Abstract
Background
Unexplained heterogeneity in response to ventricular assist device (VAD) implantation for the management of advanced heart failure impedes our ability to predict favorable outcomes, provide adequate patient and family education, and personalize monitoring and symptom management strategies. The purpose of this paper is to describe the background and design of a study entitled Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure (PREMISE).
Study Design and Methods
PREMISE is a prospective cohort study designed to a) identify common and distinct trajectories of change in physical and psychological symptom burden, b) characterize common trajectories of change in serum biomarkers of myocardial stress, systemic inflammation, and endothelial dysfunction, and c) quantify associations between symptoms and biomarkers of pathogenesis in adults undergoing VAD implantation. Latent growth mixture modeling, including parallel process and cross-classification modeling, will be used to address the study aims and will entail identifying trajectories, quantifying associations between trajectories and both clinical and quality-of-life outcomes, and identifying predictors of favorable symptom and biomarker responses to VAD implantation.
Conclusion
Research findings from PREMISE will be used to enhance shared patient and provider decision-making, and shape a much-needed new breed of interventions and clinical management strategies that are tailored to differential symptom and pathogenic responses to VAD implantation.
Introduction
Heart failure (HF) is the fastest growing cardiovascular disorder and the most common reason for re-hospitalization among older U.S. adults.1, 2 Patients with advanced HF (i.e. those with refractory symptoms despite maximal optimal medical therapy)3 live with severe symptom burden and decreased quality-of-life (QOL). Given extremely limited organ availability and restrictive eligibility for heart transplantation,4 mechanical circulatory support with a ventricular assist device (VAD) has emerged as a primary therapy as a bridge to transplantation or recovery, or as destination therapy (i.e. as a permanently implanted device) for patients with advanced HF.5
There is significant and unexplained heterogeneity in response to VAD implantation concerning clinical events,5, 6 functional capacity and physical functioning,7, 8 and health-related QOL (HRQOL).9, 10 Very little is known about how physical and psychological symptoms change after VAD implantation. We are particularly bereft of insight into how symptoms may relate to changes in underlying pathogenesis, and how symptoms and biomarkers may explain differential responses to VAD implantation. As such, we are limited in our ability to predict favorable outcomes, support adequate patient and family decision-making, provide education and anticipatory guidance, and personalize monitoring and symptom management strategies for patients undergoing VAD implantation.
The purpose of this paper is to describe the background and design of a prospective biobehavioral observational study entitled Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure (PREMISE). This study was developed to characterize common and distinct trajectories of change in symptoms and pathogenic biomarkers during the transition from pre-implantation through the first 6 months after VAD implantation, and link changes in symptoms and biomarkers over time to clinical events and HRQOL. Relevant background and the research design and methods are included in this paper. We conclude with a discussion of anticipated findings and research implications.
Background
Advanced Heart Failure: Refractory Symptoms and Limited Options
Many patients with HF have symptoms at rest or with minimal exertion that are refractory to optimal medical therapy (e.g. advanced HF).11 Up to 800,000 adults in the U.S. have advanced HF12 and they have few treatment options. First, patients may be eligible for the gold standard of cardiac transplantation. There are only 2,400 heart transplants performed each year in the U.S.,13 and fewer than 5% of patients with HF are eligible for cardiac transplantation.4 Moreover, 10% of advanced HF patients listed for transplant die each year waiting for an allograft.12 Second, continuous intravenous infusion of inotropic agents may be used to ameliorate symptoms.14 The use of inotropes conveys a 43% 6-month mortality rate,15 a survival trade-off for symptom reduction that many patients with HF are unwilling to make.16 Third, patients and providers may opt for palliation, which has become an increasingly integrated approach in advanced HF.17 Fourth, patients may choose and be eligible for mechanical support with a VAD as a bridge to transplant or if deemed not a candidate for heart transplantation, be considered for VAD support as destination therapy. With limited effectiveness of medical therapy and an inadequate number of available donor hearts, mechanical circulatory support with a VAD is an important therapeutic option for patients with advanced HF.18
Mechanical Support with Ventricular Assist Devices
Originally designed as short-term therapy to augment cardiac output,19 newer generation VADs are smaller, more durable, and associated with fewer adverse events.5 Approximately 25% of HF patients awaiting heart transplant have a VAD;20 and approximately 79% of these patients are transplanted, alive on VAD support, or have the VAD removed for myocardial recovery within 6 months.21 The use of VADs as destination therapy for patients who are not candidates for or choose not to undergo transplantation is an increasingly-utilized treatment strategy. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial provided evidence of a 27% 1-year survival benefit with VAD implantation over optimal medical therapy in advanced HF patients.7 Since that trial, the number of VAD implantations for destination therapy has increased dramatically; more than 20,000 patients are expected to receive a VAD annually and 1-year survival on contemporary devices exceeds 80%.22
Prior Research on Ventricular Assist Devices
To date, most research on VADs has focused on quantifying average event-free survival,5, 6 average restoration of functional capacity and physical functioning,7, 8 and average improvements in HRQOL associated with device implantation.8–10, 23–28 With significant heterogeneity in response to VAD implantation within these studies, however, minimal clinical insight can be gained from average changes from trials alone.29 As a recent example of the extensive heterogeneity observed in response to VAD implantation, Rogers et al.,8 reported mean ± standard deviation changes at 6 months relative to baseline in the 6-minute walk test (146±231 meters), the Minnesota Living with HF Questionnaire scores (−39±23 points), and the Kansas City Cardiomyopathy Questionnaire clinical summary score (37±25 points) for destination therapy patients. Most research reports include similarly large standard deviations that approximate or are greater than the mean, or in the case of HRQOL measures encompass almost ¼ of the possible range of these scales. Recalling that just over 68% of observed values fall within ± one standard deviation, mean estimates from this and other reports with significant heterogeneity become somewhat meaningless. Moreover, few studies have assessed psychological symptoms before and after VAD implantation and the results are variable and inconsistent,7, 23, 24 and the few studies on biomarkers before and after VAD implantation are limited to very small samples, short-term (1 to 6 week) change,30–32 and by the study of single pathogenic processes.33 Based on the current state of the science, we are extremely limited in our ability to predict favorable responses to, and provide patients with adequate information about VAD implantation without first identifying subgroups of patients with differential symptom, pathogenic, and clinical and HRQOL outcome responses to these devices.
