Abstract
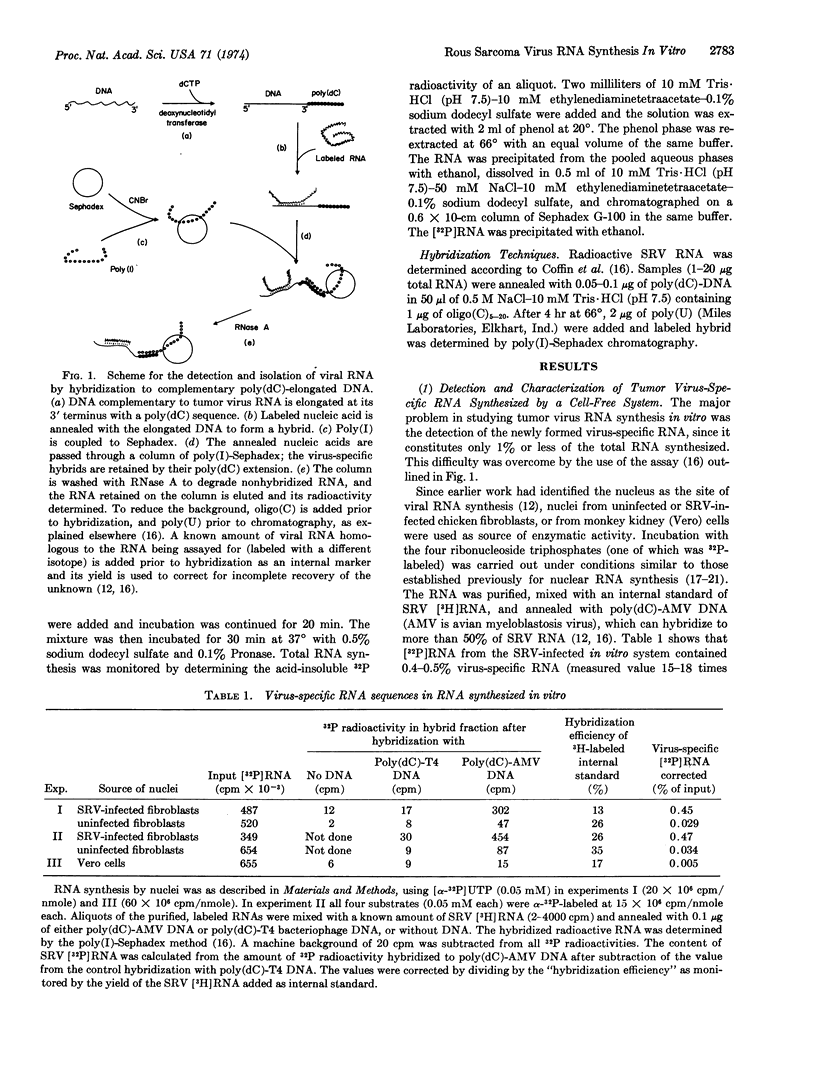
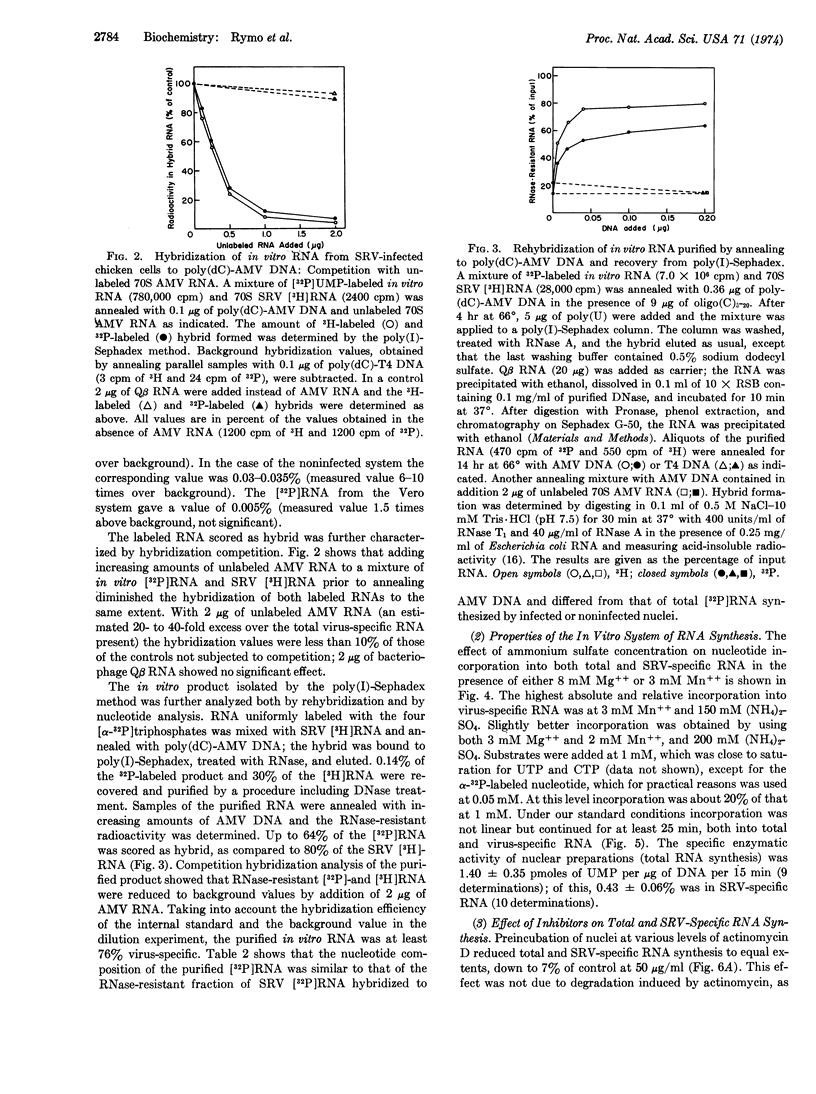
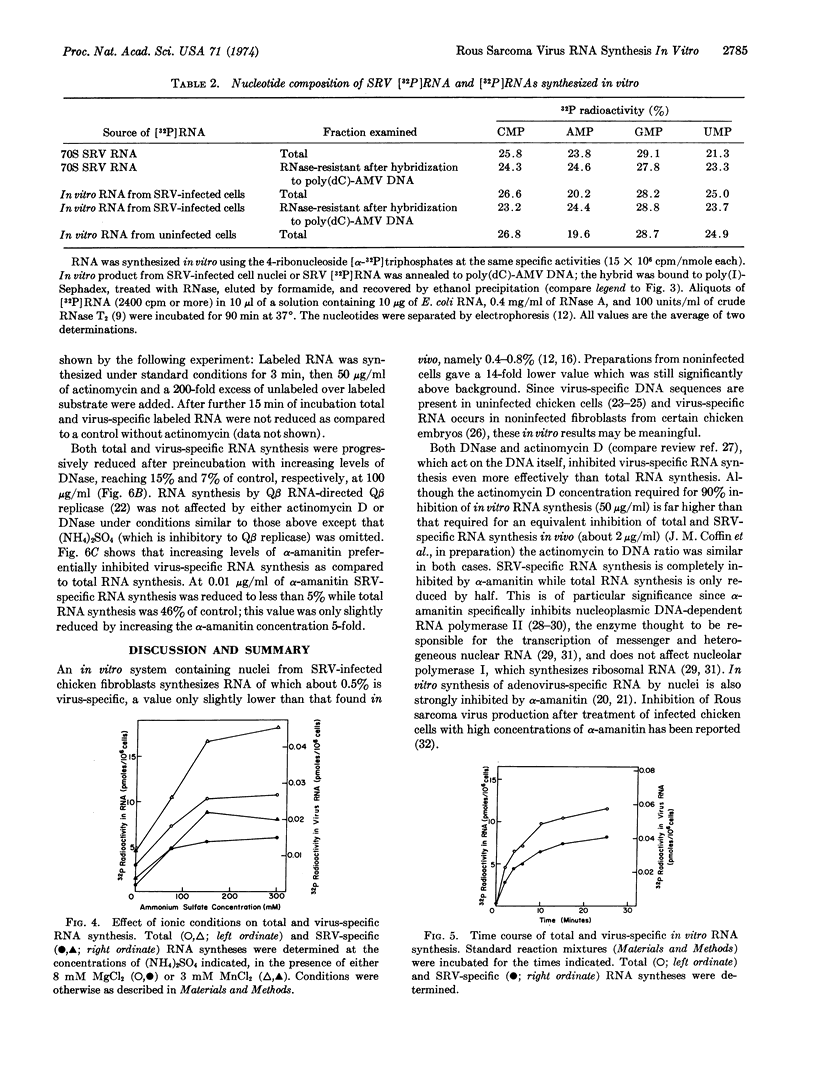
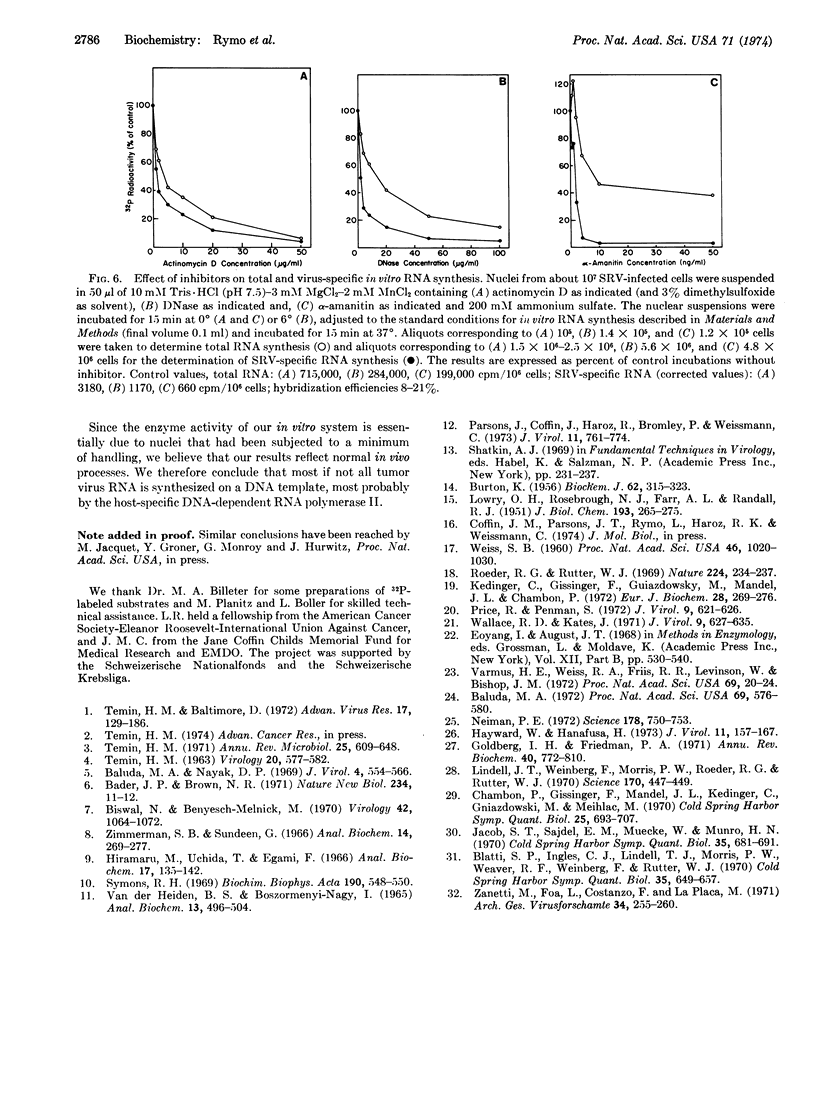
Synthesis of Rous sarcoma virus RNA was examined in vitro with a new assay for radioactive virus-specific RNA. Nuclei from infected and uninfected cells were incubated with ribonucleoside [α-32P]triphosphates, Mn++, Mg++ and (NH4)2SO4. Incorporation into total and viral RNA proceeded with similar kinetics for up to 25 min at 37°. About 0.5% of the RNA synthesized by the infected system was scored as virus-specific, compared to 0.03% of the RNA from the uninfected system and 0.005% of the RNA synthesized by monkey kidney cell nuclei. Preincubation with DNase or actinomycin D completely suppressed total and virus-specific RNA synthesis. α-Amanitin, a specific inhibitor of eukaryotic RNA polymerase II, completely inhibited virus-specific RNA synthesis, while reducing total RNA synthesis by only 50%. We conclude that tumor virus-specific RNA is synthesized on a DNA template, most probably by the host's RNA polymerase II.
