Abstract
A study was conducted to develop a Trypanosoma vivax (T. vivax) specific PCR based on the T. vivax proline racemase (TvPRAC) gene. Forward and reverse primers were designed that bind at 764–783 bp and 983–1002 bp of the gene. To assess its specificity, TvPRAC PCR was conducted on DNA extracted from different haemotropic pathogens: T. vivax from Nigeria, Ethiopia and Venezuela, T. congolense Savannah type, T. brucei brucei, T. evansi, T. equiperdum, T. theileri, Theileria parva, Anaplasma marginale, Babesia bovis and Babesia bigemina and from bovine, goat, mouse, camel and human blood. The analytical sensitivity of the TvPRAC PCR was compared with that of the ITS-1 PCR and the 18S PCR-RFLP on a dilution series of T. vivax DNA in water. The diagnostic performance of the three PCRs was compared on 411 Ethiopian bovine blood specimens collected in a former study. TvPRAC PCR proved to be fully specific for T. vivax, irrespective of its geographical origin. Its analytical sensitivity was lower than that of ITS-1 PCR. On these bovine specimens, TvPRAC PCR detected 8.3% T. vivax infections while ITS-1 PCR and 18S PCR-RFLP detected respectively 22.6 and 6.1% T. vivax infections. The study demonstrates that a proline racemase based PCR could be used, preferably in combination with ITS-1 PCR, as a species-specific diagnostic test for T. vivax infections worldwide.
Introduction
Trypanosomoses are diseases caused by species of the genus Trypanosoma. Within the Salivarian trypanosomes, Trypanosoma vivax, Trypanosoma congolense and Trypansoma brucei are the three most important pathogenic species in livestock responsible for considerable production losses and morbidity. Two subspecies of T. brucei, i.e. T.b. gambiense and T.b. rhodesiense cause human African trypanosomosis (sleeping sickness). Animal trypanosomosis caused by T. vivax is a widespread disease in Africa and South America, hindering livestock production and food self-sufficiency [1]–[3]. Compared to standard parasitological techniques, molecular diagnostic tools, and in particular the polymerase chain reaction, allow the detection of trypanosome infections with much lower parasite numbers, both in the vertebrate and in the insect host [4]–[7]. The diversity of PCR methods for identification of trypanosome taxa has also led to increased appreciation of trypanosome genetic diversity. For identification of the trypanosome species in biological specimens from mammal or insect hosts, ribosomal genes and internal transcribed spacers are often chosen as target sequences [4], [7]–[10]. For T. vivax, also other target sequences have been used such as satellite and microsatellite sequences, spliced-leader sequences and cathepsin L-like genes [6], [11]–[13]. In some studies it was shown that the DNA sequences initially used as T. vivax specific target for hybridisation or for PCR primers, were only specific for the West African form of T. vivax that also occurs in South America [2], [6], [14], [15] [16]. However, in East Africa, other T. vivax genotypes circulate [17]–[20]. In a study, carried out in Ethiopia, on bovine trypanosomosis [21] we observed that results obtained with 18S PCR [22] and TVM PCR [23] were not consistent with ITS-1 PCR [4] with respect to T. vivax detection. In order to unequivocally identify the infecting trypanosome species, we developed a PCR targeting the proline racemase (PRAC) gene. The PRAC gene, originally described for T. cruzi, was also found in the genome of the West African T. vivax ILRAD 1392 strain isolated in Nigeria and was shown to be absent in other African trypanosomes [24]. In T. cruzi, PRAC plays a crucial role in the interconversion of free L- and D-proline enantiomers. The biochemical characterisation of the corresponding protein revealed that T. vivax proline racemase (TvPRAC) exhibits characteristics and kinetic parameters comparable to T. cruzi PRAC [24]. We hereby report on the application of TvPRAC PCR to discriminate T. vivax from the other trypanosome taxa.
