Abstract
Objective
To review the effect of the fluocinolone acetonide implant (FA) in subjects with Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV), an inherited, autoimmune uveitis.
Methods
A retrospective case series was assembled from ADNIV patients who underwent fluocinolone acetonide implantation. Visual acuity and features of ADNIV, including inflammatory cells, neovascularization, fibrosis, and cystoid macular edema were reviewed.
Results
Nine eyes of five related ADNIV patients with uncontrolled inflammation were reviewed. Follow-up ranged 21.7–56.7 months. Vision at implantation ranged from 20/40 to hand motion. Preoperatively, eight eyes demonstrated vitreous cell (an eighth had a diffuse vitreous hemorrhage). Eight eyes demonstrated cystoid macular edema, seven had an epiretinal membrane, and three manifested retinal neovascularization. Following implantation, vitreous cells resolved in all eyes and neovascularization regressed or failed to develop. Central macular thickness improved in four eyes. During the postoperative course, however, visual acuity continued to deteriorate, with vision at the most recent examination ranging from 20/60 to no light perception. There was also progressive intraocular fibrosis and phthisis in one case. Four eyes underwent cataract surgery. Six of the seven eyes without previous glaucoma surgery demonstrated elevated intraocular pressure at some point, and three of these required glaucoma surgery.
Conclusions
FA implantation may inhibit specific features of ADNIV such as inflammatory cells and neovascularization, but does not stabilize long-term vision, retinal thickening, or fibrosis. All eyes in this series required cataract extraction, and more than half required surgical intervention for glaucoma. Further studies may identify additional therapies and any benefit of earlier implantation.
Introduction
Noninfectious uveitis with posterior segment inflammation can cause cataracts, vitreous opacities, vitreoretinal fibrosis, cystoid macular edema (CME), retinal neovascularization and pigmentary degeneration and lead to vision loss.1 Although uveitis accounts for as much as 10 – 15% of blindness in the U.S.,2–5 designing therapeutic studies has been difficult, since half the cases are idiopathic and outcomes may reflect the variation in disease cause rather than treatment modality. Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV) is an inherited autoimmune uveitis and vitreoretinal degeneration that maps to chromosome 11q13.6, 7 Since ADNIV patients share an identical gene defect, they provide a unique opportunity to study therapeutic effects in a highly homogenous group of eyes.
ADNIV can be divided into five disease stages, each lasting up to ten years (Table 1).6,8 The first stage of ADNIV begins in the second or third decade of life when asymptomatic patients demonstrate vitreous cells and reduction of the electroretinogram (ERG) b-wave. In the second stage, patients become symptomatic when cataract, cystoid macular edema and disc edema diminish visual acuity. In the third stage, proliferative retinal neovascularization appears, similar to that seen in diabetic retinopathy. In the fourth stage, intraocular fibrosis causes tractional retinal detachments at the macula and vitreous base. There is also an ongoing retinal degeneration during all of these stages manifesting as round pigment clumping and peripheral visual field loss. In the fifth stage, after continuous, chronic inflammation, eyes become phthisical and patients go blind. The sequelae of ADNIV are more severe than is typical for most posterior uveitis.3,8
Table 1. ADNIV Stages of disease and associated clinical findings.
| Stage | Anterior Segment | Posterior Segment | Electroretinogram |
|---|---|---|---|
| Stage I | Rare cells | Rare cells | Reduced b-wave |
| Stage II | Mild cells Mild posterior subcapsular cataract |
Moderate cells Optic disc edema Cystoid macular edema Peripheral pigmentation of the retina |
Flat b-wave, normal a-wave |
| Stage III | Moderate cells Anterior subcapsular cataract Moderate posterior subcapsular cataract Iris synechiae |
Moderate cells Vitreous traction at posterior pole Epiretinal membrane Tractional retinal detachment Posterior vitreous bands Peripheral fibrosis Peripheral neovascularization Neovascularization of the disc Pigmentary retinopathy Cystoid macular edema |
Flat b-wave, reduced a-wave |
| Stage IV | Iris bombe, seclusio pupil Peripheral anterior synechiae Angle closure glaucoma Neovascular glaucoma |
Moderate cells Vitreous hemorrhage Posterior tractional retinal detachment Anterior tractional retinal detachment |
Non-recordable ERG |
| Stage V | Phthisis | Combined tractional and rhegmatogenous retinal detachment Phthisis |
ERG, Electroretinogram
ADNIV patients do not develop any systemic autoimmune conditions. The cellular infiltrates seem to involve only the eye,9 and it is not clear whether a local or systemic aberrant immune-cell mediated process triggers the ocular damage. Past treatments have included periocular or intraocular corticosteroid injections.9 In other types of posterior uveitis, local administration of steroid can reduce the need for systemic therapy, limit inflammatory activity, and reverse macular edema or macular hyperfluorsecence on fluorescein angiography.10–13 There are also reports that suggest a potential benefit for intravitreal steroids in retinal neovascularization14–17 and proliferative vitreoretinopathy.18,19 The benefit of corticosteroid injections in ADNIV is limited however, because the condition causes chronic active inflammation.
