Abstract
Focal adhesions (FAs) are large, integrin-containing, multi-protein assemblies spanning the plasma membrane that link the cellular cytoskeleton to surrounding extracellular matrix. They play critical roles in adhesion and cell signaling and are major regulators of epithelial homeostasis, tissue response to injury, and tumorigenesis. Most integrin subunits and their associated FA proteins are expressed in skin, and murine genetic models have provided insight into the functional roles of FAs in normal and neoplastic epidermis. Here, we discuss the roles of these proteins in normal epidermal proliferation, adhesion, wound healing, and cancer. While many downstream signaling mechanisms remain unclear, the critically important roles of FAs are highlighted by the development of therapeutics targeting FAs for human cancer.
Keywords: extracellular matrix, focal adhesion, integrin, skin, squamous cell carcinoma
Introduction
The discovery of focal adhesions in the 1970s as highly conserved signal integrators that physically link the extracellular matrix (ECM) and the cytoskeleton suggested that these large protein complexes may be functionally necessary for tissue structure and the multicellularity of organisms.1 Integrins, which function as αβ heterodimers, are catalytically inactive receptors within focal adhesions that directly bind ECM ligands to initiate downstream signaling responses. There is a large number of integrin subunits (18 α subunits and 8 β subunits) and αβ heterodimers (24 total), all of which have some redundancy in ligand binding. Despite this, many individual integrin subunits are necessary for organismal viability.2-6 Relevant mouse models have revealed that certain integrin subunits or focal adhesion proteins are necessary for embryonic development, while others are required only for development and homeostasis of certain tissue types. This is very apparent in skin, where loss of specific FA proteins can lead to defects in adhesion, wound healing and proliferation.
In pathological conditions such as squamous cell carcinoma, microenvironmental changes cause disorganization of the epidermis, degradation of the basement membrane, overexpression of specific integrin subunits and altered secretion and cleavage of ECM components. These microenvironmental changes lead to altered focal adhesion formation and downstream signaling, which has been shown to enhance the ability of tumor cells to proliferate, invade and metastasize. Functional studies defining the roles of specific focal adhesion complex proteins in both normal and tumor tissues has led to a better understanding of how individual members of these complexes can be targeted therapeutically. In this review, we summarize the current understanding of the functional roles for specific integrin subunits and FA complex proteins in development, skin homeostasis, and relevant in vivo squamous cell carcinoma models. This review will also highlight some of the key FA proteins that have become the recent focus of targeted therapeutics.
Squamous Cell Carcinoma: Current Therapy
Cutaneous SCC is a generally under-appreciated public health concern, as non-melanoma skin malignancies cases are typically excluded from national cancer registries. The incidence of cSCC in the US is now estimated to be over 700 000 new cases/year, with a 4–12.5% risk of metastasis.7-9 Although this metastasis risk is lower than many other malignancies, the large burden of disease is such that in most regions of the US, total deaths due to cSCC may be as common as those from melanoma.10 While surgical excision is very effective treatment for local tumors, therapeutic options for disseminated disease are limited with few proven effective treatments. Clinical treatment regimens based on traditional chemotherapeutic agents, including cisplatin, bleomycin, doxorubicin, and fluoropyrimidines, radiation therapy, or newer targeted biologics including EGFR or general tyrosine kinases inhibitors do not result in long-term remissions in most cases.11-13 New therapeutic agents are needed to improve treatment outcomes for unresectable cSCC.
cSCC is primarily the consequence of chronic UV photodamage resulting in loss of p53 function, followed by activation of EGFR and its downstream pathways including Ras MAPK, PI3K/Akt, PLCγ/PKC, and Src kinases. Oncogenic signaling through these pathways in skin, as well as other epithelial tissues, is frequently associated with upregulation of integrin proteins. As activation of effector cascades initiating at integrin-containing focal adhesions appears to be necessary for the full malignant potential of some epithelial tumors, targeting integrins at the relatively accessible plasma membrane is an attractive option that may have clinical utility.14
Focal Adhesion Complex and Hemidesmosome Structure in Skin
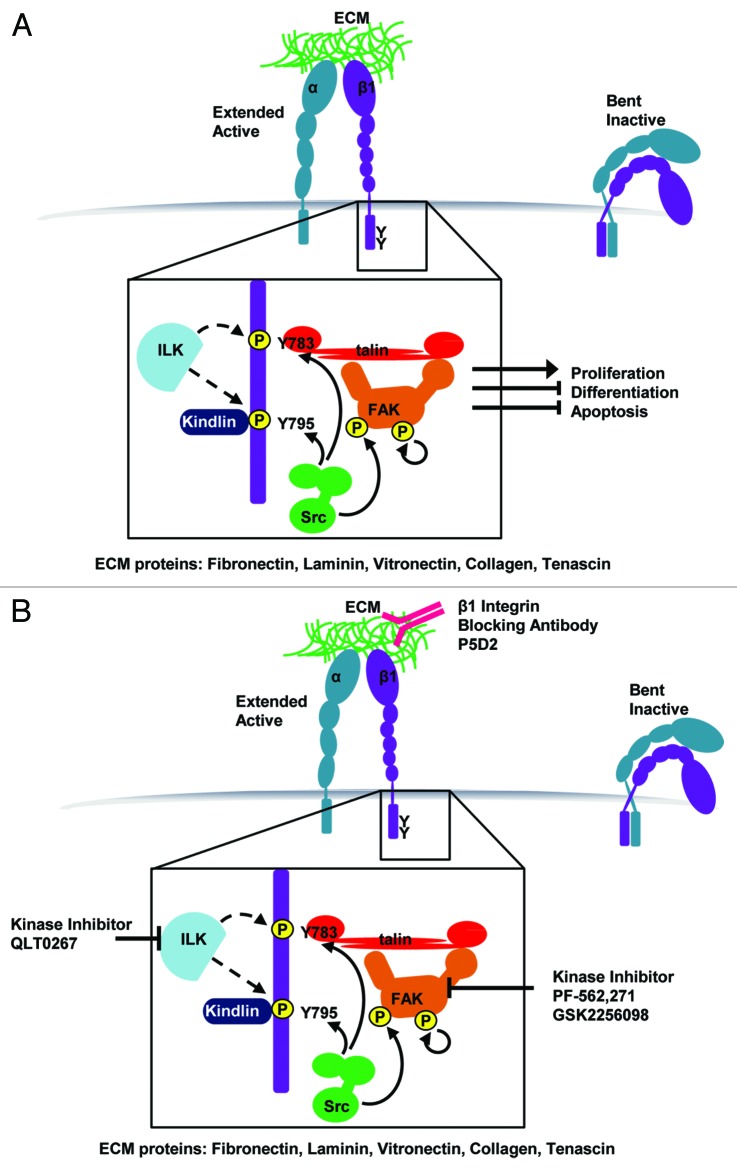
Integrins are delivered to the cellular membrane as inactive, bent heterodimers. These heterodimers are initially partially activated through binding of cytoplasmic proteins (primarily Talin and Kindlin) to the intracellular integrin tails (“inside-out” signaling). Subsequent binding to extracellular matrix ligands (“outside-in” signaling) further extends the heterodimer and generates the fully active receptor. These integrin receptors lack intrinsic catalytic activity and execute their signaling and structural roles through recruitment of other proteins to adhesion complexes at the plasma membrane (Fig. 1).