Materials and Methods
The sequence of the TvPRAC gene (1038 bp) from T.vivax ILRAD 1392 was obtained from GenBank using accession number EF175213.1. We designed primers that specifically annealed to T. vivax DNA and not to DNA from other Kinetoplastida, Bovidae, Hominoidea and Mus. TvPRAC-F forward primer 5′CGCAAGTGGACCGTTCGCCT3′ and TvPRAC-R reverse primer 5′ACGCGGGGCGAACAGAAGTG 3′ are complementary to bp 764–783 and 983–1002 of TvPRAC and generate an amplicon with an expected length of 239 bp only in T. vivax and not in T. cruzi. The primer binding sites encompass the MIII* PRAC motif and R3 (tyrosine) residue of the C-terminal half of the protein [24]. After optimisation, the following PCR protocol was retained: a 25 µL reaction volume contained 2.5 mM MgCl2 (Qiagen), 200 µM of each dNTP (Eurogentec), 0.8 µM of TvPRAC-F and TvPRAC-R primers (Biolegio), 0.5 U Hotstart Taq polymerase (Qiagen,) 0.1 mg/mL acetylated BSA (Promega) and 2.5 µL target DNA. To assess the analytical sensitivity of TvPRAC PCR, a Hotstart master mix (Qiagen) was used with 1.5 mM MgCl2 and without acetylated BSA. Cycling conditions were: 94°C for 15 min, 40 cycles of 30 sec at 94°C followed by 30 sec at 63°C and 30 sec at 72°C, final extension at 72°C for 5 min. The PCR product was electrophorised in a 2% agarose gel and stained with ethidium bromide (EtBr) for detection of the specific 239 bp band. The TvPRAC PCR products were either directly sequenced using both forward and reverse TvPRAC primers or sequenced after cloning in a pCR®4-TOPO vector followed by transformation into TOP10 chemically competent E.coli, as described in the TOPO® TA Cloning® Kit for Sequencing(Invitrogen). Sequencing was carried out at the VIB facilities at the Antwerp University. The obtained sequences were aligned to each other using CLC Sequence Viewer 6 and compared with the sequence of the TvPRAC gene of ILRAD 1392.
For assaying specificity of the TvPRAC PCR, DNA was prepared from bovine, goat, camel, mouse and human blood, and from different trypanosome species and other pathogens affecting cattle ( Table 1 ). DNA from T. vivax Sh, Anaplasma marginale, Babesia bovis and Babesia bigemina were extracted from blood of an infected calf. DNA from T. vivax LIEM 176 was extracted from blood of infected sheep. Therefore, the concentration of T. vivax DNA in these specimens remains unknown. DNA from the other pathogen strains was extracted from purified cells. To detect any contamination during extraction, a sample of DNA-free H20 ( = negative extraction control) was processed along with the other specimens. The species identity of the trypanosomes included in this study was confirmed by ITS-1 PCR that yields taxon specific amplicon lengths of 150 bp, 350 bp, 450 bp and 650 bp, for T. vivax, T. theileiri, Trypanozoon and T. congolense Savannah type, respectively.
Table 1. Pathogen strains used to extract DNA for development and evaluation of TvPRAC PCR.
| Taxon | Name | Reference | Origin |
| Trypanosoma vivax | Y486 | [26] | Nigeria |
| Trypanosoma vivax | MBOV/ET/2012/AAU-CVMA/001, alias 4337 | Unpublished | Ethiopia a |
| Trypanosoma vivax | MBOV/ET/2012/AAU-CVMA/002, alias 4338 | Unpublished | Ethiopia a |
| Trypanosoma vivax | MBOV/ET/2012/AAU-CVMA/003, alias Di | Unpublished | Ethiopia a |
| Trypanosoma vivax | MBOV/ET/2012/AAU-CVMA/004, alias Fc | Unpublished | Ethiopia b |
| Trypanosoma vivax c | MBOV/ET/2012/AAU-CVMA/005, alias Sh | Unpublished | Ethiopia b |
| Trypanosoma vivax d | LIEM 176 | [15] | Venezuela |
| Trypanosoma congolense | TRT57 (Savannah type) | [27] | Zambia |
| Trypanosoma brucei brucei | AnTar 1 | [28] | Uganda |
| Trypanosoma evansi | RoTat 1.2 | [29] | Indonesia |
| Trypanosoma equiperdum | OVI | [30] | South Africa |
| Trypanosoma theileri | MELSELE | [31] | Belgium |
| Babesia bovis c | Bbo-Gu | Unpublished | Venezuela |
| Babesia bigemina c | Bbig-Ya | Unpublished | Venezuela |
| Anaplasma marginale c | Am-Zu | Unpublished | Venezuela |
| Theileria parva | Katete | [32] | Zambia |
a Jimma zone, Chora Botor district, tsetse infested region.
b Bale Zone and West Gojjam, tsetse free region.
c DNA extracted from blood of an infected calf.
d DNA extracted from blood of an infected sheep.