The fluocinolone acetonide (FA) implant (Retisert, Bausch & Lomb, Rochester, NY) provides continuous release of intraocular corticosteroid for approximately 2.5 years.20 It is surgically implanted through the pars plana into the vitreous and sutured to the sclera. Large multi-centered clinical trials of noninfectious posterior uveitis demonstrated that this device was effective in controlling intraocular inflammation and reducing the need for systemic and local therapy.10–12 Although the original trials did not report the exact types of uveitis, we have had success using this implant in selected patients with specific types of severe uveitis such as sympathetic ophthalmia.21 FA implants were also recently shown to reverse features of diabetic retinopathy.22 ADNIV patients have responded poorly to conventional non-steroidal oral immunosuppressive medications. Periocular and intravitreal steroids may be used but require injections at regular intervals in order to control inflammation and cystoid macular edema. In this study, we describe our experience using the FA implant in patients with ADNIV to try to delay or even halt the progression of this blinding disease.
Methods
The study was approved by the Institutional Review Board for Human Subjects Research at the University of Iowa, was HIPPA compliant, and adhered to the tenets of the Declaration of Helsinki. A retrospective case series was assembled from the charts of ADNIV patients from The University of Iowa. Clinical exams for preoperative and postoperative examinations were performed by vitreoretinal specialists (authors). The diagnosis of ADNIV was made based on family history, pedigree review, and genetic mapping. All patients had inflammation in the vitreous and cystoid macular edema; all patients had no other evidence of an infection; all patients had negative TB skin tests. All visual acuities were best-corrected Snellen acuities. No standardized refractions or visual acuity measurements were used. A recurrence of inflammation after implantation was diagnosed by inflammatory cells in the aqueous or vitreous or fibrin in the vitreous and was usually accompanied by blurred vision and an increase in photopsias.
Image Analysis. Stereoscopic color fundus and fluorescein angiographic images were obtained using the Topcon TRC 50DX camera (Topcon, Pyramus, New Jersey). Images were viewed with OIS software version number 10.5.7. Over the course of the study period, optical coherence tomography (OCT) imaging was obtained from the spectral-domain Heidelberg HRA2 Spectralis, version 1.6.1 (Heidelberg Engineering Inc, Vista, CA) and Cirrus (Carl Zeiss Meditec, Dublin, CA), as well as the time-domain Stratus (Carl Zeiss Meditec, Dublin, CA). In an effort to standardize OCT data from the alternate OCT equipment, comparisons of central subfield macular thickness measurements were compared to published data.23,24
Results
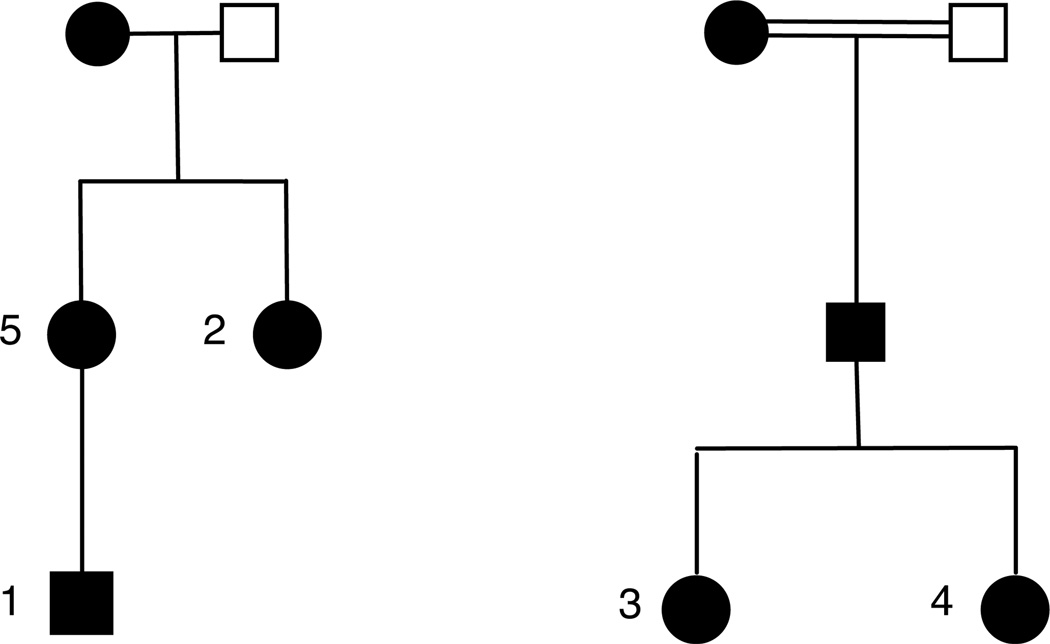
FA implantation was performed in nine eyes of five patients in two related families with ADNIV (Figure 1). Patient ages ranged from 24 years to 53 years. There were four females and one male, four right eyes and five left eyes (Table 1). Observed intraocular features prior to surgery included vitreous cell (8/9; the seventh had diffuse vitreous hemorrhage), cystoid macular edema (8/9), retinal neovascularization (3/9), vitreous hemorrhage (2/9), epiretinal membrane as evident on OCT (7/9), tractional retinal detachment (1/9), opacified vitreous secondary to uveitis (6/9), and pigmentary retinal degeneration (9/9). Glaucoma was present in 3/9 eyes, which was managed by topical medications in one eye (patient 4-OS), prior trabeculectomy in one eye (patient 3-OD), and simultaneous trabeculectomy at the time of FA implantation in one case (patient 3-OS). Three eyes underwent removal and re-implantation of exhausted FA with evidence of increased inflammatory activity (patient 3-OD [29 months], patient 3-OS [26 months], and patient 1-OS [36 months]), whereas one required removal and re-implantation during wound revision six months after primary implantation (patient 4-OS), and another failed attempted removal and re-implantation due to poor intra-operative visualization with concern that the implant was beneath a traction detachment of the retina in the inferior peiphery (patient 2-OS; 40 months). Patient 1-OD has been in place for 53 months, and patient 2-OD has been in place for 56.7 months, without need for replacement. Follow-up data for the primary implant of 21.7 months or greater was available in all patients (range = 21.7 – 56.7 months after insertion). The case of patient 1 is described in Figure 2.
