Abstract
We have investigated whether mammalian cells can repair pyrimidine dimers in their mitochondrial DNA which have been induced by ultraviolet light. The assay system is based upon the ability of the phage T4 UV endonuclease to nick covalently closed circular mitochondrial DNA that contain pyrimidine dimers. Our results show that dimers are not removed from the mitochondrial DNA of mouse L cells or human KB and HeLa cells. There is also no evidence for photoreactivation of mitochondrial DNA. Analyses of ethidium bromide-cesium chloride equilibrium density gradients of mitochondrial DNA isotopically labeled before and after exposure to ultraviolet light show that the total amount of DNA replication is depressed after exposure. In addition, an increase in the frequency of molecules banding at a position expected for intermediate replicating forms and open circular daughter molecules suggests that the rate of replication is slower (or arrested) in molecules with pyrimidine dimers. The absence of a significant amount of mixing of label incorporated before and after ultraviolet-irradiation is evidence against the occurrence of a large amount of genetic exchange between mitochondrial DNA molecules under these conditions.
Keywords: pyrimidine dimer endonuclease, DNA repair, DNA replication, density gradient centrifugation
Full text
PDF
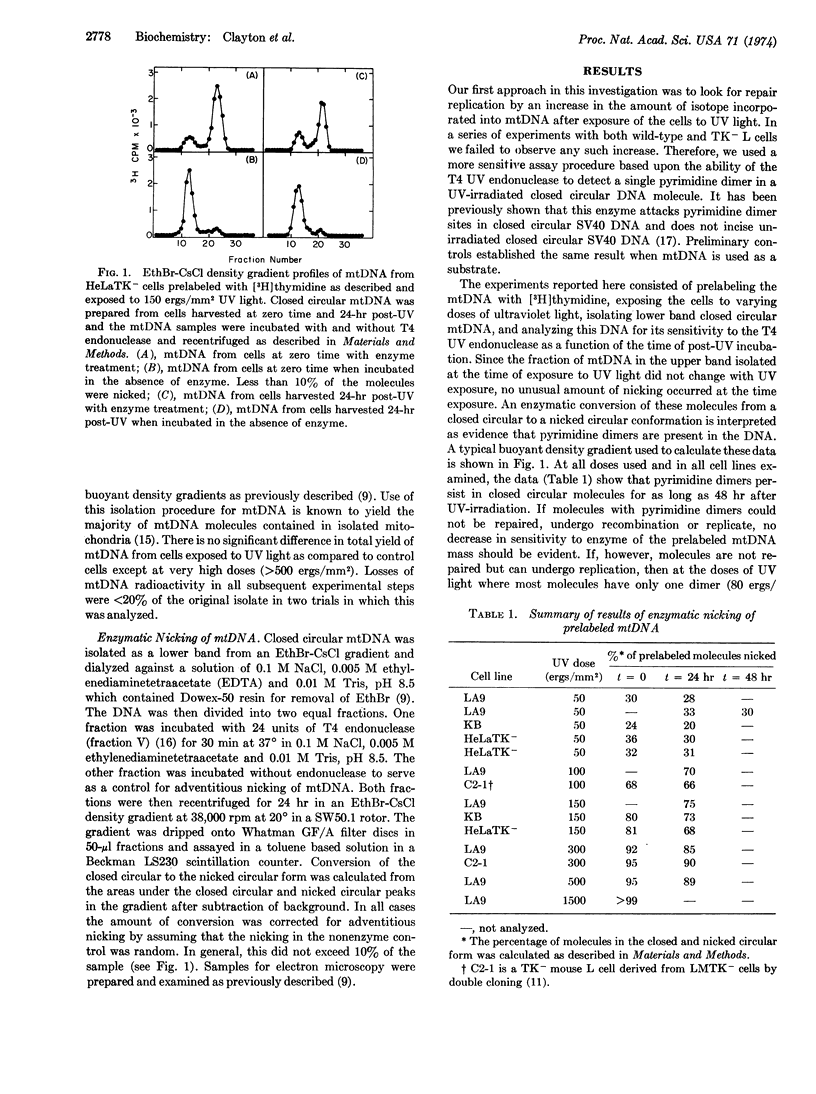
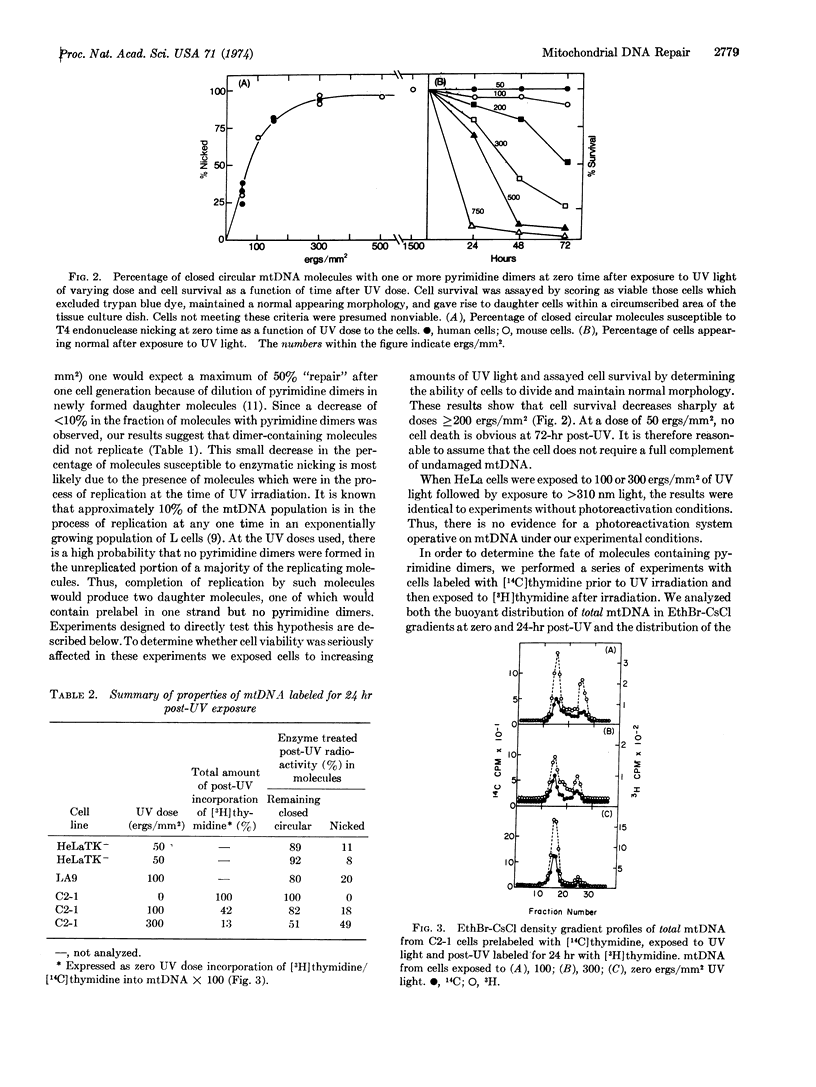
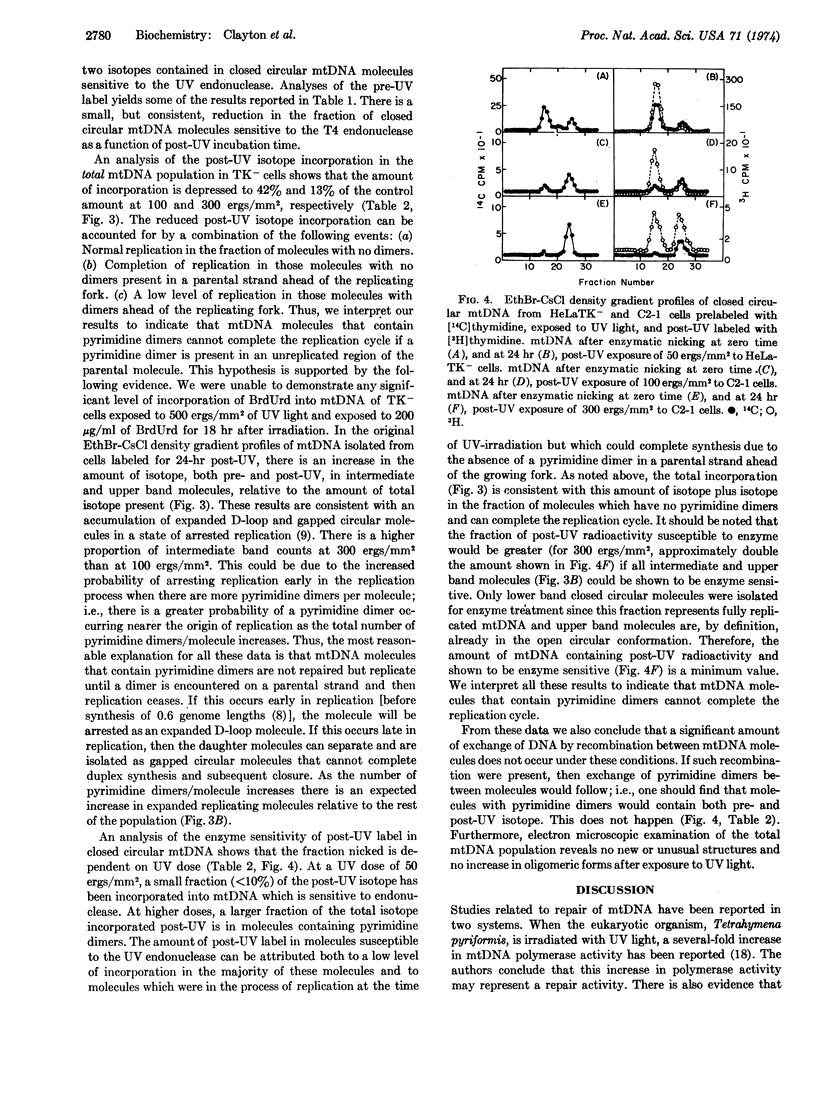

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Clayton D. A. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2722–2729. [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Teplitz R. L. Intracellular mosaicism (nuclear - -mitochondrial + ) for thymidine kinase in mouse L cells. J Cell Sci. 1972 Mar;10(2):487–493. doi: 10.1242/jcs.10.2.487. [DOI] [PubMed] [Google Scholar]
- Cook J. S. Photoreactivation in animal cells. Photophysiology. 1970;5:191–233. [PubMed] [Google Scholar]
- Friedberg E. C., Clayton D. A. Electron microscopic studies on substrate specificity of T4 excision repair endonuclease. Nature. 1972 May 12;237(5350):99–100. doi: 10.1038/237099a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Keiding J., Westergaard O. Induction of DNA polymerase activity in irradiated Tetrahymena cells. Exp Cell Res. 1971 Feb;64(2):317–322. doi: 10.1016/0014-4827(71)90082-6. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Pulse-labeled components in the replication of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Jun 25;248(12):4512–4514. [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]