The analytical sensitivity of the TvPRAC PCR was assessed on fivefold dilution series, ranging from 1 ng/µl down to 0.064 pg/µl, of DNA in water prepared from the Ethiopian T. vivax isolates 4337, 4338, Di and Fc. The same dilution series were used to assess the analytical sensitivity of ITS-1 PCR and of 18S PCR-RFLP [9]. The 18S PCR-RFLP is a semi-nested PCR, with 18S rDNA as target, followed by restriction enzyme digestion of the PCR product. The test was performed as described in [9]. The analytical sensitivity of TvPRAC PCR was further evaluated on a tenfold serial dilution of live T. vivax trypanosomes in 0.5 ml volumes of naïve mouse blood ranging from 5.106 down to 5 trypanosomes/ml.
To assess the agreement between TvPRAC PCR on the one hand and ITS-1 PCR and 18S PCR-RFLP on the other hand for diagnosis of T. vivax infection, the three PCRs were conducted on 411 bovine blood specimens collected in Ethiopia between August 2010 and April 2011. Details on collection, microscopic examination, DNA extraction and results obtained with ITS-1 PCR are described elsewhere [21].
Ethics statement
For the collection of blood specimens from bovine, camel, goat and mouse, ethical approval was obtained from the Ethics Committee for Veterinary Medicine of the Institute of Tropical Medicine Antwerp (EXT 2012-1 and EXT 2012-2). The human blood specimen was collected within a study approved by the Ethics Committee of the University Hospital of Antwerp (ITG 09415684). Written informed consent of the donor was obtained.
Data analysis
SPSS version 20 was used to analyse the data and Kappa statistics [25] was applied to assess the degree of agreement between the three PCRs.
Results
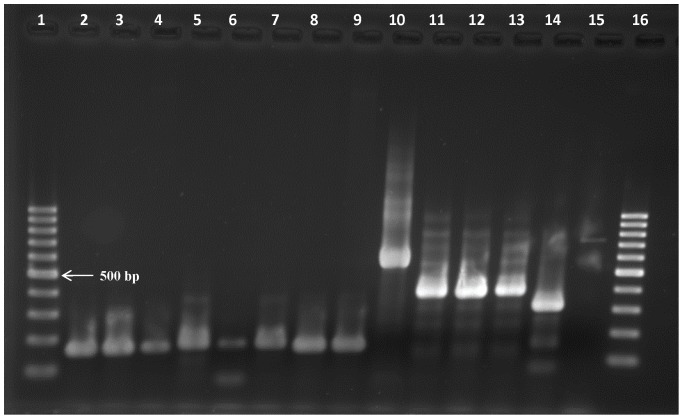
ITS-1 PCR was performed using DNA of all the trypanosome species included in this study at a concentration of 1 ng/µl except for T. vivax Sh and LIEM 176, where the specimens contained 1 ng/µl of host and T. vivax DNA together. The ITS-1 PCR yielded amplicons with the expected length thus confirming the taxon identity ( Figure 1 ).
Figure 1. Amplicons generated with ITS-1 PCR on DNA of different trypanosome taxa at 1 ng/µl, except for lanes 4, 8 and 9 where the specimen contains also host DNA.
Lanes 1 and 16 = 100 bp marker, lane 2 = T. vivax Y486, lane 3 = T. vivax Fc, lane 4 = T. vivax Sh, lane 5 = T. vivax 4337, lane 6 = T. vivax 4338, lane 7 = T. vivax Di, lanes 8 & 9 T. vivax LIEM 176, lane 10 = T. congolense, lane 11 = T. brucei brucei, lane 12 = T. evansi, lane 13 = T. equiperdum, lane 14 = T. theileri, lane 15 = negative extraction control.