Surgical objectives in placement of the primary FA implant were achieved in all eyes. Posterior segment complications such as retinal tear or dialysis, retinal detachment, suprachoroidal effusion or hemorrhage, or endophthalmitis were not observed during surgery or the immediate postoperative period. Late postoperative events included a vitreous tap and inject six months after implantation in one eye (patient 3-OS) for possible endophthalmitis. This eye had also previously undergone a trabeculectomy, but cultures were negative, and vision, inflammation, and OCT parameters returned to baseline shortly after the event. In another eye (patient 5-OS), there was progressive posterior segment fibrosis that required surgery 4.2 months after primary FA implantation, during which extensive anterior intraocular scarring incorporating the implant was discovered. This implant was removed, and the vitreous and membranes were peeled resulting in on one small hole in the superior retinal periphery. The eye developed a massive fibrin response that did not respond to 4mg intravitreal triamcinolone acetonide. The eye became hypotonous and subsequently phthisical. Another eye (patient 4-OS) required multiple revisions of the wound and eventual exchange of the implant at 5 months, due to exposure of the implant.
Preoperative vision ranged from 20/40 to hand motion, and postoperative vision at the most recent follow-up ranged from 20/60 to no light perception in the phthisical eye (patient 5-OS). The four eyes that remained phakic after initial FA implantation subsequently underwent cataract surgery.
Vitreous cells resolved in all eyes except the one with a fibrin response (5-OS). Although there was no clear trend for improvement or worsening of CME, six of the nine eyes progressively worsened in visual acuity over their postoperative period. The three eyes demonstrating an improvement of vision at their last postoperative visit improved from 20/100 to 20/80 (patient 1-OD), 20/250 to 20/200 (patient 2-OS), and 20/320 to 20/250 in the other (patient 3-OD) (Table 1). One of these eyes (patient 3-OD) also received multiple intravitreal injections of bevacizumab (Avastin, Genentech, San Francisco, CA), while patient 1-OD received a single intravitreal injection of both bevacizumab and triamcinolone acetonide 4mg (Kenalog-40, Bristol-Myers Squibb, Princeton, NJ). The degree of thickening on optical coherence tomography did not reliably correspond to visual acuity measurements (Table 1). None of the eyes achieved a central macular thickness equal to or less than the published normative means at any time during the study period.23, 24
We have carefully examined over 90 patients with ADNIV. All but one patient developed proliferative neovascularization (stage III disease). Stage III disease usually develops during the third or fourth decades, but can start earlier in some cases. Panretinal laser photocoagulation with careful treatment to the ora does not cause resolution of the neovascularization.6 In this series, three eyes demonstrated evidence of retinal neovascularization at the time of FA implantation (patient 5-OS, patient 1-OD and patient 1-OS). The neovascularization regressed in two of the eyes after device implantation and panretinal laser photocoagulation, although one eye, (patient 1-OD), had also received one dose of intravitreal bevacizumab. The third eye (patient 5-OS) developed progressive posterior segment fibrosis and eventual phthisis. Neovascularization has not developed in the six eyes without evidence of new vessels at the time of initial FA.