Figure 1. Depiction of focal adhesion structure, key phosphorylation events and therapeutics targeting individual focal adhesion proteins for treatment of cSCC. (A) In normal basal keratinocytes, integrin binding to the ECM initiates Talin binding to the membrane proximal NPxY motif (Y783) and Kindlin binding to the membrane distal NPxY motif (Y795). FAK is recruited to the adhesion and undergoes auto-phosphorylation at Y397. Src kinase phosphorylates both NPxY tyrosines on the β1 integrin tail and phosphorylates active FAK at Y925. It remains controversial whether ILK phosphorylates β1 integrin at these same sites. This adhesion assembly and phosphorylation sequence ultimately promote cell cycle progression and inhibit differentiation and apoptosis programs. (B) Three current strategies in development for treatment of cSCC are blocking β1 integrin with a P5D2 blocking antibody, inhibiting ILK kinase activity using QLT0267, and inhibiting FAK kinase activity using PF-562,271 or GSK2256098.
Integrin heterodimers are key components of 2 distinct types of adhesion complexes: FA complexes, which link the actin cytoskeleton to the ECM, and hemidesmosomes, which structurally link intermediate filaments to the ECM. Through these complexes, integrin receptors play both a structural role mediating physical attachment of epithelial cells to underlying basement membrane, and also a signaling role promoting cellular proliferation and migration. This signaling response is evident in skin tissue, where basal epidermal cells assemble on the ECM-rich basement membrane, and are given cues to proliferate. As these proliferative progenitors lose their self-renewal capacity, they detach from the basement membrane, stratify into a 5–10 cell thick epidermis and terminally differentiate, forming the epidermal barrier of human skin.15
Hemidesmosomes play a major structural role in the epidermis but have also been shown to activate several intracellular signaling pathways, including Rac1, RhoA, and Akt signaling.16 Unlike focal adhesions, hemidesmosomes are not frequently recycled and therefore serve to maintain keratinocyte anchorage to the basement membrane. Loss of the only hemidesmosomal integrin heterodimer, α6β4, leads to severe epidermal adhesion defects.17,18 Integrin α6β4 and its ligand, laminin-332, are also both required for squamous cell carcinoma formation in relevant epidermal in vivo models.19 This has been nicely reviewed by MP Marinkovich and will not be a major focus of this review.16 Because of the frequently severe skin blistering seen in patients lacking a number of different extracellular and intracellular hemidesmosomal proteins, targeting hemidesmosomal components was initially not considered to be a viable therapeutic strategy. However, it was later discovered that a specific domain of laminin-332, G45, is present only in tumor tissue and promotes tumor formation and progression.20 Blocking antibodies against G45 were shown in a pre-clinical model to be effective against SCC tumor formation through blockade of PI3K and ERK signaling, but to have no effect on normal skin homeostasis.20 This is one of the few examples of targeting specific ECM ligands for cancer therapy, but highlights the need for a deeper understanding of how the role of adhesion signaling and specific ECM ligands differ between various homeostatic and pathologic states.
In contrast to hemidesmosomes, FA complexes are dynamic adhesions that frequently assemble and disassemble, particularly during cell migration. In tissue culture, stimulation of FA complex formation using a variety of ECM ligands leads to engagement of many different signaling pathways, including PI3K/Akt activation, Rac1-mediated cytoskeleton re-organization, and NFκB pathway activation. However, the degree to which these pathways are activated upon integrin ligation and their relative functional importance in either 3-dimensional culture or in vivo tissue remains unclear. The remainder of this review will focus on the roles of specific FA complex proteins in both normal skin and epidermal squamous cell carcinoma.
Focal Adhesion Integrins
Much effort has been focused on defining roles for individual integrin subunits in epidermal homeostasis. As integrin signaling requires an intact, structurally correct basement membrane zone lacking in traditional tissue culture, much of this work has employed mouse genetic models. Efforts have been made to generate knockout mice or conditional knockout mice for each of the 26 integrin subunits. Out of these 26 subunits, 18 are expressed at a detectable level in human or mouse skin, while the others are leukocyte-specific. Knockout mice have been generated for all of these 18 subunits, but many of these mice experience embryonic lethality, and the skin-specific null phenotypes have not been determined (Table 1). Regardless, the available knockout mice have provided valuable insight into the roles of integrins in epidermal proliferation, hair follicle formation and turnover, wound healing, and susceptibility to squamous cell carcinoma. Many of these phenotypes have been nicely reviewed by Janes et al. and Margadant et al.21,22 In the next section, we provide a brief overview of these phenotypes, and discuss newly discovered roles for epidermal integrins not summarized elsewhere.
Table 1. Functional roles for focal adhesion-associated integrins in both mouse development and mouse skin.
| Gene | Knockout mouse phenotype | Conditional skin knockout phenotype (K5 or K14 promoter) | Role in SCC |
|---|---|---|---|
|
Integrin α1 |
-viable, fertile and no apparent abnormalities
130 |
N/A
|
N/A
|
|
Integrin α2 |
-Healthy, viable, fertile41 -No change in re-epithelialization or BM deposition, but increase in neoangiogenesis during wound healing42,44 |
N/A |
-K14-HPV mice crossed with α2-null mice shows decreased lymph node metastases and tumor formation45 |
|
Integrin α3 |
-Survive until birth, but die shortly after due to kidney and lung defects33 -Minor blistering of the epidermis, but normal stratification33 |
-Disorganized BM34,131 -Blistering at epidermal-dermal junction34,131 -Spatial and temporal differentiation is intact34,131 -Hair loss and impaired hair follicle growth35 -Enhanced re-epithelialization during wound healing36 -Enhanced epidermal turnover38 |
-Significantly reduced papilloma formation upon DMBA-TPA treatment of mice lacking α3 in the epidermis38 -SCCs that form are more poorly differentiated38 |
|
Integrin α4 (VCAM-1) |
-Required for formation of umbilical cord and placenta during development3 -Mostly embryonic lethal E8.5, but a small number of viable, fertile mice3 |
N/A |
N/A |
|
Integrin α5 |
-Mesodermal defects and embryonic death at E10–11132 |
N/A |
N/A |
|
Integrin α6 |
-Embryonic lethal at E14.5-E18.518 -Skin blistering similar to epidermolysis bullosa18 -Normal differentiation and stratification of the epidermis18,131 |
-mild hyperproliferation, blistering and inflammation upon tamoxifen-induced deletion in epidermis133 |
See integrin β4 |
|
Integrin α7 |
-Viable, fertile mice134 -Muscular dystrophy135 -Defective axonal elongation135 |
N/A |
N/A |
|
Integrin α8 |
-Death immediately after birth likely due to renal deficiencies136 |
N/A |
N/A |
|
Integrin α9 |
-Normal at birth, but die at day 6–12 due to respiratory failure137 -Edema and lymphocyte infiltration into chest wall137 |
-Poor re-epithelization during wound healing39 |
N/A |
|
Integrin α10 |
-viable, fertile138 -stunted growth of long bones138 |
N/A |
N/A |
|
Integrin α11 |
-Viable, fertile139 -Dwarfism and defective tooth movement139 |
N/A |
N/A |
|
Integrin αv |
-Mostly embryonic lethal at E9.5, but 20% of mice born alive5 -Defects in placental function5 -Intracerebral and intestinal hemorrhage5 -Cleft palates5 |
N/A |
N/A |
|
Integrin β1 |
-Embryonic lethal2,4 -Die immediately after attaching to uterine epithelia and invading the stroma, around E52,4 |