Research Design and Methods
Biobehavioral Research Framework
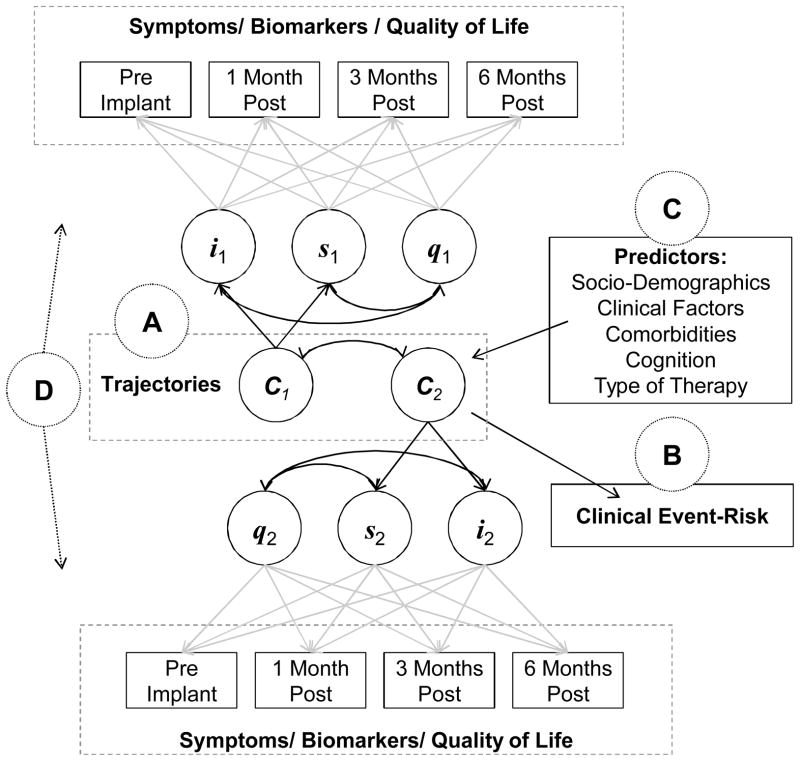
The research framework for this study was adapted from Lenz’s Theory of Unpleasant Symptoms, with respect to interactions among multiple symptoms (i.e. physical and psychological), multiple influential pathophysiological mechanisms (i.e. myocardial stress, systemic inflammation, and endothelial dysfunction), situational factors (i.e. transition from advanced HF to VAD implantation that is likely to change both symptoms and marker of underlying HF pathogenesis), and performance (e.g. HRQOL and clinical event-risk).34, 35 This research framework was customized to overcome limitations of extant approaches to symptom research and includes a symptom biochemistry element to gain insight into pathogenic mechanism that may or may not underlie concomitant changes in symptoms (Figure 1).
Figure 1. Biobehavioral Research Framework.
The interaction between symptoms and the appropriate handling of the temporal nature of symptoms in response to pathophysiological and situational factors are major omission from many symptom models. Using latent growth mixture modeling, common and distinct trajectories of change among symptoms and biomarkers over time will be identified in response to ventricular assist device implantation (Figure 1-A). Identified symptom and biomarker trajectories (C1 and C2) may have different intercepts (i), slopes (s), and non-linear patterns of change (q) over time. Our framework was developed to codify the clinical relevance of differential responses to ventricular assist device implantation in the context of both patient-oriented and clinical outcomes. As such, associations between symptoms and biomarkers and the outcomes of health-related quality of life (Figure 1-A) and 6-month clinical event-risk (Figure 1-B) will be quantified. Our framework also includes predictors of favorable and unfavorable symptom and biomarker responses. We will explore a number of factors in order to identify patients at greater risk for symptom burden, worse pathogenic responses, poor health-related quality of life, and/or greater event-risk (Figure 1-C). Figure 1A-C match with our identify, associate, predict statistical approach to specific aims 1 and 2. To effectively capture interactions among symptoms and indices of pathogenesis, patterns of association among multiple symptoms and among multiple pathogenic biomarkers over time will be quantified using parallel process modeling in a symptom biochemistry element (Figure 1-D). In sum, our biobehavioral research framework has elements of symptom science, heart failure pathophysiological research, symptom biology/biochemistry, and clinical and patient-oriented outcomes research.
Study Design
A prospective descriptive cohort design will be used to characterize common trajectories of change in symptoms and biomarkers of pathogenesis during the transition from advanced HF through the first 6 months after VAD implantation. Subjective data on physical (physical symptoms, pain, and daytime sleepiness) and psychological (depression, anxiety, and hostility) symptoms will be collected from a cohort of 120 adults with advanced HF prior to, and at 1, 3, and 6 months after VAD implantation. Serum biomarkers will be collected synchronously with symptom data prior to and at 1, 3, and 6 months after VAD implantation. Corresponding data on HRQOL prior to and after VAD implantation, and clinical event data throughout 6 months of follow-up will also be collected to observe common clinical and/or adverse events and the early and sustained improvements in HRQOL described previously.26 Latent growth mixture modeling and extensions thereof will be used to identify multiple trajectories of change in these biobehavioral factors over time; changes in biobehavioral factors will be linked to concomitant changes in HRQOL throughout the duration of the study, and with differential clinical event-risk at 6 months.
Study Specific Aims and Hypotheses
This overarching goal of this to characterize common and distinct trajectories of change in symptoms and pathogenic biomarkers during the transition from pre-implantation through the first 6 months after VAD implantation, and link changes in symptoms and biomarkers over time to clinical events and HRQOL.
Specific Aim 1)
Identify common trajectories of change in physical and psychological symptom burden in adults undergoing VAD implantation.