Each of the six eyes with visual potential and no previous glaucoma surgery demonstrated elevated intraocular pressure following FA implantation. Three underwent glaucoma surgery (patient 4-OS, patient 1-OD and patient 1-OS), one was placed on long-term topical intraocular pressure-lowering medication (patient 4-OD), and two required topical therapy for a limited time (patient 2-OD and patient 2-OS).
Treatment with systemic immunomodulatory agents was attempted in four patients, but appeared to have no effect, so only two patients remained on therapy at the time of FA implantation. At the time of implantation, Patient 5 was treated with 200mg azathioprine and 5mg prednisone daily. The azathioprine was discontinued soon after surgery, and the prednisone was discontinued after the eye proceeded toward phthisis. Patient 1 was initially on 60mg oral prednisone, but this was tapered and discontinued soon after FA implantation (patient 1-OS). This patient was treated briefly with oral prednisone for all subsequent intraocular procedures (OD and OS) and for a brief time while exhibiting evidence of a mild central retinal vein occlusion (OD). Patients 2, 3 and 4 were not treated with further systemic immunomodulation at any time.
Discussion
Although the number of eyes described in this report is limited, they all share an identical genetic defect and provide a unique insight into uveitis therapy. ADNIV is a difficult condition to treat because the inflammation is often severe and chronic, neovascularization develops leading to hemorrhage, fibrosis causes traction retinal detachments, and there is an ongoing outer retinal degeneration. Immunohistopathological findings support the concept that an underlying ocular immune dysfunction is present in these eyes.9 Profound vision loss results from photoreceptor degeneration, cataract, cystoid macular edema, vitreous hemorrhage, dense membrane formation leading to tractional retinal detachment, and neovascular glaucoma.6 Patients universally undergo early cataract surgery and many require glaucoma surgery. By stage III disease, however, retinal neovascularization develops at the disc and retinal periphery, and patients seem to pass a point of no return with complications leading to complete blindness and phthisis bulbi. There is only a partial response to laser photocoagulation of the peripheral retina to neovascularization of the retina and iris. Although vitrectomy surgery repaired several detachments in the original report,6 long-term results have been disappointing with recurrent membranes and detachments. FA device implantation can reduce the need for systemic therapy,13 but systemic medication, including oral steroids, methotrexate, and anti-tumor necrosis factor agents and other nonsteroidals, has shown either limited or no benefit in ADNIV patients (unpublished observations). The observation that the FA causes resolution of neovascularization is important, however. The one patient who never developed neovascularization even into her ninth decade had severely restricted fields and 20/400 vision in both eyes. She is the only patient however, who did not develop NLP vision in both eyes by the eighth decade. Thus the FA implant may limit the neovascular changes and alter the course of ADNIV into that of other more common retinal degenerations that develop constricted visual fields but retain limited vision in the center.
Although the inflammatory uveitis response of the ADNIV patients may be similar to cases of severe idiopathic posterior uveitis, the visual outcome of ADNIV patients worse than published reports of other posterior uveitides treated with FA implantation.10–12,20,21 Patel and colleagues, in their report examining the treatment of pediatric uveitis, reported that inflammation was well-controlled in all eyes,25 while Pavesio et al demonstrated a delayed onset of uveitis recurrence and lower onset of recurrence compared with standard therapy (18.2 versus 63.5%).12 Callanan et al demonstrated that recurrence of inflammation during the year prior to 0.59 mg FA implantation was 62% versus 4%, 10%, and 20% at 1, 2, and 3 years post-implantation, respectively.10 Pavesio and colleagues demonstrated that mean VA decreased transiently relative to the patient’s baseline immediately after surgery and from 15–21 months, but was otherwise at baseline by 24 months after surgery.12
Patel et al found that three of six eyes improved at least 3 lines while being followed up to 39 months.25 Callanan et al found that FA implants improved, or at least stabilized, eyes with posterior uveitis.10 One of the nine ADNIV eyes that were given the FA implant went NLP and only two had vision at 24 months that was equal to or better than the immediate pre-implantation vision (patient 1-OD and patient 2-OS). Therefore ADNIV eyes did worse than eyes with other types of uveitis. Our study does not address whether implantation during earlier stages of ADNIV might show improved visual acuity benefit.