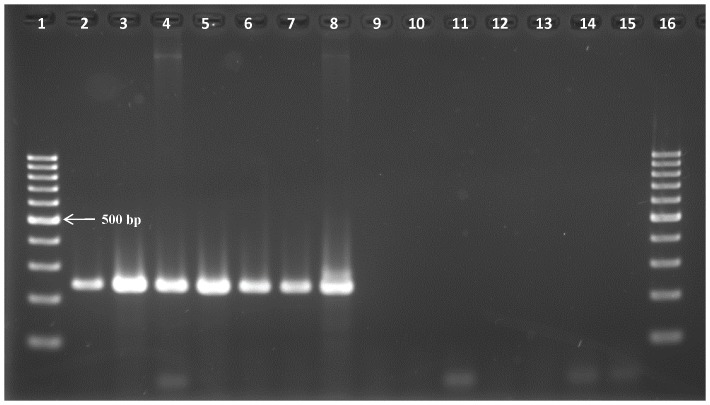
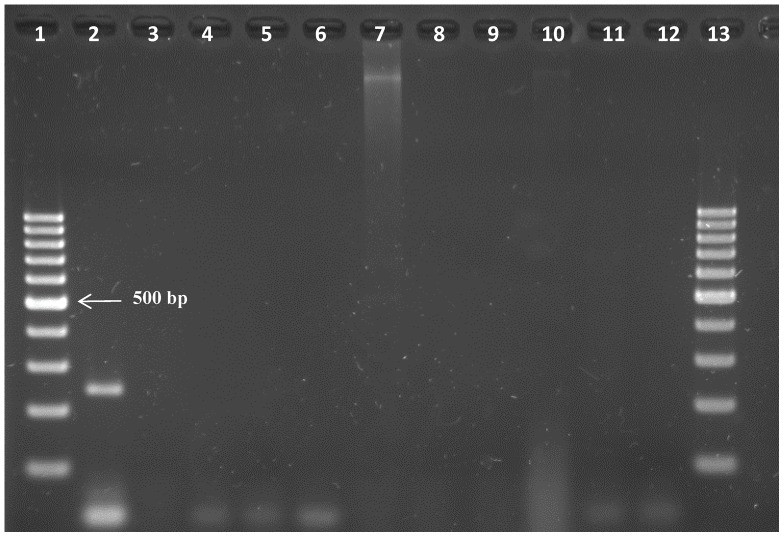
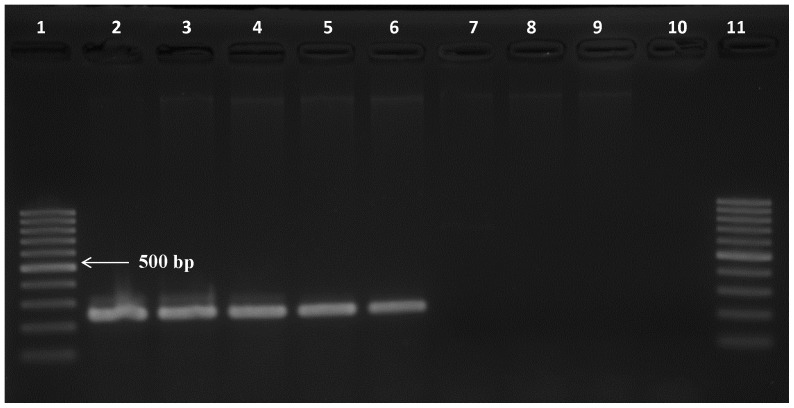
The specificity of the TvPRAC PCR was tested on the same trypanosome DNA collection, on DNA of other haemoparasites of cattle, and on host DNA of mammals and human. Only with T. vivax DNA, irrespective of the strain, TvPRAC PCR yielded the expected 239 bp amplicon ( Figure 2 ). Note that lane 9 remained negative since the PCR reaction contained <1 µl of template DNA. No amplicons were generated with non-target DNA ( Figure 3 ).
Figure 2. Amplicons generated with TvPRAC PCR on DNA of different trypanosome taxa at 1 ng/µl, except for lanes 4, 8 and 9 where the specimen contains also host DNA.
Lanes 1 and 16 = 100 bp marker, lane 2 = T. vivax Y486, lane 3 = T. vivax Fc, lane 4 = T. vivax Sh, lane 5 = T. vivax 4337, lane 6 = T. vivax 4338, lane 7 = T. vivax Di, lanes 8 & 9 T. vivax LIEM 176, lane 10 = T. congolense, lane 11 = T. brucei brucei, lane 12 = T. evansi, lane 13 = T. equiperdum, lane 14 = T. theileri, lane 15 = negative extraction control.
Figure 3. Amplicons generated with TvPRAC PCR on DNA of non-target species at 1 ng/µl.
Lanes 1 & 13 = 100 bp marker, lane 2 = positive control (T. vivax Y486), lane 3 = Theileria parva, lane 4 = Anaplasma marginale, lane 5 = Babesia bovis, lane 6 = Babesia bigemina, lane 7 = bovine, lane 8 = goat, lane 9 = camel, lane 10 = mouse, lane 11 = human, lane 12 = negative extraction control.