Keywords: actinomycin D, α-amanitin, assay of virus-specific RNA
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Benyesh-Melnick M. Characterization of the complementary nuclear RNA of murine sarcoma-leukemia virus. Virology. 1970 Dec;42(4):1064–1072. doi: 10.1016/0042-6822(70)90354-5. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramaru M., Uchida T., Egami F. Ribonuclease preparation for the base analysis of polyribonucleotides. Anal Biochem. 1966 Oct;17(1):135–142. doi: 10.1016/0003-2697(66)90016-9. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Gniazdowski M., Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. 1. Large-scale solubilization and separation of A and B calf-thymus RNA-polymerase activities. Eur J Biochem. 1972 Jul 13;28(2):269–276. doi: 10.1111/j.1432-1033.1972.tb01910.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Preparation of [alpha-32P]nucleoside and deoxynucleoside 5'-triphosphates from 32Pi and protected and unprotected nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):548–550. doi: 10.1016/0005-2787(69)90105-1. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Vanderheiden B. S., Boszormenyi-Nagy I. Preparation of 32-P-labeled nucleotides. Anal Biochem. 1965 Dec;13(3):496–504. doi: 10.1016/0003-2697(65)90343-x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. B. ENZYMATIC INCORPORATION OF RIBONUCLEOSIDE TRIPHOSPHATES INTO THE INTERPOLYNUCLEOTIDE LINKAGES OF RIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1020–1030. doi: 10.1073/pnas.46.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M., Foa L., Costanzo F., La Placa M. Specific inhibition of Rous sarcoma virus by -amanitin. Arch Gesamte Virusforsch. 1971;34(4):255–260. doi: 10.1007/BF01242970. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]