Previous reports have shown FA implants to be efficacious in controlling inflammation, but the implant almost universally causes cataracts and many eyes develop elevated intraocular pressure. Two studies reported that 45%26 and 40%10 of eyes with FA implants needed glaucoma surgery. Other studies reported that 21.2%12 and 26.2%13 required glaucoma surgery. Five of nine eyes in this study required glaucoma surgery. Two of the nine eyes had received glaucoma surgery prior to FA implantation and three additional eyes required glaucoma surgery after implantation. Another eye in this study has required long-term topical pressure-lowering drops. Previous reports stated that 80.4 to 93% of phakic patients will require cataract surgery after implantation.10,12,13 All four ADNIV eyes in this report that remained phakic after the initial FA implantation required cataract surgery. One eye that underwent FA implant placement subsequently required vitrectomy surgery for severe preretinal membranes and a tractional retinal detachment. This eye developed a severe fibrin response and became phthisical (case 5-OS). There is a report of the formation of visually significant vitreous bands after FA implantation requiring further surgery.27
One possible explanation for understanding the pathological processes in ADNIV is that the constellation of findings is all a consequence of ocular inflammation. The observations here suggest otherwise. FA implantation reversed vitreous cell and neovascularization and prevented future neovascularization, which did not develop in any previous unaffected eyes. However, CME on OCT did not change consistently over the long term, and visual acuity results, while better than the previously described course of vision in ADNIV eyes,6 did not stabilize as had been hoped, indicating the possibility that photoreceptors continue degenerating even when CME is under control. Electroretinography demonstrates early retinal and photoreceptor dysfunction.6,7 Interestingly, the fibrotic response progressed despite inflammatory control with the development of proliferative vitreoretinopathy, which even required explantation in the most severe case. If however, FA prevents neovascularization and alters the course of ADNIV toward that seen in more common pigmentary retinal degeneration, this would be of benefit. Older ADNIV patients may then retain at least some vision instead of losing light perception with phthisis in both eyes.
Unfortunately, prolonged inflammatory suppression with the FA implant only partially controls the disease progression in ADNIV. Although the exact mechanisms of immune suppression are not known, steroid-based transcriptional regulation within the eye may be sufficient to inhibit cytokine signals that activate the cell-mediated immune responses and neovascularization, but not photoreceptor death or intraocular fibrosis. Effectively targeting each pathway may require different therapeutic modalities, and the study of ADNIV patients may reveal the molecular signals involved in vitreous inflammation, retinal neovascularization, photoreceptor degeneration, and proliferative vitreoretinopathy.
Figure 1. ADNIV Pedigrees.
Two related families with clinical features of Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV) exhibited a dominant pattern of inheritance. Black symbols represent clinically affected subjects. Numbers correspond to patients that underwent fluocinolone acetonide implantation. Open symbols represent unaffected subjects.
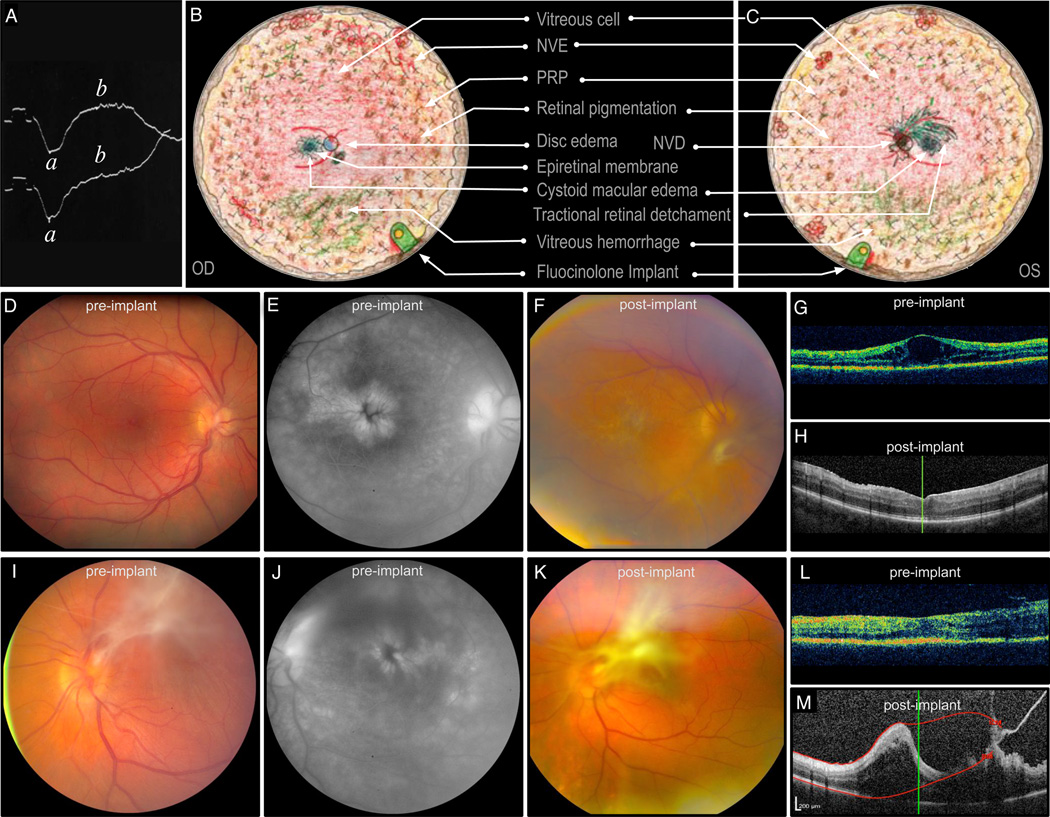
Figure 2. ADNIV Case Report.