-Severe hair loss23,24 -Reduced numbers of hemidesmosomes23,24 -Disruption in BM and blistering23,24 -K14-Cre model shows normal spatial and temporal differentiation, but K5-Cre model shows enhanced differentiation23,24 -K14-Cre model shows epidermal thinning, but K5-Cre model shows epidermal thickening23,24 -Poor re-epithelialization during wound healing140 -K14-CreER 4-OHT excision in adult epidermis has no apparent phenotype25 |
-Activating mutation T1881β1 stimulated conversion of papillomas to SCCs upon DMBA-TPA141 -Blocking antibodies against integrin β1 block tumor formation and progression in a human tissue graft model of SCC14 |
|
Integrin β3 |
-Enhanced re-epithelialization during wound healing142 |
N/A |
N/A |
|
Integrin β4 |
-Die shortly after birth due to respiratory and intestinal failure and skin fragility17 -Skin blistering defects similar to epidermolysis bullosa17 -Normal stratification of the epidermis17 |
-Loss of hemidesmosomes, skin blistering, but normal differentiation and proliferation131,143 |
-β4 knockout or blocking antibodies prevented Ras-driven tumorigenesis in human tissue graft model of SCC19 |
|
Integrin β5 |
-Viable, fertile and no apparent abnormalities43 |
N/A |
N/A |
|
Integrin β6 |
-Hair loss144 -Inflammation of skin and lungs144 |
-Retarded hair follicle regression after depilation40 -Enhanced keratinocyte proliferation40 |
N/A |
| Integrin β8 | -65% die at midgestation due to insufficient vasculogenesis6 -35% die shortly after birth due to intracerebral hemorrhage6 -Leaky brain capillaries and endothelial hyperplasia6 |
N/A | N/A |
β1 integrin and its phosphorylation
Severe phenotypes are seen in β1-null mouse skin. Loss of β1 during development leads to severe epidermal defects, including skin blistering and hair loss.23,24 There are differences in these phenotypes depending on the promoter used for Cre-mediated recombination. K5-Cre-induced β1 deletion leads to differentiation defects, skin thickening, and mouse death at approximately 6 weeks after birth, potentially due to hypoproliferation in the esophagus.23 K14-Cre-induced β1 deletion results in normal differentiation, but significant epidermal hypoproliferation and perinatal death within days after birth.24 Despite these severe developmental defects, β1 loss in adult mouse skin has no apparent deleterious phenotype.14,25 Using gene expression profiling and network topology analysis, integrin β1 was identified as a key oncogenic hub in a human skin graft model of squamous cell carcinoma.14 Subsequently, antibody-mediated blockade of integrin β1 both prevented tumor formation and slowed tumor progression, with no deleterious effects on normal human skin tissue or overall mouse health (Fig. 1B).14
Binding of Talin to β1 cytoplasmic tails disrupts a salt bridge between the α and β cytoplasmic tails, helping to separate the tails and enhance integrin binding affinity to ECM ligands. This is thought to be the first step in “inside-out” integrin activation. With subsequent integrin activation and clustering, Src phosphorylation of β1 tyrosines in the cytoplasmic NPxY motifs is thought to reduce binding of the adaptor proteins Talin and Kindlin.26 This is consistent with the rounded morphology and loss of adhesion seen in v-Src-transformed cells, which have high levels of β1 tyrosine phosphorylation.27 In addition, focal adhesion kinase (FAK), which plays a role in promoting oncogenic transformation, is activated in response to β1 integrin phosphorylation (Fig. 1).28
Complicating this understanding, determined largely from cell culture systems, are in vivo studies suggesting that the tyrosine residue itself, and not its phosphorylation, is most important for β1 function.29 Mutation of tyrosine to alanine in either the membrane proximal NPxY motif (Y783, Talin binding motif) or the membrane distal NPxY motif (Y795, kindlin binding motif) results in embryonic lethality.30 However, mutation of either of these residues to phenylalanines, which contains the aromatic ring but is unable to be phosphorylated, results in viable, fertile mice with no apparent abnormalities.29,30
Mice with keratinocyte-restricted expression of both Y783A and Y795A (YY/AA) using the K5-Cre promoter phenocopy mice with keratinocyte-restricted deletion of β1.30 These mice experience impaired hair follicle morphogenesis, abnormal skin pigmentation, skin blistering, and thickened epidermis.30 In vitro, keratinocytes with the YY/AA mutation have no β1 integrin activation, and decreased expression of other integrin subunits, including β4, α6, and α2.30 Mice with keratinocyte-restricted expression of either Y783A or Y795A have much less severe epidermal defects.31 These individual mutations lead to patchy hair loss but normal proliferation, epidermal adhesion, and hemidesmosome localization.31 In vitro, keratinocytes containing the Y783A mutation experience adhesion and spreading defects and rapid terminal differentiation, implying that binding of Talin to β1 inhibits keratinocyte differentiation.31 Mice containing both Y783F and Y795F mutations (YY/FF mice) develop normally and have normal skin.32 However, these mice are less susceptible to DMBA-TPA induced skin tumorigenesis.32 Mutation of each residue alone does not have any effect on susceptibility to tumor formation.32 Although Talin1, Talin2, Kindlin1, and Kindlin2 preferentially bind to wild-type β1 over YY/FF β1 in vitro, binding to the mutants is only reduced by approximately 50%.32 This indicates that the YY/FF mouse may have hypomorphic β1 activity, which is sufficient to block tumor formation but not to affect normal skin homeostasis.
Other focal adhesion integrins
Integrin α3-null mice exhibit skin blistering and basement membrane disorganization as well as kidney and lung defects that lead to lethality shortly after birth.33 Mice with epidermis-specific ablation of integrin α3 exhibit the same skin blistering defect and additional hair follicle and wound healing abnormalities.34-36 Previously, human skin disease had not been associated with mutations in integrin α3 or any other FA integrin. Recently, however, homozygous mutations in the integrin α3 gene were described in 3 patients with skin blistering disease.37 These 3 mutations are different, but are all predicted to lead to loss of integrin α3 function.37 All 3 of these patients died within 2 years of birth due to infection or multi-organ failure associated with reduced kidney and lung barrier function, similar to the phenotype seen in integrin α3-null mice.33,37 It was also recently shown that mice with a skin-specific deletion of integrin α3 have significantly reduced susceptibility to tumor formation upon DMBA-TPA treatment.38 The authors show that this reduced susceptibility to tumor development is the result of increased epidermal turnover seen in mouse epidermis lacking α3, leading to increased differentiation and shedding of the cells that accumulate mutations upon carcinogen treatment.38 Despite this reduced tumor formation, the squamous cell carcinomas that do form in mouse skin lacking α3 show reduced differentiation, a marker for increased malignancy, suggesting that integrin α3 plays dual roles in tumor formation and progression.38
As summarized in Table1, several other integrins are utilized in specific epidermal contexts. For instance, integrin α9 plays a crucial role in enhancing keratinocyte migration and proliferation during wound healing.39 Loss of β6 in mouse epidermis leads to retarded hair growth after depilation and enhanced keratinocyte proliferation.40 Integrin α2 and β5 are not essential for mouse epidermis, since corresponding knockout or conditional knockout mice have no apparent skin abnormalities.41-44 However, integrin α2 was shown to play a key role in HPV-driven SCC tumorigenesis and metastasis.45 K14-HPV16/ITGA2−/− mice had reduced lymph node metastases in comparison to K14-HPV16/ITGA2+/+ mice.45 In addition, SCC cell lines developed from tumors in the K14-HPV16/ITGA2−/− mice had reduced tumor growth and increased tumor latency compared with SCC lines derived from K14-HPV16/ITGA2+/+ mice.45 While it remains to be seen whether this metastasis phenotype is microenvironment-dependent, this study indicates that targeting integrin α2 may be a viable therapeutic target for HPV-driven SCC.