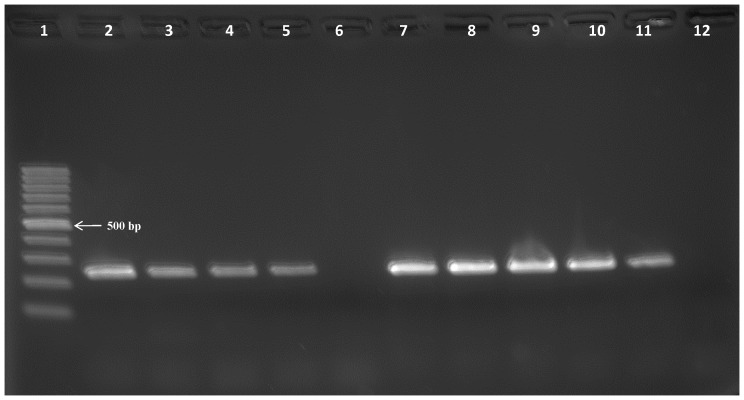
The analytical sensitivities of the TvPRAC PCR, ITS-1 PCR and 18S PCR-RFLP, were assessed on fivefold dilution series of T. vivax DNA in water ( Figure 4 and Table 2 ). As shown in Figure 4 for T. vivax 4337 and Fc, the lower detection limit of TvPRAC PCR ranges from 8 pg/µl to 1.6 pg/µl depending on the strain. From Table 2, it appears that for three isolates, 18S PCR-RFLP remained negative at 1000 pg/µl. Depending on the strain, the TvPRAC PCR showed either a lower or higher detection limit than ITS-1 PCR. With T. vivax Y486 that grows in mice, we were able to assess also the analytical sensitivity of TvPRAC on DNA extracted from blood containing live parasites and found that it was 500 parasites/ml ( Figure 5 ).
Figure 4. Assessment of the lower detection limit of TvPRAC PCR on a fivefold DNA dilution series in water.
Lane 1 = 100 bp marker, lanes 2 to 6: T. vivax 4337 DNA at 1000, 200, 40, 8, 1.6 pg/µl, lanes 7 to 11: T. vivax Fc DNA at 1000, 200, 40, 8, 1.6 pg/µl, 12 = negative extraction control.
Table 2. Lower detection limits expressed as pg/µl of TvPRAC PCR, ITS-1 PCR and 18S PCR-RFLP assessed on DNA dilutions of four Ethiopian T. vivax isolates.
| Lower detection limit (pg/µl) | |||
| T. vivax isolates | TvPRAC PCR | ITS-1 PCR | 18S PCR-RFLP |
| 4337 | 8 | 1.6 | >1000 |
| 4338 | 40 | 1000 | >1000 |
| Di | 8 | 40 | >1000 |
| Fc | 1.6 | 0.32 | 8 |
Figure 5. Assessment of the lower detection limit of TvPRAC PCR on a tenfold dilution series of parasites in mouse blood.
Lane 1 = 100 bp marker, lanes 2 to 8: T. vivax Y486 parasites at 5.106 down to 5 trypanosomes/ml, lane 9 = naïve mouse blood, lane 10 = negative extraction control, lane 11 = .100 bp marker.
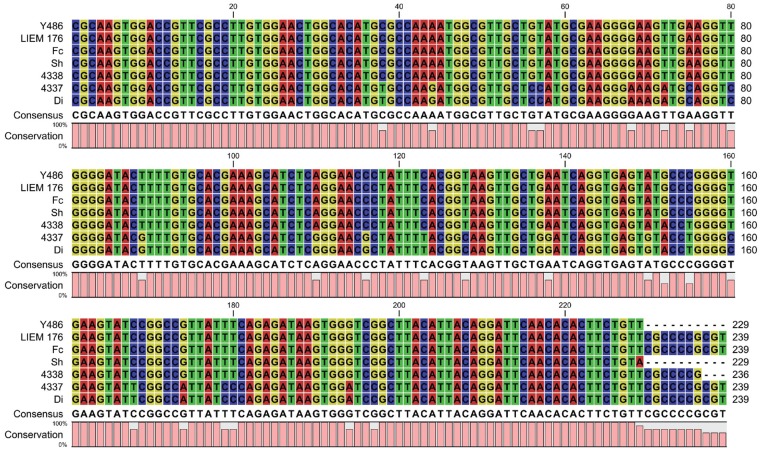
The TvPRAC PCR products from the different T. vivax strains were sequenced, directly from the PCR product (4337, 4338, Fc, Sh, Di, Y486) or after cloning into E. coli (LIEM 176). Alignment with the ILRAD 1392 sequence published in GenBank reveals that the amplicon sequences of Y486, the two Ethiopian isolates from tsetse free region (Fc and Sh), and the Venezuelan isolate LIEM 176, are almost identical to each other and to the corresponding sequence of ILRAD 1392, as shown in Figure 6 . There are sequence variations among the Ethiopian T. vivax isolates from tsetse infested regions (4338, 4337 and Di). The amplicons of strains 4338, 4337 and Di are respectively 99%, 90% and 90% similar to the corresponding sequence of ILRAD 1392.