A. An electroretinogram showing a diminished b-wave helped diagnose patient 1 with ADNIV at age 17. B, C. Composite fundus drawings show vitreoretinal pathology overlaid with treatment that took place over several years beginning at age 21 when his visual acuity was 20/100 OU. He developed aggressive ADNIV with cystoid macular edema, an epiretinal membrane, and small punctuate areas of peripheral neovascularization and vitreous hemorrhage OD. There was a central vitreous hemorrhage secondary to neovascularization of the disc with count fingers vision OS. Traction was noted in the periphery OS. Both eyes showed characteristic peripheral pigmentary changes. Oral steroids and panretinal scatter photocoagulation initially stabilized the patient to 20/30 OD and 20/50 OS. He subsequently lost vision due to continuous inflammation, CME, and recurrent vitreous hemorrhage while on steroid-sparing agents over the next two years, including methotrexate and infliximab, and moderate doses of prednisone. At age 24, fluocinolone acetonide implants were placed in each eye. D. Preoperative fundus image OD shows an epiretinal membrane and CME (VA 20/100). E. CME was confirmed by fluorescein angiography. F. Postoperative fundus image shows worsening membrane OD. G, H. Pre and postoperative OCT shows CME that resolved soon after device implantation. Within one month after surgery, a mild CRVO developed, and the patient was treated with oral steroids. A small area of retinal neovascularization was noted at that time, precipitating a dose of intravitreal bevacizumab and further laser photocoagulation. All vascular changes regressed in six months. Cataract surgery and trabeculectomy OD were performed one and two years after implant placement, respectively. The right eye also required a laser peripheral iridotomy for pupillary block, but inflammation was controlled nearly four years after device implantation without need for replacement. I. Preoperative fundus image OS shows regressed NVD, preretinal fibrosis, and epiretinal membrane. J. Findings consistent with recurrent CME are apparent on preoperative fluorescein angiography. K. Postoperative fundus image shows progressive fibrosis OS. L. The immediate preoperative OCT shows early tractional membranes superiorly (right side of OCT), without traction on the fovea or significant CME. M. Postoperative OCT shows increased tractional membranes. Implantation OS was followed nearly immediately by elevated intraocular pressure that was controlled initially with topical glaucoma medication. Initially, the vision OS stabilized to 20/60 without recurrent CME, neovascularization, or hemorrhage. One year later, cataract surgery was performed, and two years later the implant was replaced and a trabeculectomy was performed. The only systemic immunomodulatory medications this patient received at any time after initial implant surgeries were short courses of prednisone for a mild central retinal vein occlusion OD and any subsequent intraocular surgeries. Despite control of neovascularization and vitreous cell, there was worsening of preretinal fibrosis and tractional membranes and deterioration of visual acuity to 20/80 OD and 20/200 OS. (ADNIV, autosomal dominant neovascular inflammatory vitreoretinopathy; CME, cystoid macular edema; CRVO, central retinal vein occlusion; NVD, neovascularization of the disc; OCT, optical coherence tomography; OD, right eye; OS left eye; VA, visual acuity.)
Table 2. Summary of Clinical Findings.
The first column lists the patient, age (years) at which the fluocinolone acetonide implanatation was first performed. The “preop” column contains data from the month prior to implantation. (Preop, preoperative; OD, right eye; OS, left eye; Logmar, logarithm of the minimal angle of resolution; VA, visual acuity; M, male; F, female; OCT, optical coherence tomography; Vit cells, vitreous cells; n/a, not available; HM, Hand motion; VH, vitreous hemorrhage).