Downstream of integrins, dozens of proteins participate in FA dynamics. However, it remains unclear which different integrin heterodimers form complexes with different FA proteins, and which integrin functions are mediated by each FA complex protein. The remainder of this review will cover the roles of FA complex proteins in epidermal morphogenesis, hair follicles, wound healing, and squamous cell carcinoma, with an emphasis on insights gained from transgenic mouse models. We will focus on focal adhesion kinase (FAK), integrin-linked kinase (ILK), and Kindlin, because of their well-characterized epidermal roles.
Focal Adhesion Kinase (FAK)
Focal adhesion kinase (FAK) in normal skin
Focal adhesion kinase (FAK) has been shown to be both a signaling kinase and an adaptor protein that helps link integrin adhesion complexes to the actin cytoskeleton.46,47 While FAK is only indirectly associated with β integrin cytoplasmic domains through binding to Paxillin and Talin, it is rapidly recruited to focal adhesions and auto-phosphorylated upon cellular adhesion to ECM proteins (Fig. 1). This auto-phosphorylation can lead to recruitment and activation of a variety of downstream signaling proteins.46,47
FAK is required for mouse development, since FAK-null mice die during embryogenesis at about E8.5, with mesodermal defects.48,49 This phenotype is highly similar to the phenotype of the fibronectin-knockout mouse, which also shows specific defects in mesoderm development.50 This suggests that FAK is essential for focal adhesions involving the fibronectin-binding integrins, including α4β1, α5β1, αIIbβ3, αvβ3, αvβ6, and αvβ8. Autophosphorylation is required for FAK function in vitro; however, mice lacking the autophosphorylation site of FAK have a slightly different phenotype than FAK knockout mice.51 Mice lacking exon 15 of FAK, which contains the Y397 autophosphorylation site, proceed through embryonic development until E12.5, 5 days longer than FAK-null mice.51 These mutant mice display hemorrhage, edema, and vascular remodeling defects at E12.5.51 While it remains clear that this autophosphorylation plays an essential role in development, this study highlights the fact that FAK likely plays an important scaffolding role as well.
Skin-specific deletion of FAK leads to hair cycle irregularities, sebaceous gland hypoplasia and slight epidermal thinning.52 Isolated keratinocytes from these mice undergo apoptosis in culture, potentially due to inability to adhere to tissue culture plastic.52 Furthermore, loss of FAK in the epidermis seems to have no effect on wound healing.52 The phenotypes of FAK loss are not nearly as striking as the phenotype of β1 loss in the epidermis, indicating that FAK is only responsible for mediating a fraction of β1 integrin function in skin.23,24
Focal adhesion kinase (FAK) in squamous cell carcinoma
FAK expression and activity is elevated in multiple epithelial cancers, including squamous cell carcinoma. In a mouse model of SCC driven by loss of TGFβRII in the mouse epidermis, enhanced integrin-FAK-Src signaling and keratinocyte migration was observed.53 Further, loss of only one FAK allele significantly reduces papilloma formation upon DMBA-TPA treatment.54 Loss of both alleles prevents papilloma progression to SCC.55 FAK was shown to be necessary for phosphorylation of ERK downstream of Ras in cultured cells, and loss of FAK reduced migration of keratinocytes in vitro.54,55 Use of a FAK kinase inhibitor, PF-562,271, also blocked tumor cell migration, anchorage-independent growth, and SCC xenograft growth (Fig. 1B).56 This inhibition of FAK activity correlated with a decrease in phosphorylation of Src at tyrosine 416.56 Skin-specific loss of FAK also prevented phorbol ester-induced skin carcinogenesis, potentially due to prevention of β-catenin-induced stem cell mobilization in the bulge of the hair follicle.57 Inhibition of Src, a kinase that intricately associates with FAK at FA complexes also showed the same effect on preventing stem cell mobilization.57
In other types of carcinomas, FAK signaling has been intricately associated with PI3K/Akt signaling, providing a potential mechanism by which FAK acts to promote tumor growth and progression to malignancy. In a neuroblastoma tumor model, treatment with Y15, a FAK inhibitor, or with a FAK siRNA blocks gastrin-releasing peptide receptor (GRP-R)-mediated tumor growth and metastasis to the liver.58 This loss of FAK activity correlates with decreased phosphorylation of both Akt and ERK 1/2.58 Similarly, in melanoma, FAK interaction with the insulin-like growth factor receptor-1, IGF-1R, is necessary for downstream phosphorylation of Akt.59 Abrogation of the FAK/IGF-1R interaction led to melanoma regression in an orthotopic mouse model.59 Furthermore, in glioblastoma, FAK activity is dependent on PI3K/Akt activation, since knockdown of either the PIK3CA or PIK3R1 gene reduces FAK phosphorylation and tumor cell-invasive properties.60 PI3K/Akt signaling is upregulated in the vast majority of HNSCC cases due to direct mutation of PIK3CA or PTEN, or increased signaling flux due to other oncogenic mutations.61,62 The potential interplay between PI3K/Akt and FAK signaling in SCC thus merits further investigation.
While FAK clearly plays a pro-tumorigenic role, loss of FAK in SCC also results in increased resistance to radiation therapy.63 This resistance appeared dependent on p53-mediated induction of p21.63 In many contexts, FAK has been shown to both bind p53 and mediate its degradation.64-66 While further studies are required to verify this phenomenon in an orthotopic in vivo context, this result suggests that the viability of FAK as a therapeutic target may depend on p53 status.
Integrin Linked Kinase (ILK)
Integrin-linked kinase (ILK) in normal skin
Integrin-linked kinase (ILK) was discovered as a β1-integrin-interacting protein using a 2-hybrid screen.67 ILK was further shown to phosphorylate the β1 NPxY cytoplasmic motifs in vitro.67 It has been proposed in recent literature that ILK acts as a pseudo-kinase, because structural studies of its presumed kinase domain do not predict activity, and kinase-active or kinase-dead mutants of ILK were shown to block protein–protein interactions.68,69 However, integrin-linked kinase has been shown to directly phosphorylate GSK-3 in vitro and modulate its activity in cultured cells.70 Furthermore, kinase-active, but not kinase-deficient ILK can phosphorylate PKB/Akt on serine-473.70 The phenotypes of knockout and knock-in mice have provided more insight into ILK’s potential kinase role in vivo. ILK-knockout mice exhibit embryonic lethality at the peri-implantation stage due to epiblast failure.71 Surprisingly, when endogenous ILK is replaced with either a kinase-dead ILK (S343A), or a hyperactive ILK (S343D), mice are normal, viable, and fertile, with no apparent skin abnormalities and normal phosphorylation of Akt and GSK3β.72 However, mice containing K220A or K220M mutations in ILK’s ATP binding site die due to kidney failure but do not exhibit the severe embryonic lethality seen in ILK-null mice.72 Neither of these mutations altered phosphorylation of MBP, Akt, or GSK3β, but did reveal diminished binding to the FA adapters β- and α-parvin, which could be mediating this phenotype.72
Epidermal targeted ILK loss results in epidermal detachment, blister formation, and extension of proliferating keratinocytes to suprabasal layers.73,74 These mice also exhibit hair loss, attributed to the inability of proliferating progenitor cells in the follicle to migrate and replenish the hair matrix required for hair cycling.73 In culture, ILK-null keratinocytes have a severe migration defect and de-stabilized lamellipodia.73,74 These defects in cellular spreading and movement can be rescued through re-expression of active Rac1 or RhoG.74 Surprisingly, this skin phenotype does not appear dependent on either ILK’s presumed kinase activity or ATP-binding, since mice expressing S343A, S343D, K220A, or K220M mutant ILK display normal skin.72 ILK loss in the stem cells of the mouse hair follicle bulge using a K15-Cre promoter revealed a novel role for ILK in regenerating the epidermis after injury.75 Recently, a role was described for ILK in mediating keratinocyte phagocytosis, which could explain the abnormal pigmentation also seen in ILK-deficient epidermis. ILK-deficient keratinocytes are unable to engulf fluorescent microspheres in response to keratinocyte growth factor (KGF), similar to β1-null keratinocytes.76 It remains unclear exactly how ILK regulates downstream signaling pathways to mediate skin development. It is possible that ILK does have true kinase activity, but that this kinase activity is not essential for mouse development and can be compensated for through other pathways. It is also possible that the phosphorylation of substrates seen in culture is largely artifactual, and that ILK serves primarily as a scaffold protein in vivo.