-
Hypothesis 1.1
Distinct trajectories of change in physical and psychological symptoms can be identified, and will be associated with significant differences in clinical event-risk and HRQOL.
-
Hypothesis 1.2
Socio-demographic and clinical predictors of symptom responses can be identified.
Specific Aim 2)
Characterize common trajectories of change in serum biomarkers of myocardial stress, systemic inflammation, and endothelial dysfunction in adults undergoing VAD implantation.
-
Hypothesis 2.1
Distinct trajectories of change in biomarkers of pathogenesis can be identified, and will be associated with differences in clinical event-risk and HRQOL.
-
Hypothesis 2.2
Socio-demographic and clinical predictors of biomarker responses can be identified.
Specific Aim 3)
Quantify associations between symptoms and biomarkers of pathogenesis in adults undergoing VAD implantation.
-
Hypothesis 3.1
More severe myocardial stress, systemic inflammation, and endothelial dysfunction will be associated with worse symptoms.
-
Hypothesis 3.2
Combined data on patient-reported symptoms and serum biomarkers will improve clinical event-risk prediction and explain more variability in HRQOL than either factor independently.
Sample
The sampling frame for the proposed research is adult women and men with advanced HF who are responsible for their own healthcare decisions, and are undergoing the implantation of a continuous flow VAD as a bridge to transplant or as destination therapy. All participants will meet the criteria for and receive a commercially available and Food and Drug Administration-approved continuous flow VAD for the management of advanced HF. All eligible patients will be approached for voluntary participation by investigators who are not involved directly with patient care. Up to 120 participants from a single advanced HF clinic will be enrolled. The Institutional Review Board approved all study procedures, and both the study sponsor and the Institutional Review Board approved the Data and Safety Monitoring Plan. Inclusion and Exclusion criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria
Inclusion Criteria:
|
Exclusion Criteria:
|
Centers for Medicare and Medicaid Services Ventricular Assist Device (VAD) eligibility: Patients who are listed as heart transplant candidates can have a VAD implanted as a bridge to heart transplantation. For destination therapy, patients must meet all the following criteria; a) documented ineligibility for heart transplantation, b) end stage HF, c) peak oxygen consumption less than or equal to 14ml/kg per minute, and d) New York Heart Association (NYHA) class IV HF for at least 60 days, or NYHA class III/IV for at least 28 days and on intra-aortic balloon pump for at least 14 of those days, or dependent on IV inotropic medications, with two weaning attempts.
Measurement
Well-established measures and methods in clinical research will be used to capture physical and psychological symptoms, serum biomarkers, HRQOL, and factors that may influence these variables over time including HF history and diagnostics, comorbidities, hemodynamic profile, socio-demographics, functional capacity, and mild cognitive dysfunction. All study measures are presented in Table 2.
Table 2.
Schedule of Assessments
| Clinical Characteristics | Measurement | Validity/Reliability | Baseline | 30/90/180 days |
|---|---|---|---|---|
| HF History and Diagnostics | Chart Abstraction Tool (based on risk36 and 37) | - | x | x |
| Comorbidities | Charlson Comorbidity Index38 (via record) | - | x | |
| Hemodynamic Profile | Output, pressure, O2 extraction etc. (via record) | - | x | x |
| VAD Support | Flow, rotations, power, pulse index etc. (via record) | - | x | |
| Other Potentially Influential Factors (Exploratory) | ||||
| Socio-demographics | Socio-demographic Questionnaire | - | x | |
| Perceived Control | Control Attitudes Scale-Revised39 | α .73 | x | x |
| Perceived Social Support | Perceived Social Support Scale40 | α .84 | x | x |
| Mild Cognitive Dysfunction | Montreal Cognitive Assessment41 | 100/83%‡ | x | x |
| Physical Symptoms | ||||
| Physical Symptoms | Heart Failure Somatic Perception Scale42 | α .90¥ | x | x |
| Pain Intensity/Interference | Brief Pain Inventory Short Form43 | α .84–.94 | x | x |
| Daytime Sleepiness | Epworth Sleepiness Scale44 | α .83¥ | x | x |
| Psychological Symptoms | ||||
| Depression and Severity | Patient Health Questionnaire-945 | α .86¥ | x | x |
| Anxiety | Brief Symptom Inventory46 6 Anxiety Items | α .87¥ | x | x |
| Hostility | Brief Symptom Inventory46 5 Hostility Items | α .83¥ | x | x |
| Serum Biomarkers | ||||
| Myocardial Stress | Amino terminal pro-B-type natriuretic peptide | 11.75pg/mL* | x | x |
| Systemic Inflammation | Soluble tumor necrosis factor alpha receptor | 0.77ng/mL* | x | x |
| Endothelial Dysfunction | Soluble endothelial-leukocyte adhesion molecule 1 | 0.009ng/mL* | x | x |
| Outcomes | ||||
| HF Health-Related QOL | Kansas City Cardiomyopathy Questionnaire47 | α .78–.95 | x | x |
| Health-Related QOL | European Quality of Life 5 Dimensions48 | θ | x | x |
| Clinical Events | Clinical Event Adjudication | - | x | |
sensitivity/specificity for mild cognitive dysfunction with cut-off of 24;
quantified using data from this same advanced HF population;51
minimal detection limits; θ mean of 0.78±0.18 in mild to 0.51±0.21 in severe HF.50
Abbreviations: HF, heart failure; QOL, quality-of-life; VAD, ventricular assist device.