Figure 6. Alignment of the TvPRAC PCR amplicon sequences of the different T. vivax isolates with T. vivax ILRAD 1392 as reference sequence in GenBank.
The agreement between TvPRAC PCR and ITS-1 PCR and 18S PCR-RFLP, assessed on 411 specimens from cattle under natural infection conditions, is represented in Table 3 . TvPRAC PCR detects T. vivax infection in 8.3% of the tested specimens compared to 22.6% and 6.1% detected with ITS-1 PCR and 18S PCR-RFLP respectively. Here it is important to note that 9 specimens were positive in TvPRAC PCR but negative in ITS-1 PCR. The degree of agreement between the TvPRAC PCR and the other two PCRs is fair with K = 0.4 between TvPRAC PCR and 18S PCR-RFLP and K = 0.3 between TvPRAC PCR and ITS-1 PCR.
Table 3. Cross tabulation of results obtained with the three PCRs on 411 specimens from cattle under natural infection conditions.
| Test | TvPRAC PCR | ||||||
| Positive | Negative | Total | |||||
| n | % | n | % | N | % | ||
| 18S PCR-RFLP | Positive* | 13 | 3.2 | 12 | 2.9 | 25 | 6.1 |
| Negative | 21 | 5.1 | 365 | 88.8 | 386 | 93.9 | |
| ITS-1 PCR | Positive* | 25 | 6.1 | 68 | 16.5 | 93 | 22.6 |
| Negative | 9 | 2.2 | 309 | 75.2 | 318 | 77.4 | |
| Total | 34 | 8.3 | 377 | 91.7 | 411 | ||
for the T. vivax-specific band.
Discussion
This study aimed to develop a PCR that is able to unequivocally identify T. vivax from diverse geographical origin with particular interest in strains circulating in East Africa. The TvPRAC PCR is based on a T. vivax specific proline racemase gene described in the West African T. vivax ILRAD 1392 strain [24]. In contrast with other species-specific molecular tests, the TvPRAC PCR developed in this study detected T.vivax from Nigeria (T. vivax Y486), from Venezuela (LIEM 176) as well as from Ethiopia (4337, 4338, Fc, Di, Sh). Furthermore, alignment of TvPRAC PCR amplicons with the corresponding sequence of T. vivax ILRAD 1392 in GenBank showed none or limited sequence variations among the different strains that were tested. These findings make the TvPRAC PCR a valuable candidate as a molecular diagnostic tool for T. vivax infection worldwide. The specificity of the primers proved excellent since TvPRAC PCR remained negative on DNA from other African trypanosome taxa and from other cattle infecting pathogens like Babesia bovis, B. bigemina, Theileria parva and Anaplasma marginale. TvPRAC PCR remained also negative with T. cruzi (data not shown). In addition, DNA from natural and laboratory host species as well as from man was not amplified, thus eliminating the possibility of false positive results due to contamination with host or manipulator DNA.
Using purified DNA in water, the analytical sensitivity of the TvPRAC PCR varied among the Ethiopian strains with a factor of 25 from 1.6 to 40 pg/µl while with the ITS-1 PCR, this parameter varied with a factor of 3125 from 0.32 to 1000 pg/µl. With the 18S PCR-RFLP three out of four strains remained even negative at the highest DNA concentration of 1000 pg/µl. The latter finding is consistent with the low number of T. vivax infections (6.1%) detected with the 18S PCR-RFLP in the bovine blood collection in this study. Up to now, the variation of analytical sensitivity of the TvPRAC PCR and of the ITS-1 PCR among the tested strains remains unexplained. It might be attributed to different copy numbers of the target sequences in the respective genomes although the proline racemase gene has been described as a single copy gene in the T. vivax ILRAD 1392 strain [24]. Another possibility could be small mismatches between the primers and the target sequences, both in the TvPRAC PCR and the ITS-1 PCR. The different copy number of the proline racemase gene and the ITS-1 sequences most probably underlies the differences in observed prevalence in the bovine blood collection (8.3% with TvPRAC PCR and 22.6% with ITS-1 PCR).