ILK in squamous cell carcinoma
ILK’s role in tumorigenesis has not been extensively explored in relevant epidermal SCC mouse models. However, there are a few studies that suggest a role for ILK in epithelial cancer. ILK expression was shown to be elevated in ~70% of lung SCC cases. This expression is associated with both reduced E-cadherin expression and poor patient prognosis.77 In oral, keratinocyte-derived SCC, ILK protein expression is increased in approximately 90% of primary samples and 100% of tumor metastases, with expression strongly correlated with enhanced tumor invasion and metastasis, higher tumor grade, poor outcome, and increased risk of recurrence.78 ILK expression also correlates with increased expression of EMT markers, such as Snail and N-cadherin.78 In vitro, SCCHN cell lines respond to ILK kinase inhibition (using QLT0267) by undergoing cell cycle arrest and apoptosis, associated with a decrease in Akt phosphorylation (Fig. 1B).79 These data are highly suggestive that ILK plays a key role in tumor malignancy, and that targeting ILK’s kinase activity may be an effective therapeutic strategy. Perhaps ILK kinase activity plays a more important role during tumorigenesis than normal skin homeostasis. However, further studies using relevant in vivo models are required.
Kindlin in Skin
Kindler syndrome (KS) is an autosomal recessive disease characterized by a variety of skin phenotypes, including: blistering, sensitivity to UV, abnormal pigmentation, and skin fragility.80 Linkage analysis of a group of Panamanian patients in 2003 led to the discovery of the gene responsible for this syndrome, KIND1, which encodes for the protein Kindlin-1.81 Many additional mutations in the KIND1 gene have been described that lead to loss of protein expression or function.82 There are three Kindlin isoforms—Kindlin-1, Kindlin-2, and Kindlin-3—that have tissue-specific expression, which can explain why Kindler syndrome results only from mutations in KIND1. Kindlin-1 is expressed exclusively in epithelial tissue, Kindlin-3 is expressed exclusively in hematopoietic lineages, and Kindlin-2 is expressed ubiquitously. Loss of KIND2 in mice leads to peri-implantation lethality, presumably because Kindlin-2 is absolutely required for Talin-induced activation of integrin signaling.83 As expected, loss of KIND1 in mice leads to some of the defects seen in KS patients, including skin atrophy.84 However, this phenotype is associated with severe intestinal dysfunction, leading to perinatal lethality in these mice.84 Ulcerative colitis is a common feature in a fraction of KS patients, but it does not lead to death in humans so early in life. This indicates that either there are significant differences between Kindlin-1 function in mouse and human colons, or that perhaps the mutations seen in KS patients do not result in complete loss of KIND1 activity.
Epidermis deficient in Kindlin-1 displays defects in basal keratinocyte polarity and proliferation, and enhanced apoptosis.85 Keratinocytes lacking Kindlin-1 also show reduced proliferation and adhesion in culture, and undirected cellular motility and plasma membrane protrusion.85 More thorough analysis of FA complexes revealed that Kindlin-1 is phosphorylated, and that it co-immunoprecipitates with both Kindlin-2 and Migfilin in the skin.85,86 However, Kindlin-1 in keratinocytes is not required for KIND2 or FBLIM1 (Migfilin) gene expression or protein localization.86 Furthermore, it was shown that Kindlin-1 forms a complex with β1 integrin, α-actinin, Migfilin, and FAK and regulates cell shape and migration by controlling lamellipodia formation.87 Kindlin-1 expression is required for activation of motility-related proteins, including Rac1, RhoA, Cdc42, p21-activated kinase 1 (PAK1), LIM kinase, and Cofilin.87 KS primary keratinocytes, or keratinocytes lacking Kindlin-1, show reduced clonogenicity and upregulation of cell senescence markers, potentially explaining the skin fragility seen in these patients.88 Kindlin-1 and Kindlin-2 were shown to have some overlapping functions in skin; however, loss of Kindlin-2 alone in cultured keratinocytes led to reduction of cell motility and formation of cell–cell adhesions.89 Irradiation of keratinocytes led to loss of Kindlin-2 protein expression, which could explain why patients with KS experience enhanced UV sensitivity.89
The function of Kindlin-1 or -2 has not been directly assessed in mouse models of SCC; however, there is a higher incidence of skin cancer in patients affected with KS.90 Given the relative fitness disadvantage of nontumorigenic Kindlin-1 mutant cells, this increased cancer susceptibility seems somewhat surprising. However, keratinocytes lacking Kindlin-1 show reduced epithelial features and signs of epithelial–mesenchymal transition, including reduced α6β4, E-cadherin, and collagen XVII expression, and increased levels of mesenchymal markers vimentin and fibronectin, along with increased secretion of MMPs and pro-inflammatory cytokines.90 This suggests an anti-tumor and anti-invasive role for Kindlin-1 in normal keratinocytes, though in vivo studies are required to verify this possibility.
Other Focal Adhesion Proteins
Many other scaffolds, adaptor and signaling proteins exist within Focal adhesions and are also essential for mouse viability. Most of these proteins have no known functional role in skin in vivo, as conditional knockout mice have not been generated. Here we review what is known about the role of additional FA proteins in keratinocytes or squamous cell carcinoma cell lines.
α-actinin is a cytoskeletal protein that binds directly to actin to link integrins to the cellular cytoskeleton. This protein exists in 4 isoforms that are restricted to specific cell types. α-actinin-2 and -3 are muscle-specific, while α-actinin-1 and -4 are expressed more ubiquitously. ACTN1 (the gene encoding α-actinin-1) has not been knocked out in a mouse, and there is no information on any role for α-actinin-1 in keratinocytes. However, the ACTN4-knockout mouse has been created and exhibits lung and kidney failure, leading to death several months after birth.91 In culture, knockdown of ACTN4 in keratinocytes leads to loss of polarity, decreased directional migration, and decreased activity of cofilin, an actin remodeling protein.92 Additionally, the focal contact area in these cells is increased and the hemidesmosome proteins in these cells are mislocalized.92 This could indicate that skin lacking ACTN4 may have a structural or wound healing defect, but in vivo data are needed to verify this.