Measures Pertinent to Trajectory Identification
In the absence of a single comprehensive symptom measure in HF, multiple symptom measures were chosen to capture common physical and psychological domains experienced in HF, alleviate item overlap among the multiple symptom and HRQOL measures, and because of the established and solid psychometric properties and frequent use in HF research. Furthermore, profiles among these symptom measures were recently reported to predict 1-year event-free survival in moderate to advanced HF.51
Physical symptoms will be measured using the 18-item Heart Failure Somatic Perception Scale (HFSPS).52 Based on the theory of unpleasant symptoms, the HFSPS asks about how much the participant was bothered by 18 common HF symptoms during the last week and provides six response options ranging from 0 (not at all) to 5 (extremely bothersome). Theta reliability of the HFSPS total score that will be used in the analysis was 0.71–0.78 in the original psychometric evaluations;42 Cronbach’s alpha on the HFSPS total score was most recently reported at 0.90 in an advanced HF sample.51
The Brief Pain Inventory (BPI) will be used as a quick assessment of pain location, intensity and interferences.43 The BPI consists of 4 questions about pain intensity and 7 questions about pain interference (in addition to questions on pain location and treatment). Respondents rate their worst, least, average, and current pain intensity and also rate the degree to which pain interferes with 7 domains of functioning on a scale of 0 (no pain or does not interfere) to 10 (as bad as you could imagine or interferes completely). Summary scores of pain interference will be generated and used in the analysis as a primary metric of pain.43
Daytime sleepiness will be measured using the 8-item Epworth Sleepiness Scale (ESS).44 The ESS asks respondents to rate how likely they would be to doze off or fall asleep in 8 situations by choosing response options that range from 0 (would never doze) to 3 (high chance). The ESS correlates significantly with sleep latency measures, and scores distinguish normal sleep patterns, obstructive sleep apnea syndrome, narcolepsy, idiopathic hypersomnia, and insomnia.44
Depressive symptoms will be measured using the 9-Item Patient Health Questionnaire (PHQ9).45 The PHQ9 scores each of the 9 related DSM-IV criteria providing four response options ranging from 0 (not at all) to 3 (nearly every day). The PHQ9 has 88% sensitivity and specificity for major depression (score ≥ 10); scores of 5, 10, 15, and 20 are indicative of, respectively, mild, moderate, moderately severe, and severe depression.45
Anxiety and hostility will be measured using the Brief Symptom Inventory (BSI).46 The BSI asks about feelings during the past seven days and provides five response options ranging from 0 (not at all) to 4 (extremely). Subscale scores (ranging from 0 to 4) are calculated by adding the ratings and dividing the total by the number of items in the subscale, with higher scores indicating higher distress. We will only administer the 11 items that factor in subscale scores for anxiety and hostility.
Based on current recommendations for selecting biomarkers,53, 54 biomarkers of 3 pathogenic processes shown by others to be influenced by VAD implantation will be measured.30–33 All biomarkers will be quantified by a National Center for Advancing Translational Sciences-sponsored core laboratory using commercially available enzyme-linked immunosorbent assay kits. Amino terminal pro-B-type natriuretic peptide (NT-proBNP) will be measured as an index of myocardial stress55 and a confirmatory biomarker of hemodynamic congestion in HF.56 The detection limit of NT-proBNP is < 11.75 pg/ml; intra- and inter-assay coefficient of variation (CV) is estimated at less than 8% and 10% respectively (CUSABIO®, Wuhan, China). Soluble tumor necrosis factor alpha receptor 1 (sTNFR1) will be measured as an index of systemic inflammation triggered by direct antigenic stimulation,57 endothelial disruption,58 and direct hemodynamic stress.59 The detection limit of sTNFR1 is < 0.77 ng/ml; intra- and inter-assay CV is estimated at less than 5% and 9% respectively (R&D Systems, Minneapolis MN). Endothelial-leukocyte adhesion molecule 1 (E-selectin), a member of the selectin family of cell adhesion molecules,60 will be measured as an index of endothelial dysfunction. Circulating soluble forms of cell adhesion molecules reflect enhanced expression and/or shedding,61 due to endothelial perturbations.62 The detection limit of E-selectin is < 0.009 ng/ml; intra- and inter-assay CV is estimated at less than 7% and 9% respectively (R&D Systems, Minneapolis MN).
Clinical Events and Adjudication
Patients will be followed clinically and research coordinators will complete a review of the electronic medical record prior to, and at 1, 3, and 6 months after VAD implantation, looking specifically for all-cause a) emergency room visits, b) unplanned hospitalizations, c) mortality, as well as d) heart transplantation (censored event) or e) being alive on a VAD without an event. Secondary adverse events will be classified in accordance with definitions used in the Interagency Registry of Mechanically Assisted Circulatory Support manual of operations.63 The event adjudication committee will determine final classification of all events.
Statistical Analysis Plan
Standard descriptive statistics of frequency, central tendency, and dispersion will be used to describe all measures in the study under consideration of applicable levels of measurement. Comparisons of characteristics between/among observed trajectories will be made using Student’s t, Mann-Whitney U, Fisher’s exact or Kruskal-Wallis tests, or Pearson χ2 analysis or ANOVA where appropriate. StataMP v11 (College Station, Texas) will be used for all descriptive and comparative statistics.
Overview of Trajectory Identification using Growth Mixture Modeling
Latent growth mixture modeling (GMM) will be used to address all hypotheses. GMM is an approach to modeling that identifies distinct trajectories of change that vary around different means, have unique estimates of variance and homogenous within-trajectory growth. Based on conditional probabilities and not absolute certainty, cases are assigned to the “most likely” trajectory, or pattern of change over time. Changes in factors over time are modeled as random effects, non-linear patterns of change are accommodated quite well, there are several metrics (specified below) to help judge comparative fit between models, and data need not be measured at the same test occasion or at evenly-spaced time intervals in GMM.64 Our approach to model specification in GMM is based on common procedures.65 In each instance, we will use several metrics to support the number of trajectories within the sample. The Lo-Mendell-Rubin adjusted likelihood ratio test,66 parametric bootstrapped likelihood ratio test, Bayesian Information Criterion (BIC),67 convergence (entropy closest to zero), the proportion of sample in each trajectory (≥ 5%), and posterior probabilities (average probability of belonging in “most likely” trajectory close to 1.0) will be used to compare alternative models (e.g. k vs. k-1 trajectories).68,69 Mplus v6.0 (Muthén & Muthén, Los Angeles, CA) will be used to perform all GMM. The default method of mitigating bias due to missing data in Mplus is full-information maximum likelihood estimation (FIMLE), which handles effectively most data that are missing at random. Principled methods of multiple imputation,70 such as the method of incremental chained equations in Stata,71 will be used to complement and sensitivity test the effectiveness of FIMLE in our modeling. In addition, pattern mixture modeling72 will be used to assess and account for dropout patterns (i.e. data not missing at random) given the longitudinal nature of the research.