Depending on the situation, we believe that the combined application of both ITS-1 PCR and TvPRAC PCR may take advantage of the characteristics of both tests. ITS-1 PCR can detect multiple species, including mixed infections, in one single reaction and has a high diagnostic sensitivity, although in some instances, it will not detect T. vivax infections that are detectable with TvPRAC PCR. The latter test, on the other hand, has the advantage that it is species specific and that it reacts with T. vivax strains from diverse geographical origin with a lower detection limit of about 500 trypanosomes/ml. Further improvement in primer design might increase the analytical sensitivity of the TvPRAC PCR which may be very important for studying the prevalence of T. vivax in areas where tsetse do not naturally occur or where this vector has been eradicated.
Acknowledgments
We acknowledge the University of Addis Ababa and Oromia Regional State, Ethiopia for supporting laboratory and field work.
Funding Statement
The PhD fellowship of Fikru Regassa was financed by the Belgian Directorate General for Development Cooperation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Abebe G, Jobre Y (1996) Trypanosomiasis: a threat to cattle production in Ethiopia. Rev Méd Vét 147: 897–902. [Google Scholar]
- 2. Davila AM, Silva RA (2000) Animal trypanosomiasis in South America. Current status, partnership, and information technology. Ann N Y Acad Sci 916: 199–212. [DOI] [PubMed] [Google Scholar]
- 3. Oluwafemi A, Ilemobade A, Laseined E (2007) The impact of African animal trypanosomosis and tsetse on the livelihood and wellbeing of cattle and their owners in the BICOT study area of Nigeria. Sc Res Essays 2: 380–383. [Google Scholar]
- 4. Desquesnes M, McLaughlin G, Zoungrana A, Davila AM (2001) Detection and identification of Trypanosoma of African livestock through a single PCR based on internal transcribed spacer 1 of rDNA. Int J Parasitol 31: 610–614. [DOI] [PubMed] [Google Scholar]
- 5. Thumbi SM, Jung'a JO, Mosi RO, McOdimba FA (2010) Spatial distribution of African animal trypanosomiasis in Suba and Teso districts in Western Kenya. BMC Res Notes 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masiga DK, Smyth AJ, Hayes P, Bromidge TJ, Gibson WC (1992) Sensitive detection of trypanosomes in tsetse flies by DNA amplification. Int J Parasitol 22: 909–918. [DOI] [PubMed] [Google Scholar]
- 7. Adams ER, Malele II, Msangi AR, Gibson WC (2006) Trypanosome identification in wild tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Trop 100: 103–109. [DOI] [PubMed] [Google Scholar]
- 8. Njiru ZK, Constantine CC, Guya S, Crowther J, Kiragu JM, et al. (2005) The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol Res 95: 186–192. [DOI] [PubMed] [Google Scholar]
- 9. Geysen D, Delespaux V, Geerts S (2003) PCR-RFLP using Ssu-rDNA amplification as an easy method for species-specific diagnosis of Trypanosoma species in cattle. Vet Parasitol 110: 171–180. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton PB, Adams ER, Malele II, Gibson WC (2008) A novel, high-throughput technique for species identification reveals a new species of tsetse-transmitted trypanosome related to the Trypanosoma brucei subgenus, Trypanozoon . Infect Genet Evol 8: 26–33. [DOI] [PubMed] [Google Scholar]
- 11. Ventura RM, Paiva F, Silva RAMS, Takeda GF, Buck GA, et al. (2001) Trypanosoma vivax: characterization of the spliced-leader gene of a Brazilian stock and species-specific detection by PCR amplification of an intergenic spacer sequence. Exp Parasitol 99: 37–48. [DOI] [PubMed] [Google Scholar]
- 12. Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS, et al. (2009) Cathepsin L-like genes of Trypanosoma vivax from Africa and South America—characterization, relationships and diagnostic implications. Mol Cell Probes 23: 44–51. [DOI] [PubMed] [Google Scholar]