| Patient | Eye | Exam | Preop | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 (24-M) |
OD | Logmar VA Snellen VA |
0.70; 20/100 |
1.10; 20/250 |
0.90*; 20/160 |
0.70; 20/100 |
0.54; 20/70 |
0.70; 20/100 |
0.60; 20/80 |
0.70; 20/100 |
0.70; 20/100 |
0.60; 20/80 |
| OCT† | 663 (469) |
718 (524) |
323 (129) |
460 (207) |
323 (53) |
n/a | n/a | n/a | n/a | n/a | ||
| Vit cells | 2+ | trace | 0 | 0 | trace | 0 | trace | 0 | 0 | 0 | ||
| OS | Logmar VA Snellen VA |
0.48; 20/60 |
0.48; 20/60 |
0.48; 20/60 |
0.48‡; 20/60 |
0.80; 20/125 |
1.10; 20/250 |
1.00; 20/200 |
||||
| OCT | n/a | 431 (237) |
411 (217) |
485 (291) |
n/a | n/a | n/a | |||||
| Vit cells | 1+ | 0 | 0 | 0 | 0 | 0 | 0 | |||||
|
2 (44-F) |
OD | Logmar VA Snellen VA |
0.30; 20/40 |
0.40; 20/50 |
0.40; 20/50 |
0.40^; 20/50 |
0.40; 20/50 |
0.40; 20/50 |
0.48; 20/60 |
0.48; 20/60 |
0.40; 20/50 |
0.48; 20/60 |
| OCT | 393 (199) |
230 (36) |
256 (62) |
312 (59) |
313 (43) |
285 (15) |
n/a | n/a | n/a | n/a | ||
| Vit cells | 1+ | 0 | 0 | 0 | 0 | 0 | Trace | 0 | 0 | 0 | ||
| OS | Logmar VA Snellen VA |
1.10; 20/250 |
1.10; 20/250 |
0.70^; 20/100 |
1.10; 20/250 |
1.00; 20/200 |
0.90; 20/160 |
1.00; 20/200 |
||||
| OCT | 624 (430) |
227 (33) |
276 (82) |
369 (175) |
403 (209) |
414 (161) |
404 (134) |
|||||
| Vit Cells | 2+ | 0 | 0 | 0 | 0 | 0 | 0 | |||||
|
3 (36-F) |
OD | Logmar VA Snellen VA |
1.20; 20/320 |
0.60; 20/80 |
1.00; 20/200 |
1.10**; 20/250 |
1.30**; 20/400 |
1.10**; 20/250 |
||||
| OCT | 542 (348) |
325 (55) |
443 (173) |
486 (216) |
431 (161) |
434 (164) |
||||||
| Vit cells | 2+ | trace | 0 | 0 | trace | 1+ | ||||||
| OS | Logmar VA Snellen VA |
0.90; 20/160 |
0.90; 20/160 |
1.00; 20/200 |
1.00; 20/200 |
1.30; 20/400 |
||||||
| OCT | 296 (26) |
299 (29) |
304 (34) |
309 (39) |
318 (48) |
|||||||
| Vit cells | 1+ | 0 | 0 | 0 | 0 | |||||||
|
4 (38-F) |
OD | Logmar VA Snellen VA |
0.40; 20/50 |
0.48; 20/60 |
0.70; 20/100 |
n/a | 1.10; 20/250 |
|||||
| OCT | 306 (36) |
346 (76) |
302 (32) |
n/a | n/a | |||||||
| Vit cells | 3+ | trace | 0 | n/a | 0 | |||||||
| OS | VA Logmar VA Snellen VA |
0.30; 20/40 |
0.30; 20/40 |
0.48; 20/60 |
0.54; 20/60 |
0.48; 20/70 |
||||||
| OCT | 323 (129) |
n/a | n/a | 426 (156) |
320 (50) |
|||||||
| Vit cells | 2+ | 0 | 0 | 0 | 0 | |||||||
|
5 (53-F) |
OS | Logmar VA Snellen VA |
3.00; HM |
3.00; HM |
||||||||
| OCT | n/a | n/a | ||||||||||
| Vit cells | Dense VH |
0 |
Uncomplicated cataract surgery was performed during the preceding interval, as well as intravitreal injections of bevacizumab and triamcinolone acetonide
Data is presented first in microns, then in (microns greater than normative published mean for the imaging equipment and software utilized)
intravitreal bevacizumab administered during preceding interval
uncomplicated cataract surgery performed during preceding interval
uncomplicated cataract surgery performed with (OD) and without (OS) intravitreal injection of triamcinolone acetonide during preceding interval
Acknowledgments
Financial Support: The authors are supported by NIH Grants K08EY020530 (VBM), The Judith (Gardner) and Donald H. Beisner Professorship of Vitreoretinal Diseases and Surgery, (JCF); R01EY016822 and the Howard Hughes Medical Institute (EMS); the Roy J. Carver Charitable Trust, and Research to Prevent Blindness (New York, NY).
Footnotes
The authors have no commercial or financial interests associated with this article.