Filamin A is another actin binding protein that participates in organization of the actin cytoskeleton. The FLNA-knockout mouse is embryonic lethal due to cardiac failure and defects in blood vessel formation.93 Filamin A has been shown to be required for calcium-induced keratinocyte differentiation in culture, though the mechanism by which this occurs is unknown.94 Migfilin, also known as Filamin binding LIM protein 1, also binds to Filamin A and plays a role in the actin cytoskeleton. However, the FBLIM1 (gene that encodes Migfilin) knockout mouse is normal with no noticeable defect in skin or tissue morphology.95 Primary keratinocytes isolated from Migfilin deficient mice exhibited a migration defect in culture which appeared to be independent of Filamin binding.95
P130cas, encoded by the BCAR1 gene, is a direct substrate of FAK and is involved in cellular migration, survival, and invasion. The BCAR1-knockout mouse experiences embryonic lethality due to cardiac defects and improper blood vessel formation, similar to the phenotype seen in the FLNA knockout mouse.96 Nothing is known about the role of this protein in skin. However, in lung squamous cell carcinoma, phosphorylation of p130cas is associated with increased invasiveness, and knockdown of p130cas could reverse the increased invasion induced by a mutation in the EphA2 gene.97 This suggests a possible role for this protein in SCC invasion.
Paxillin is a signal adaptor protein that is recruited to the β1 integrin cytoplasmic domain following attachment of SCC cells to type IV collagen.98 Paxillin may also play a role in SCC invasion, since overexpression of this protein stimulated in vitro invasive properties of an SCC cell line.98 There is not any in vivo data on the role of this protein in skin or SCC, since the PXN-knockout mouse has disrupted development of mesodermal structures, leading to embryonic lethality at E9.5.99
PINCH1 and PINCH2 are LIM domain-containing proteins that bind directly to ILK.100 The PINCH1-knockout mouse exhibits embryonic lethality between E6.5–E9.5, with abnormal epiblast polarity and impaired cavitation, similar to the ILK-null mouse.100,101 The PINCH2-knockout mouse, however, is viable and fertile, with no apparent phenotype.102 Both expressed at high levels in mouse skin, but their functional roles are unknown.103
Several additional secreted glycoproteins have been reported to bind to and activate integrin αv heterodimers. These proteins comprise the SIBLING (small integrin binding ligand N-linked glycoprotein) family, which consists of proteins such as dentin sialophosphoprotein (DSPP), bone sialoprotein (BSP), and osteopontin (OPN). These proteins play major roles in mineral deposition in the bone and have been shown to bind and activate matrix metalloproteases. Though it is not known whether these proteins play a role in skin homeostasis, their expression is highly upregulated in clinical cases of OSCC.104 Furthermore, DSPP and OPN are expressed at histologically negative margins of OSCCs, and their expression predicts recurrence following tumor resection.105
Tensin is a FA protein that is phosphorylated upon integrin activation or upon transformation by oncogenes such as v-Src. Tensin also binds and cross-links actin filaments. Two additional Tensin isoforms, Tensin-2 and Tensin-3, were discovered more recently. The Tensin knockout mouse is normal at birth, but develops kidney abnormalities.106 Some Tensin-3-knockout mice exhibit growth retardation and postnatal lethality, which is associated with incomplete development of the small intestine, lung, and bone.107 Tensin-3 has been shown to be required for tumorigenesis and metastasis in a number of different cell lines, though its role in squamous cell carcinoma specifically has not been determined.108
Finally, Zyxin is an adaptor protein containing both LIM domains and SH3 domains. Zyxin has a punctate staining in the cytoplasm of keratinocytes within the normal epidermis; however, this staining re-localizes the the edge of migrating keratinocyte sheets during wound healing.109 The Zyxin knockout mouse is normal, which could potentially be due to functional redundancy between the Zyxin family members LPP or TRIP6.110
Therapeutic Targeting
Inhibitors targeting integrin activation and the focal adhesion kinases FAK and ILK are in various stages of development, with clinical trials currently ongoing for integrin inhibitors, integrin blocking antibodies, and FAK inhibitors. Although in vitro data support the use of ILK kinase inhibitors for therapy, no clinical trials have been started for those compounds.
Cilengitide (EMD 121974) is an RGD-based peptide targeted against αvβ3 and αvβ5 integrins, which are upregulated on blood vessels during tumor angiogenesis.111 In preclinical studies, this drug showed induction of apoptosis of angiogenic endothelial cells and additional direct anti-tumor activity.112 While preclinical models showed promise, only a fraction of glioblastoma patients respond to therapy, and there is variability in response of patients with other types of tumors to the drug.112 Treatment of patients with squamous cell carcinomas of the head and neck with cilengitide resulted in partial response or stable disease for all patients tested, and randomized phase II clinical trials are currently in progress.113 More recently, it was shown that low concentrations of this inhibitor actually stimulate tumor growth by promoting VEGFR-2 trafficking to the endothelial cell surface.114 It is therefore possible that dose of the drug is highly important in tumor response, which could account for some of the variability seen thus far.
CNTO-95 (intetumumab) is a fully humanized anti-αv-integrin monoclonal antibody that was also developed to target angiogenic blood vessels and some primary tumors, but broadly binds to all αv heterodimers.115 The expression of αv integrins has been shown to be essential for survival of melanoma cells in 3-dimensional cultures, and thus most of the clinical trials for this antibody have been for stage IV melanoma patients.116 This therapy was well tolerated in phase I trials and has shown variable success in phase II trials.117-119 There is a trend toward improved survival, but it is not yet significant, and studies with larger patient cohorts may be necessary.
In preclinical studies, inhibition of FAK kinase activity showed promising anti-tumor activity for a variety of different types of malignancies. A specific inhibitor, PF-00562271, has shown safety and some efficacy in phase I trials for advanced solid tumors (including head and neck tumors).120 Phase I trials are currently ongoing for a second FAK kinase inhibitor, GSK2256098.
Another potential strategy for targeting integrin activation in cancer is to target specific extracellular matrix ligands. Secretion of proteases during tumorigenesis can lead to cleavage of ECM components to generate new ligands with distinct structure and binding affinity for specific integrin heterodimers. Cleavage of type IV collagen into 2 epitopes, HU177 and HUIV26, occurs in the extracellular matrix surrounding melanoma tumors. This collagen cleavage exposes additional integrin-binding motifs within these epitopes that enhance signaling of αvβ3 within angiogenic blood vessels.121 Blocking antibodies against these epitopes have shown anti-angiogenic, anti-tumor and anti-metastasis efficacy.122 Additionally, increased shedding of HU177 is observed in melanoma patient sera and has been shown to correlate with poor prognosis and disease progression.123,124 More work is required to determine if additional epitopes are released, and if this differs between tumor types.
Conclusions and Future Directions
It has become increasingly clear that FA complexes anchoring epidermal keratinocytes to the basement membrane are essential for many aspects of skin homeostasis, including basal keratinocyte proliferation, differentiation, barrier function, migration and wound healing, and hair follicle morphogenesis. Many of the individual FA complex proteins, however, are not absolutely required for skin, but play key roles during epithelial cell carcinogenesis. It is possible to exploit this biological information to target FA complexes from outside the cell, using blocking antibodies, RGD-based peptides, or small-molecule inhibitors to block integrin activation.125 However, a better understanding of individual integrin subunit roles in both normal skin and SCC is required for development of effective therapy. Maximizing the medical relevance of these studies will require functional experiments in native 3-dimensional environments, as traditional 2-dimensional experiments utilizing cultured cells results in different responses that are not seen in vivo.126 While functional roles for some integrin subunits, FAK, ILK, and Kindlin have been established in mouse epidermis, there exist many other FA complex proteins with no known in vivo functional role in skin. Loss of most of these proteins results in embryonic lethality (Table 2), and additional studies using conditional knockout strategies or human organotypic skin reconstructs are required to determine the roles these proteins play in skin homeostasis.