Analytic Procedures to Address Specific Aims 1 and 2
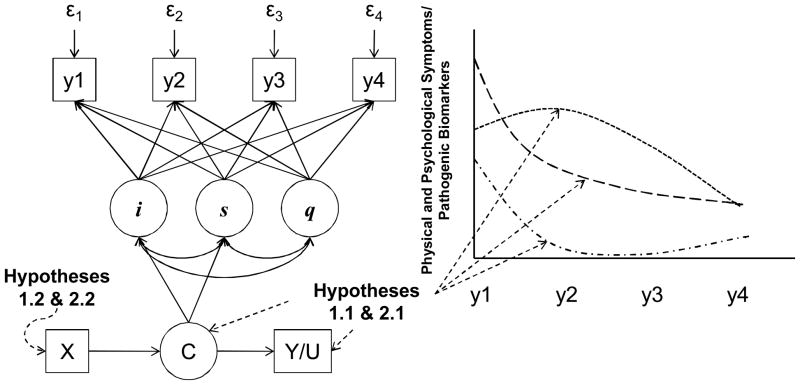
In concert with our biobehavioral research framework, an identify, associate, and predict approach to GMM will be used to address specific aims 1 and 2. Accordingly, the first step will be to develop separate growth mixture models for each symptom measure (Figure 2). Then GMMs that account for changes in all symptom measures over time will be developed. The product of this step is the identification of multiple trajectories of change in physical and psychological symptoms in response to VAD implantation.
Figure 2. Growth Mixture Modeling: Specific Aims 1 and 2.
Following an identify, associate, and predict approach to growth mixture modeling, this example model includes growth curves for a symptoms/biomarkers (y) as observed at 4 time points, with intercepts (i) slopes (s), a non-linear pattern of change (q) a categorical variable indicating “most likely” trajectory (C), outcomes that are associated with trajectory membership such as the continuous outcome of health-related quality of life (Y) or categorical outcome such as hospitalization (U) (relevant to hypotheses 1.1 and 2.1), and predictors of trajectory membership (X) (relevant to hypotheses 1.2 and 2.2). This model only contains change in one symptom/biomarker for economy of presentation; growth mixture modeling is quite flexible and can accommodate change in many factors concomitantly.
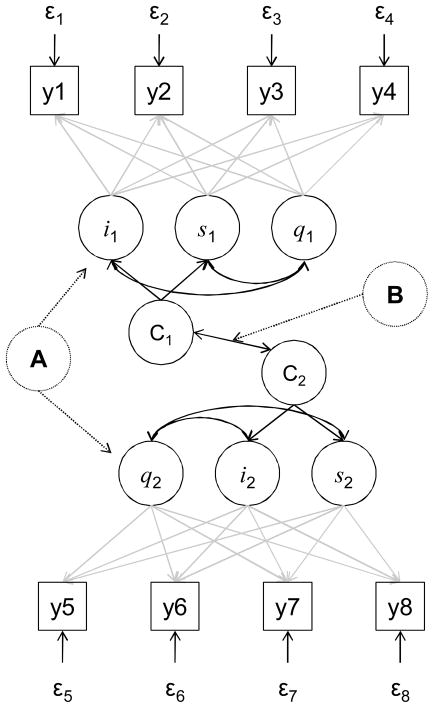
The second step will be to model associations among symptom trajectories and both 6-month event-free survival and HRQOL. Using GMM, both discrete-time73 and continuous time survival74 will be modeled following recent guidance on competing risks75 and analyze event-free survival from all-cause emergency room visit, hospitalization, or death rather than cause-specific or cumulative incidence functions. Associations between symptom trajectories and concomitant changes in HRQOL will be quantified using parallel process modeling96, 97 (Figure 3-A). Specifically, repeated measures congruence between symptoms and HRQOL will be quantified using fit statistics similar to those employed in structural equation modeling. Additionally, we will determine if trajectories of physical and psychological symptoms are similar to trajectories of HRQOL over time (Figure 3-B) using cross-classification extensions of GMM. That is, we will test if trajectories of HRQOL are solely a function of concomitant trajectories in symptoms. The product of this step will be the estimates of association between symptom trajectories and event-free survival and HRQOL.
Figure 3. Parallel Process and Cross Classification Modeling: Specific Aim 3.
This example model includes growth curves for two continuous measures (y) as observed at 4 time points, each with an intercept (i) slope (s), non-linear pattern of change (q) and a categorical variable indicating “most likely” common and distinct trajectory (C). Parallel process modeling (Figure 3-A) entails quantifying repeated measures congruence between changes in the two measures over time using fit statistics to determine how much change in one variable is explained by change in the other (χ2, comparative fit index, Tucker-Lewis index, and root mean square error of approximation, as well as parameter estimates). Cross classification modeling (Figure 3-B) involves comparing distinct trajectories of change in one variable (C1) compared with distinct trajectories of the other (C2). For example, the “most likely” trajectory membership based on change in a symptom and the “most likely” trajectory membership based on change in a serum biomarker are compared using odds ratios or χ2 tests.
The third step will be to identify predictors of symptom responses. Bivariate associations (Student’s t, Mann-Whitney U, Fisher’s exact or Kruskal-Wallis tests, Pearson χ2 analysis, ANOVA, Pearson’s r, Spearman’s rho, mixed-effects, or repeated measures ANOVA where appropriate) between baseline socio-demographics, clinical characteristics, comorbidities, and other influential factors (based on the literature and exploratory analyses) and symptom measures over time will be quantified to identify candidate predictors. Restricted fence methods will then be employed for final longitudinal model covariate selection. In brief, restricted fence methods combine the ideas of a statistical barrier designed to minimize model misspecification and of restricted maximum likelihood that is designed to negate the influence of nuisance parameters.77, 78 Restricted fence methods were developed specifically to overcome limitations of the traditional methods of model covariate selection that are not appropriate in complex modeling such as GMM.78 The product of this step is a robust and parsimonious list of predictors of symptom responses to VAD implantation.