References
- 1.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 3.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 4.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Uveitis Society. [Accessed February 1, 2012];Frequently Asked Questions. 2002 http://www.uveitissociety.org/pages/faq.html.
- 6.Bennett SR, Folk JC, Kimura AE, Russell SR, Stone EM, Raphtis EM. Autosomal dominant neovascular inflammatory vitreoretinopathy. Ophthalmology. 1990;97(9):1125–1135. doi: 10.1016/s0161-6420(90)32447-8. discussion 1135-1126. [DOI] [PubMed] [Google Scholar]
- 7.Stone EM, Kimura AE, Folk JC, et al. Genetic linkage of autosomal dominant neovascular inflammatory vitreoretinopathy to chromosome 11q13. Hum Mol Genet. 1992;1(9):685–689. doi: 10.1093/hmg/1.9.685. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan VB, Folk JC, Fingert JH, et al. Genetic Analysis and Phenotypic Staging of Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy. ARVO. 2011 May 1:62/A175. [Google Scholar]
- 9.Mahajan VB, Vallone JG, Lin JH, et al. T-cell infiltration in autosomal dominant neovascular inflammatory vitreoretinopathy. Mol Vis. 2010;16:1034–1040. [PMC free article] [PubMed] [Google Scholar]
- 10.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6):1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology. 2010;117(3):567–575. doi: 10.1016/j.ophtha.2009.11.027. 575 e561. [DOI] [PubMed] [Google Scholar]
- 13.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandello F, Polito A, Pognuz DR, Monaco P, Dimastrogiovanni A, Paissios J. Triamcinolone as adjunctive treatment to laser panretinal photocoagulation for proliferative diabetic retinopathy. Arch Ophthalmol. 2006;124(5):643–650. doi: 10.1001/archopht.124.5.643. [DOI] [PubMed] [Google Scholar]
- 15.Chan CK, Ip MS, Vanveldhuisen PC, et al. SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion. Ophthalmology. 2011;118(7):1364–1372. doi: 10.1016/j.ophtha.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001;131(4):468–471. doi: 10.1016/s0002-9394(00)00882-5. [DOI] [PubMed] [Google Scholar]
- 17.Munir WM, Pulido JS, Sharma MC, Buerk BM. Intravitreal triamcinolone for treatment of complicated proliferative diabetic retinopathy and proliferative vitreoretinopathy. Can J Ophthalmol. 2005;40(5):598–604. doi: 10.1016/S0008-4182(05)80052-3. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Chen H, Hou P, Fok A, Hu Y, Lam DS. Midterm results of low-dose intravitreal triamcinolone as adjunctive treatment for proliferative vitreoretinopathy. Retina. 2011;31(6):1137–1142. doi: 10.1097/IAE.0b013e3181fe5427. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadieh H, Feghhi M, Tabatabaei H, Shoeibi N, Ramezani A, Mohebbi MR. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology. 2008;115(11):1938–1943. doi: 10.1016/j.ophtha.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112(7):1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan VB, Gehrs KM, Goldstein DA, Fischer DH, Lopez JS, Folk JC. Management of sympathetic ophthalmia with the fluocinolone acetonide implant. Ophthalmology. 2009;116(3):552–557. doi: 10.1016/j.ophtha.2008.10.024. e551. [DOI] [PubMed] [Google Scholar]
- 22.Pearson PA, Comstock TL, Ip M, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118(8):1580–1587. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (Spectralis) Am J Ophthalmol. 2009;148(2):266–271. doi: 10.1016/j.ajo.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Abedi G, Patal P, Doros G, Subramanian ML. Transitioning from stratus OCT to cirrus OCT: a comparison and a proposed equation to convert central subfield macular thickness measurements in healthy subjects. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1353–1357. doi: 10.1007/s00417-011-1725-6. [DOI] [PubMed] [Google Scholar]
- 25.Patel CC, Mandava N, Oliver SC, Braverman R, Quiroz-Mercado H, Olson JL. Treatment of Intractable Posterior Uveitis in Pediatric Patients with the Fluocinolone Acetonide Intravitreal Implant (Retisert) Retina. 2011 doi: 10.1097/IAE.0b013e31822058bb. [DOI] [PubMed] [Google Scholar]
- 26.Bollinger K, Kim J, Lowder CY, Kaiser PK, Smith SD. Intraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitis. Ophthalmology. 2011;118(10):1927–1931. doi: 10.1016/j.ophtha.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Galor A, Margolis R, Kaiser PK, Lowder CY. Vitreous band formation and the sustained-release, intravitreal fluocinolone (Retisert) implant. Arch Ophthalmol. 2007;125(6):836–838. doi: 10.1001/archopht.125.6.836. [DOI] [PubMed] [Google Scholar]