta Y. A case report of portal-systemic encephalopathy with normal portal vein pressure and non-cirrhosis of the liver. Nihon Shokakibyo Gakkai Zasshi. 1987;84:1684–9. [PubMed] [Google Scholar]
- 29.Ohtomo K, Furui S, Saito M, Kokubo T, Itai Y, Iio M. Enormous intrahepatic communication between the portal vein and the hepatic vein. Clin Radiol. 1986;37:513–4. doi: 10.1016/s0009-9260(86)80085-x. [DOI] [PubMed] [Google Scholar]
- 30.Gupta V, Kalra N, Vyas S, Sodhi KS, Thapa BR, Khandelwal N. Embolization of congenital intrahepatic porto-systemic shunt by n-butyl cyanoacrylate. Indian J Pediatr. 2009;76:1059–60. doi: 10.1007/s12098-009-0202-2. doi: 10.1007/s12098-009-0202-2. [DOI] [PubMed] [Google Scholar]