Abstract
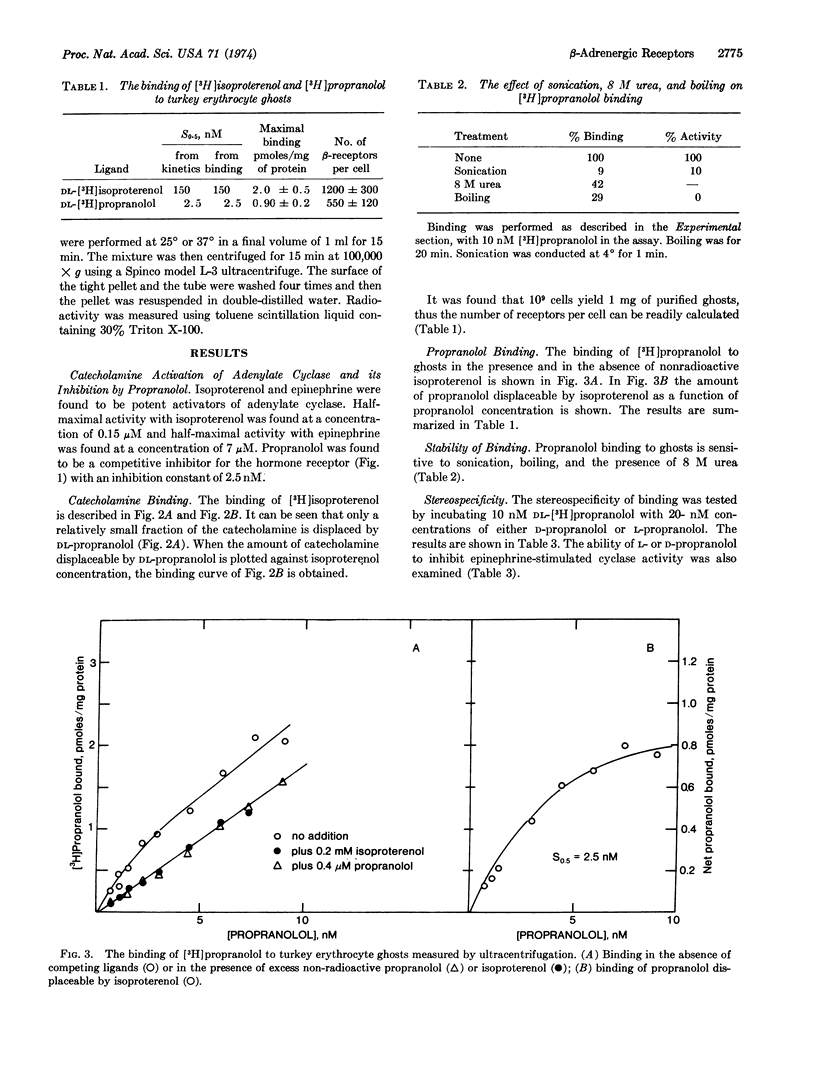
Turkey erythrocyte ghosts (empty membranes) possess a class of receptors that can bind both L-[3H]isoproterenol and DL-[3H]propranolol. The binding of [3H]isoproterenol to these receptors occurs with a dissociation constant of 0.15 μM and can be fully inhibited by 1 μM propranolol. The binding of [3H]propranolol occurs with a dissociation constant of 2.5 nM and can be fully inhibited by 0.2 mM DL-isoproterenol. Ligand binding is sensitive to sonication, boiling, and 8 M urea. The cells possess 500 to 1000 β-adrenergic receptors per cell. Binding of propranolol to the β-receptor was found to be stereospecific for the L stereoisomer. If one assumed a 1:1 relationship between β-adrenergic receptors and adenylate cyclase, the turnover number of this adenylate cyclase would be close to 100 min-1.
Keywords: β-receptors, isoproterenol, propranolol, equilibrium dialysis, ultracentrifugation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilezikian J. P., Aurbach G. D. A beta-adrenergic receptor of the turkey erythrocyte. II. Characterization and solubilization of the receptor. J Biol Chem. 1973 Aug 25;248(16):5584–5589. [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P., Sica V., Parikh I., Chang K. J. Noradrenaline binding and the search for catecholamine receptors. Nature. 1974 Jan 11;247(5436):92–97. doi: 10.1038/247092a0. [DOI] [PubMed] [Google Scholar]
- Dunnick J. K., Marinetti G. V. Hormone action at the membrane level. 3. Epinephrine interaction with the rat liver plasma membrane. Biochim Biophys Acta. 1971 Oct 12;249(1):122–134. doi: 10.1016/0005-2736(71)90089-7. [DOI] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E. A fraction of the ventricular myocardium that has the specificity of the cardiac beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1773–1777. doi: 10.1073/pnas.68.8.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E., O'Hara D. Identification of the cardiac beta-adrenergic receptor protein: solubilization and purification by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2828–2832. doi: 10.1073/pnas.69.10.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., O'Hara D. S., Warshaw J. Binding of catecholamines to receptors in cultured myocardial cells. Nat New Biol. 1973 Jul 18;244(133):79–80. doi: 10.1038/newbio244079a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Negative co-operativity in clustered receptors as a possible basis for membrane action. J Theor Biol. 1974 Apr;44(2):367–372. doi: 10.1016/0022-5193(74)90167-2. [DOI] [PubMed] [Google Scholar]
- Schramm M., Feinstein H., Naim E., Lang E., Lasser M. Epinephrine binding to the catecholamine receptor and activation of the adenylate cyclase in erythrocyte membranes (hormone receptor- -adrenergic receptor-cyclic AMP-turkey). Proc Natl Acad Sci U S A. 1972 Feb;69(2):523–527. doi: 10.1073/pnas.69.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]