Table 2. Functional roles for intracellular focal adhesion proteins in mouse development.
| Gene | Knockout mouse phenotype |
|---|---|
|
α-Actinin-4 |
-Die at several months of age due to glomerular disease and proteinuria91 |
|
FAK |
-Embryonic lethal E8.548,49 -Defect in mesodermal development48,49 -Able to implant and initiate gastrulation48,49 |
|
FLNA (Filamin A) |
-Embryonic lethal E13.5–14.593 -Cardiac and vascular failure, and impaired brain development93 |
|
ILK |
-Die shortly after implantation, E8.571,72 -Fail to form epiblast71,72 -Mice with kinase-dead ILK mutants (S343A) or kinase hyperactive ILK mutants (S343D) have no developmental or skin defects71,72 -Mice with mutation in ATP-binding site of kinase domain (K220A or K220M) die shortly after birth due to kidney defects, but have normal skin71,72 |
|
KIND-1 (Kindlin-1) |
-Skin atrophy84 -Intestinal dysfunction resulting in perinatal lethality84 |
|
KIND-2 (Kindlin-2) |
-Peri-implantation lethality at E783 -Detachment of endoderm and epiblast from basement membrane83 |
|
Migfilin |
-Viable, fertile and no apparent abnormalities95 |
|
Cas (p130Cas) |
Embryonic lethality at E11.5–12.596 -Poor development of heart and blood vessel dilation96 |
|
Paxillin |
-Embryonic lethality at E9.599 -Disrupted development of mesodermal structures99 |
|
PINCH1 |
-Embryonic lethality at E6.5 or E9.5100,101 -Disorganized egg cylinder, and significant cell death100,101 -Abnormal epiblast polarity, and impaired cavitation into basement membrane100,101 |
|
PINCH2 |
-Viable, fertile with no apparent phenotype102 |
|
Talin |
-Embryonic lethality at E8.5–9.5145 -Disorganization of embryos at gastrulation145 -Defective embryonic ectoderm145 |
|
Tensin |
-Normal at birth, but develop kidney abnormalities that lead to lethality106 |
|
Tensin-3 |
-Growth retardation and postnatal lethality in 1/3 of mice that is associated with impaired development of the intestine, lung and bone107 |
|
Vinculin |
-Embryonic lethality around E8–10146 -Attenuated cranial nerve, spinal nerve and heart development146 -Defective ectoderm development146 |
| Zyxin | -Viable, fertile and no apparent abnormalities110 |
Generation of such a large number of integrin-knockout mice is expensive, time-consuming, and can lead to misleading conclusions due to strain-specific defects (as shown in the case of β1 integrin). Furthermore, there are many structural differences between mouse and human skin.127 More recently, organotypic skin substitutes have been developed that contain intact and structurally correct human basement membrane and human epidermal and dermal cells.128 These epidermal cells can be genetically engineered to overexpress or knockdown expression of multiple genes of interest. This type of experimental platform can greatly accelerate research on the functional roles of integrins and FA proteins in human skin and SCC. This tissue can also be xenografted onto mice, allowing for the study of human skin and SCC in vivo.129 This type of experimental platform is a potentially useful approach for translating the developmental and biological insight we have gained from mouse models into therapy for human disease. While clinical trials for integrin antagonists or blocking antibodies, and FAK kinase inhibitors are ongoing, there remains much to learn regarding integrin signaling in tissue, and how it changes in disease conditions. A better understanding of both integrin signaling and the different roles of each FA kinase protein in vivo will hopefully lead to the development of more effective cancer therapies (Table 3).
Table 3. Functional roles for intracellular focal adhesion proteins in mouse skin.
| Gene | Conditional skin knockout phenotype (K5 or K14 promoter) | Role in SCC |
|---|---|---|
|
FAK |
-Hair cycle irregularities52 -Loss of proliferation in sebaceous gland52 -Slight epidermal thinning, but no change in wound healing52 |
-FAK+/− mice show reduced papilloma formation, but no change in conversion of papillomas to SCCs upon DMBA-TPA treatment54 -K14-CreER 4-OHT induced deletion of FAK in the epidermis both prevented tumor formation and stopped tumor progression upon DMBA-TPA treatment55 |
| ILK | -Epidermal blistering73 -Movement of proliferative cells to suprabasal layers73 -Hair loss73 |
N/A |
Acknowledgments
TWR is supported by a grant from the NIH/NCI (RO1 CA163566). EKD is supported by an NIH/NIAMS training grant (5-T32-AR0007465-30). We would like to thank Dr Sarah Millar at the University of Pennsylvania for critical pre-submission manuscript review.
Glossary
Abbreviations:
- cSCC
cutaneous squamous cell carcinoma
- DMBA-TPA
7,12-dimethylbenz(a)anthracene 12-O-tetradecanoylphorbol-13-acetate
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal regulated kinase
- FA
focal adhesion
- FAK
focal adhesion kinase
- GRPR
gastrin releasing peptide receptor
- GSK3β
glycogen synthase kinase 3 beta
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- IGF1R
insulin like growth factor receptor 1
- ILK
integrin linked kinase
- K5
Keratin 5
- K14
Keratin 14
- KS
Kindler syndrome
- MAPK
mitogen activated protein kinase
- MBP
myelin basic protein
- NFκB
nuclear factor kappa light chain enhancer of activated B cells
- OSCC
oral squamous cell carcinoma
- PI3K
phosphoinositide 3 kinase
- PKB
protein kinase B
- PKC
protein kinase C
- PLCγ
phospholipase C gamma
- PTEN
phosphatase and tensin homolog
- SH3
Src homology 3
- TGFβRII
Transforming Growth Factor β Receptor II
- UV
ultraviolet radiation
- VEGFR2
vascular endothelial growth factor receptor 2
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26385
References
- 1.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–40. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 3.Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 5.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–19. doi: 10.1016/S0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 8.Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, Breuninger H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–20. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 9.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28:268–73. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 10.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–66. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist. 2010;15:1320–8. doi: 10.1634/theoncologist.2009-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, Certain A, Duval X, Crickx B, Buffard V, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–26. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 13.Preneau S, Rio E, Brocard A, Peuvrel L, Nguyen JM, Quéreux G, Dreno B. Efficacy of cetuximab in the treatment of squamous cell carcinoma. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2012.751481. [DOI] [PubMed] [Google Scholar]
- 14.Reuter JA, Ortiz-Urda S, Kretz M, Garcia J, Scholl FA, Pasmooij AM, Cassarino D, Chang HY, Khavari PA. Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell. 2009;15:477–88. doi: 10.1016/j.ccr.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–80. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 17.Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–72. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–3. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 19.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 20.Tran M, Rousselle P, Nokelainen P, Tallapragada S, Nguyen NT, Fincher EF, Marinkovich MP. Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res. 2008;68:2885–94. doi: 10.1158/0008-5472.CAN-07-6160. [DOI] [PubMed] [Google Scholar]
- 21.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–83. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 22.Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–52. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- 23.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–60. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Rovira T, Silva-Vargas V, Watt FM. Different consequences of beta1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J Invest Dermatol. 2005;125:1215–27. doi: 10.1111/j.0022-202X.2005.23956.x. [DOI] [PubMed] [Google Scholar]