The above three steps will be followed for the analysis of pathogenic biomarker trajectories. That is, the first step will be to develop separate models for each biomarker (i.e. NT-proBNP, sTNFR1, and E-selectin) and then multiple biomarkers. The second step will be to model 6-month event-free survival and HRQOL as a function of biomarker trajectories. Finally, the third step will be to identify predictors of favorable biomarker responses to VAD implantation.
Analytic Procedures to Address Specific Aim 3
In the final symptom biochemistry aim, congruence between symptoms and biomarkers will be quantified using parallel process and cross-classification modeling (Figure 3). Specifically, parallel process models for each symptom/serum biomarker combination will be developed to identify additive (limited association) vs. redundant (strong associations) information to incorporate into further modeling. Additionally, we will test if trajectories of symptoms are solely a function of concomitant trajectories in biomarkers. A final trajectory model that contains both symptoms and biomarkers will be developed and quantified in association with event-free survival and HRQOL. Finally, models from aims 1 and 2 will be compared with this final model that contain both symptoms and biomarkers regarding associations with event-free survival and HRQOL using comparative fit statistics (χ2 tests, Harrell’s C, BIC etc.).
Sample Size Justification
No formal approach has been taken for sample size considerations in GMM. With 6 primary indices of physical and psychological symptoms and 3 pathogenic markers, however, the n-to-items ratio exceeds sample size recommendations for related factor analysis.76 Regarding group comparisons, assuming 80% power, two-sided α of 0.05, mean QOL scores of 76±21 (preliminary data on 273 advanced HF patients from our institution), we will detect differences in scores as small as 8.5 (range 0–100) between two equal groups of 50 using t or Mann-Whitney tests; assuming 80% power, one-sided α of 0.05, and a mean event rate of 13%,6 we will detect differences in clinical events of 23% using Fisher’s Exact test. Cox proportional modeling, and by extension survival modeling in GMM, is resilient to small sample sizes when there are strong, independent relationships. 120 participants is a feasible number and will allow for each aim to be addressed.
Anticipated Results
Among adults with advanced HF, mixture modeling has been useful in identifying previously unobserved subgroups with respect to physical and psychological symptom burden51 and self-care behaviors,77 as well as medication adherence78 and cognitive function79 over time. Anticipated symptom science results of PREMISE include the identification of multiple trajectories of change in physical and psychological symptoms in response to VAD implantation that are associated with clinical and patient-oriented outcomes and can be predicted based on demographics, clinical characteristics, and other influential factors. It may also be that there are considerable trade-offs in symptoms. For example, there may be reductions in shortness of breath with short-term increase in post-surgical pain intensity. Minimally, it is expected that a favorable symptom and an unfavorable symptom trajectory can be identified. Anticipated HF pathophysiological results of PREMISE include the identification of unique trajectories of change in pathogenic biomarkers in response to VAD implantation that are associated with clinical and patient-oriented outcomes and can be predicted based on multiple pre-implant factors. Similar to prior research,33 we may find that endothelial dysfuntion is increased after VAD implantation and to a greater degree in a subgroup of participants. We expect to identify at least two biomarker trajectories that reflect a better and a worse pathogenic response to VAD implantation, and that distiguish gradients of clinical event-risk and HRQOL. Anticipated symptom biochemistry results from PREMISE include a state-of-the art understanding of the temporal relationships between HF pathogenesis and physical and psychological symptoms in response to VAD implantation. Finally, we anticipate that having information on both symptoms and biomarkers will be much more informative than having either measure in explaining event-risk and HRQOL.
Conclusion
Unexplained heterogeneity in response to VAD implantation impedes our ability to predict favorable outcomes, provide adequate patient and family education and anticipatory guidance, and personalize monitoring and symptom management strategies. Patient-oriented and clinical outcomes of current and future advanced HF populations will be improved with a better understanding of the complex interplay between physical and psychological symptoms and multiple underlying pathogenic processes. Research findings from PREMISE will be used to enhance patient and provider shared decision-making regarding VAD implantation, and shape a much-needed new breed of interventions and clinical management strategies that are tailored to differential symptom and pathogenic responses to VAD implantation.
What is New?
Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure (PREMISE) is a prospective cohort study designed to a) identify common and distinct trajectories of change in physical and psychological symptom burden, b) characterize common trajectories of change in serum biomarkers of myocardial stress, systemic inflammation, and endothelial dysfunction, and c) quantify associations between symptoms and biomarkers of pathogenesis, and trajectories thereof, in adults undergoing VAD implantation.
Research findings from PREMISE will be used to enhance shared patient and provider decision-making, and shape a much-needed new breed of interventions and clinical management strategies that are tailored to differential symptom and pathogenic responses to VAD implantation.
Acknowledgments
Funding Acknowledgment: This work was supported by the National Institutes of Health/National Institute of Nursing Research (1R01NR013492). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Contributor Information
Christopher S. Lee, Oregon Health & Science University Schools of Nursing and Medicine, Portland, OR.
James O. Mudd, Oregon Health & Science University School of Medicine, Portland, OR.
Jill M. Gelow, Oregon Health & Science University School of Medicine, Portland, OR.
Thuan Nguyen, Oregon Health & Science University School of Medicine, Portland, OR.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
Jennifer K. Green, Oregon Health & Science University School of Nursing, Portland, OR.
Quin E. Denfeld, Student Oregon Health & Science University School of Nursing, Portland, OR.
Julie T. Bidwell, Student Oregon Health & Science University School of Nursing, Portland, OR.