- 13. Nakayima J, Nakao R, Alhassan A, Mahama C, Afakye K, et al. (2012) Molecular epidemiological studies on animal trypanosomiases in Ghana. Parasit Vectors 5: e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickin SK, Gibson WC (1989) Hybridisation with a repetitive DNA probe reveals the presence of small chromosomes in Trypanosoma vivax. Mol Biochem Parasitol 33: 135–142. [DOI] [PubMed] [Google Scholar]
- 15. González LE, Garcia JA, Núñez C, Perrone TM, González-Baradat B, et al. (2005) Trypanosoma vivax: a novel method for purification from experimentally infected sheep blood. Exp Parasitol 111: 126–129. [DOI] [PubMed] [Google Scholar]
- 16. Silva RAMS, Egüez A, Morales G, Eulert E, Montenegro A, et al. (1998) Bovine trypanosomiasis in Bolivian and Brazilian lowlands. Mem Inst Oswaldo Cruz 93: 29–32. [DOI] [PubMed] [Google Scholar]
- 17.Hoare CA (1972) The trypanosomes of mammals. Oxford: Blackwell Scientific Publications. 749 p. [Google Scholar]
- 18. Masake RA, Majiwa PAO, Moloo SK, Makau JM, Njuguna JT, et al. (1997) Sensitive and specific detection of Trypanosoma vivax using the polymerase chain reaction. Exp Parasitol 85: 193–205. [DOI] [PubMed] [Google Scholar]
- 19. Gibson W (2007) Resolution of the species problem in African trypanosomes. Int J Parasitol 37: 829–838. [DOI] [PubMed] [Google Scholar]
- 20. Steverding D (2008) The history of African trypanosomiasis. Parasit Vectors 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fikru R, Goddeeris BM, Delespaux V, Moti Y, Tadesse A, et al. (2012) Widespread occurrence of Trypanosoma vivax in bovines of tsetse- as well as non-tsetse-infested regions of Ethiopia: a reason for concern? Vet Parasitol 190: 355–361. [DOI] [PubMed] [Google Scholar]
- 22. Deborggraeve S, Lejon V, Ali Ekangu R, Mumba Ngoyi D, Pyana PP, et al. (2011) Diagnostic accuracy of PCR in gambiense sleeping sickness diagnosis, staging and post-treatment follow-up: a 2-year longitudinal study. PLoS Negl Trop Dis 5: e972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morlais I, Ravel S, Grébaut P, Dumas V, Cuny G (2001) New molecular marker for Trypanosoma (Duttonella) vivax identification. Acta Trop 80: 207–213. [DOI] [PubMed] [Google Scholar]
- 24. Chamond N, Cosson A, Coatnoan N, Minoprio P (2009) Proline racemases are conserved mitogens: characterization of a Trypanosoma vivax proline racemase. Mol Biochem Parasitol 165: 170–179. [DOI] [PubMed] [Google Scholar]
- 25. Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Family Medicine 37: 360–363. [PubMed] [Google Scholar]
- 26. Leeflang P, Buys J, Blotkamp C (1976) Studies on Trypanosoma vivax: infectivity and serial maintenance of natural bovine isolates in mice. J Parasitol 6: 413–417. [DOI] [PubMed] [Google Scholar]
- 27. Delespaux V, Geysen D, Van den Bossche P, Geerts S (2008) Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitology 24: 236–242. [DOI] [PubMed] [Google Scholar]
- 28. Van Meirvenne N, Janssens PG, Magnus E (1975) Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. I. Rationalization of the experimental approach. Ann Soc Belg Méd Trop 55: 1–23. [PubMed] [Google Scholar]
- 29. Bajyana Songa E, Hamers R (1988) A card agglutination test (CATT) for veterinary use based on an early VAT RoTat 1/2 of Trypanosoma evansi . Ann Soc Belg Méd Trop 68: 233–240. [PubMed] [Google Scholar]
- 30. Barrowman PR (1976) Observations in the transmission, immunology, clinical signs and chemotherapy of dourine (Trypanosoma equiperdum infection) in horses, with special reference to cerebrospinal fluid. J Vet Res 43: 55–66. [PubMed] [Google Scholar]
- 31. Verloo D, Brandt J, Van Meirvenne N, Büscher P (2000) Comparative in vitro isolation of Trypanosoma theileri from cattle in Belgium. Vet Parasitol 89: 129–132. [DOI] [PubMed] [Google Scholar]
- 32.Geysen D (2000) The application of molecular techniques to analyse diversity in Theileria parva populations in Zambia [dissertation]. Brunel University, UK. 268 p. [Google Scholar]