Kathleen L. Grady, Feinberg School of Medicine, Northwestern University, Chicago, IL.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. The New England Journal of Medicine. 2009;360:1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Zannad F. Clinical definition and epidemiology of advanced heart failure. Am Heart J. 1998;135:S204–215. doi: 10.1016/s0002-8703(98)70251-0. [DOI] [PubMed] [Google Scholar]
- 4.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the heartmate ii left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–1234. doi: 10.1016/j.athoracsur.2008.06.030. discussion 1234–1225. [DOI] [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Miller K, Myers TJ, Robertson K, Shah N, Delgado RM, 3rd, Gregoric ID. Quality of life in bridge-to-transplant patients with chronic heart failure after implantation of an axial flow ventricular assist device. Congest Heart Fail. 2004;10:226–229. doi: 10.1111/j.1527-5299.2004.03258.x. [DOI] [PubMed] [Google Scholar]
- 10.Grady KL, Meyer PM, Mattea A, Dressler D, Ormaza S, White-Williams C, Chillcott S, Kaan A, Loo A, Todd B, Klemme A, Piccione W, Costanzo MR. Change in quality of life from before to after discharge following left ventricular assist device implantation. J Heart Lung Transplant. 2003;22:322–333. doi: 10.1016/s1053-2498(02)00668-x. [DOI] [PubMed] [Google Scholar]
- 11.Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Bohm M, Anker S, Dargie H, Brutsaert D, Komajda M. Advanced chronic heart failure: A position statement from the study group on advanced heart failure of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Russell SD, Miller LW, Pagani FD. Advanced heart failure: A call to action. Congest Heart Fail. 2008;14:316–321. doi: 10.1111/j.1751-7133.2008.00022.x. [DOI] [PubMed] [Google Scholar]
- 13.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 15.Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096, e1091–1098. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, Schramm EE, O’Connor CM. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Pina I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Strueber M, O’Driscoll G, Jansz P, Khaghani A, Levy WC, Wieselthaler GM. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Fang JC. Rise of the machines--left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 20.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: Twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 22.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The fourth intermacs annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Dew MA, Kormos RL, Winowich S, Harris RC, Stanford EA, Carozza L, Griffith BP. Quality of life outcomes after heart transplantation in individuals bridged to transplant with ventricular assist devices. J Heart Lung Transplant. 2001;20:1199–1212. doi: 10.1016/s1053-2498(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 24.Wray J, Hallas CN, Banner NR. Quality of life and psychological well-being during and after left ventricular assist device support. Clin Transplant. 2007;21:622–627. doi: 10.1111/j.1399-0012.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz AJ, Weinberg AD, Oz MC, Williams DL. Quality of life with an implanted left ventricular assist device. Ann Thorac Surg. 1997;64:1764–1769. doi: 10.1016/s0003-4975(97)01000-x. [DOI] [PubMed] [Google Scholar]
- 26.Grady KL, Meyer PM, Dressler D, Mattea A, Chillcott S, Loo A, White-Williams C, Todd B, Ormaza S, Kaan A, Costanzo MR, Piccione W. Longitudinal change in quality of life and impact on survival after left ventricular assist device implantation. Ann Thorac Surg. 2004;77:1321–1327. doi: 10.1016/j.athoracsur.2003.09.089. [DOI] [PubMed] [Google Scholar]
- 27.Grady KL, Meyer P, Mattea A, Dressler D, Ormaza S, White-Williams C, Chillcott S, Kaan A, Loo A, Todd B, Klemme A, Piccione W, Costanzo M. Change in physical and psychosocial domains of quality of life from before to after discharge post left ventricular assist device implantation. J Heart Lung Transplant. 2001;20:203. doi: 10.1016/s1053-2498(00)00434-4. [DOI] [PubMed] [Google Scholar]
- 28.Allen JG, Weiss ES, Schaffer JM, Patel ND, Ullrich SL, Russell SD, Shah AS, Conte JV. Quality of life and functional status in patients surviving 12 months after left ventricular assist device implantation. J Heart Lung Transplant. 2010;29:278–285. doi: 10.1016/j.healun.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 30.Sodian R, Loebe M, Schmitt C, Potapov EV, Siniawski H, Muller J, Hausmann H, Zurbruegg HR, Weng Y, Hetzer R. Decreased plasma concentration of brain natriuretic peptide as a potential indicator of cardiac recovery in patients supported by mechanical circulatory assist systems. J Am Coll Cardiol. 2001;38:1942–1949. doi: 10.1016/s0735-1097(01)01677-1. [DOI] [PubMed] [Google Scholar]
- 31.Thompson LO, Skrabal CA, Loebe M, Lafuente JA, Roberts RR, Akgul A, Jones V, Bruckner BA, Thohan V, Noon GP, Youker KA. Plasma neurohormone levels correlate with left ventricular functional and morphological improvement in lvad patients. J Surg Res. 2005;123:25–32. doi: 10.1016/j.jss.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Sareyyupoglu B, Boilson BA, Durham LA, McGregor CG, Daly RC, Redfield MM, Edwards BS, Frantz RP, Pereira NL, Park SJ. B-type natriuretic peptide levels and continuous-flow left ventricular assist devices. ASAIO J. 2010;56:527–531. doi: 10.1097/MAT.0b013e3181f127a7. [DOI] [PubMed] [Google Scholar]
- 33.John R, Panch S, Hrabe J, Wei P, Solovey A, Joyce L, Hebbel R. Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg. 2009;88:1171–1179. doi: 10.1016/j.athoracsur.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 34.Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA. Collaborative development of middle-range nursing theories: Toward a theory of unpleasant symptoms. ANS Adv Nurs Sci. 1995;17:1–13. doi: 10.1097/00012272-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS Adv Nurs Sci. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 37.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Goff DC, Grover FL, Malenka DJ, Peterson ED, Redberg RF. Acc/aha key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: A report of the american college of cardiology/american heart association task force on clinical data standards (writing committee to develop heart failure clinical data standards): Developed in collaboration with the american college of chest physicians and the international society for heart and lung transplantation: Endorsed by the heart failure society of america. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Moser DK, Riegel B, McKinley S, Doering LV, Meischke H, Heo S, Lennie TA, Dracup K. The control attitudes scale-revised: Psychometric evaluation in three groups of patients with cardiac illness. Nurs Res. 2009;58:42–51. doi: 10.1097/NNR.0b013e3181900ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimet G, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. Journal of Personality Assessment. 1990;55:610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 41.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 42.Jurgens CY, Fain JA, Riegel B. Psychometric testing of the heart failure somatic awareness scale. J Cardiovasc Nurs. 2006;21:95–102. doi: 10.1097/00005082-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 44.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derogatis LR, Melisaratos N. The brief symptom inventory: An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 47.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 48.Shaw JW, Johnson JA, Coons SJ. Us valuation of the eq-5d health states: Development and testing of the d1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 49.McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the montreal cognitive assessment (moca) as a screening test for mild cognitive impairment (mci) in a cardiovascular population. J Geriatr Psychiatry Neurol. 2011;24:33–38. doi: 10.1177/0891988710390813. [DOI] [PubMed] [Google Scholar]
- 50.Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the eq-5d in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee CS, Gelow JM, Denfeld QE, Mudd JO, Burgess D, Green JK, Hiatt SO, Jurgens CY. Physical and psychological symptom profiling and event-free survival in adults with moderate to advanced heart failure. J Cardiovasc Nurs. 2013 doi: 10.1097/JCN.0b013e318285968a. epub-ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29:74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 53.Allen LA, Michael Felker G. Multi-marker strategies in heart failure: Clinical and statistical approaches. Heart Fail Rev. 2009 doi: 10.1007/s10741-009-9144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apple FS, Wu AH, Jaffe AS, Panteghini M, Christenson RH. National academy of clinical biochemistry and ifcc committee for standardization of markers of cardiac damage laboratory medicine practice guidelines: Analytical issues for biomarkers of heart failure. Clin Biochem. 2008;41:222–226. doi: 10.1016/j.clinbiochem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 56.Apple FS, Wu AH, Jaffe AS, Panteghini M, Christenson RH, Cannon CP, Francis G, Jesse RL, Morrow DA, Newby LK, Storrow AB, Tang WH, Pagani F, Tate J, Ordonez-Llanos J, Mair J. National academy of clinical biochemistry and ifcc committee for standardization of markers of cardiac damage laboratory medicine practice guidelines: Analytical issues for biomarkers of heart failure. Circulation. 2007;116:e95–98. doi: 10.1161/CIRCULATIONAHA.107.185266. [DOI] [PubMed] [Google Scholar]
- 57.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–8C. doi: 10.1016/j.amjcard.2005.03.006. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 58.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: Potential therapeutic implications. Ann Med. 2005;37:74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- 59.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 60.Bauersachs J, Schafer A. Endothelial dysfunction in heart failure: Mechanisms and therapeutic approaches. Curr Vasc Pharmacol. 2004;2:115–124. doi: 10.2174/1570161043476447. [DOI] [PubMed] [Google Scholar]
- 61.Yin WH, Chen JW, Jen HL, Chiang MC, Huang WP, Feng AN, Lin SJ, Young MS. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003;5:507–516. doi: 10.1016/s1388-9842(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 62.Chong AY, Lip GY, Freestone B, Blann AD. Increased circulating endothelial cells in acute heart failure: Comparison with von willebrand factor and soluble e-selectin. Eur J Heart Fail. 2006;8:167–172. doi: 10.1016/j.ejheart.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Interagency registry of mechanically assisted circulatory support manual of operations. Adverse Event Definitions. 2008 [Google Scholar]
- 64.Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent growth curve modeling. Thousand Oaks, California: Sage; 2008. [Google Scholar]
- 65.Ram N, Grimm KJ. Methods and measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33:565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 67.Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 68.Jung T, Wickrama KA. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Compass. 2008;2:302–317. [Google Scholar]
- 69.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 70.Kenward M, Carpenter J. Multiple imputation: Current perspectives. Statistical Methods in Medical Research. 2007;16:199–218. doi: 10.1177/0962280206075304. [DOI] [PubMed] [Google Scholar]
- 71.Royston P. Multiple imputation of missing values: Update of ice. The Stata Journal. 2005;5:527–187. [Google Scholar]
- 72.Wilkins KJ, Fitzmaurice GM. A marginalized pattern-mixture model for longitudinal binary data when nonresponse depends on unobserved responses. Biostatistics. 2007;8:297–305. doi: 10.1093/biostatistics/kxl010. [DOI] [PubMed] [Google Scholar]
- 73.Muthen B, Masyn K. Discrete-time survival mixture analysis. Journal of Educational and Behavioral Statistics. 2005;30:27–58. [Google Scholar]
- 74.Asparouhov T, Masyn K, Muthen B. Continuous time survival in latent variable models. ASA Biometrics Section. 2006:180–187. [Google Scholar]
- 75.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: A review of statistical methods and clinical applications. Med Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 76.MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychological Methods. 1999;4:84–99. [Google Scholar]
- 77.Riegel B, Lee CS, Albert N, Lennie T, Chung M, Song EK, Bentley B, Heo S, Worrall-Carter L, Moser DK. From novice to expert: Confidence and activity status determine heart failure self-care performance. Nurs Res. 2011;60:132–138. doi: 10.1097/NNR.0b013e31820978ec. [DOI] [PubMed] [Google Scholar]
- 78.Riegel B, Lee CS, Ratcliffe SJ, De Geest S, Potashnik S, Patey M, Sayers SL, Goldberg LR, Weintraub WS. Predictors of objectively measured medication nonadherence in adults with heart failure. Circ Heart Fail. 2012;5:430–436. doi: 10.1161/CIRCHEARTFAILURE.111.965152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riegel B, Lee CS, Glaser D, Moelter ST. Patterns of change in cognitive function over six months in adults with chronic heart failure. Cardiol Res Pract. 2012;2012:631075. doi: 10.1155/2012/631075. [DOI] [PMC free article] [PubMed] [Google Scholar]