- 26.Anthis NJ, Haling JR, Oxley CL, Memo M, Wegener KL, Lim CJ, Ginsberg MH, Campbell ID. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J Biol Chem. 2009;284:36700–10. doi: 10.1074/jbc.M109.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai T, Jove R, Fässler R, Mosher DF. Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proc Natl Acad Sci U S A. 2001;98:3808–13. doi: 10.1073/pnas.240456398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennerberg K, Armulik A, Sakai T, Karlsson M, Fässler R, Schaefer EM, Mosher DF, Johansson S. The cytoplasmic tyrosines of integrin subunit β1 are involved in focal adhesion kinase activation. Mol Cell Biol. 2000;20:5758–65. doi: 10.1128/MCB.20.15.5758-5765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Zou Z, Sarratt KL, Zhou D, Zhang M, Sebzda E, Hammer DA, Kahn ML. In vivo beta1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 2006;20:927–32. doi: 10.1101/gad.1408306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czuchra A, Meyer H, Legate KR, Brakebusch C, Fässler R. Genetic analysis of beta1 integrin “activation motifs” in mice. J Cell Biol. 2006;174:889–99. doi: 10.1083/jcb.200604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meves A, Stremmel C, Böttcher RT, Fässler R. β1 Integrins with Individually Disrupted Cytoplasmic NPxY Motifs Are Embryonic Lethal but Partially Active in the Epidermis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meves A, Geiger T, Zanivan S, DiGiovanni J, Mann M, Fässler R. Beta1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc Natl Acad Sci U S A. 2011;108:15213–8. doi: 10.1073/pnas.1105689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 34.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–42. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conti FJ, Rudling RJ, Robson A, Hodivala-Dilke KM. alpha3beta1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci. 2003;116:2737–47. doi: 10.1242/jcs.00475. [DOI] [PubMed] [Google Scholar]
- 36.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–88. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 37.Has C, Spartà G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, Waters A, He Y, Anikster Y, Esser P, et al. Integrin α3 mutations with kidney, lung, and skin disease. N Engl J Med. 2012;366:1508–14. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachs N, Secades P, van Hulst L, Kreft M, Song JY, Sonnenberg A. Loss of integrin α3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proc Natl Acad Sci U S A. 2012;109:21468–73. doi: 10.1073/pnas.1204614110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol. 2009;129:217–28. doi: 10.1038/jid.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, McElwee KJ, Owen GR, Häkkinen L, Larjava HS. Integrin β6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation. J Invest Dermatol. 2012;132:547–55. doi: 10.1038/jid.2011.381. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–44. doi: 10.1016/S0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM. Wound healing in the alpha2beta1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–66. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Griffiths M, Wu J, Farese RV, Jr., Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–9. doi: 10.1128/MCB.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, Leutgeb B, Krieg T, Eckes B. Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol. 2007;127:467–78. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]
- 45.Tran T, Barlow B, O’Rear L, Jarvis B, Li Z, Dickeson K, Dupont W, Zutter M. Loss of the α2β1 integrin alters human papilloma virus-induced squamous carcinoma progression in vivo and in vitro. PLoS One. 2011;6:e26858. doi: 10.1371/journal.pone.0026858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 47.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 48.Furuta Y, Ilić D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–95. [PubMed] [Google Scholar]
- 49.Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 50.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 51.Corsi JM, Houbron C, Billuart P, Brunet I, Bouvrée K, Eichmann A, Girault JA, Enslen H. Autophosphorylation-independent and -dependent functions of focal adhesion kinase during development. J Biol Chem. 2009;284:34769–76. doi: 10.1074/jbc.M109.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essayem S, Kovacic-Milivojevic B, Baumbusch C, McDonagh S, Dolganov G, Howerton K, Larocque N, Mauro T, Ramirez A, Ramos DM, et al. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene. 2006;25:1081–9. doi: 10.1038/sj.onc.1209130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–27. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLean GW, Brown K, Arbuckle MI, Wyke AW, Pikkarainen T, Ruoslahti E, Frame MC. Decreased focal adhesion kinase suppresses papilloma formation during experimental mouse skin carcinogenesis. Cancer Res. 2001;61:8385–9. [PubMed] [Google Scholar]
- 55.McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serrels A, McLeod K, Canel M, Kinnaird A, Graham K, Frame MC, Brunton VG. The role of focal adhesion kinase catalytic activity on the proliferation and migration of squamous cell carcinoma cells. Int J Cancer. 2012;131:287–97. doi: 10.1002/ijc.26351. [DOI] [PubMed] [Google Scholar]
- 57.Ridgway RA, Serrels B, Mason S, Kinnaird A, Muir M, Patel H, Muller WJ, Sansom OJ, Brunton VG. Focal adhesion kinase is required for β-catenin-induced mobilization of epidermal stem cells. Carcinogenesis. 2012;33:2369–76. doi: 10.1093/carcin/bgs284. [DOI] [PubMed] [Google Scholar]
- 58.Lee S, Qiao J, Paul P, O’Connor KL, Evers MB, Chung DH. FAK is a critical regulator of neuroblastoma liver metastasis. Oncotarget. 2012;3:1576–87. doi: 10.18632/oncotarget.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ucar DA, Kurenova E, Garrett TJ, Cance WG, Nyberg C, Cox A, Massoll N, Ostrov DA, Lawrence N, Sebti SM, et al. Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle. 2012;11:3250–9. doi: 10.4161/cc.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber GL, Parat MO, Binder ZA, Gallia GL, Riggins GJ. Abrogation of PIK3CA or PIK3R1 reduces proliferation, migration, and invasion in glioblastoma multiforme cells. Oncotarget. 2011;2:833–49. doi: 10.18632/oncotarget.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herzog A, Bian Y, Vander Broek R, Hall B, Coupar J, Cheng H, Sowers AL, Cook JD, Mitchell JB, Chen Z, et al. PI3K/mTOR inhibitor PF-04691502 anti-tumor activity is enhanced with induction of wild-type TP53 in human xenograft and murine knockout models of head and neck cancer. Clin Cancer Res. 2013;19:3808–19. doi: 10.1158/1078-0432.CCR-12-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham K, Moran-Jones K, Sansom OJ, Brunton VG, Frame MC. FAK deletion promotes p53-mediated induction of p21, DNA-damage responses and radio-resistance in advanced squamous cancer cells. PLoS One. 2011;6:e27806. doi: 10.1371/journal.pone.0027806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem. 2005;280:25008–21. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 65.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Front Biosci (Landmark Ed) 2010;15:901–12. doi: 10.2741/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell. 2009;36:819–30. doi: 10.1016/j.molcel.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29:281–91. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–6. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fässler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–40. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 2009;461:1002–6. doi: 10.1038/nature08468. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fässler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–13. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakrieko KA, Welch I, Dupuis H, Bryce D, Pajak A, St Arnaud R, Dedhar S, D’Souza SJ, Dagnino L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell. 2008;19:1462–73. doi: 10.1091/mbc.E07-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakrieko KA, Rudkouskaya A, Irvine TS, D’Souza SJ, Dagnino L. Targeted inactivation of integrin-linked kinase in hair follicle stem cells reveals an important modulatory role in skin repair after injury. Mol Biol Cell. 2011;22:2532–40. doi: 10.1091/mbc.E11-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sayedyahossein S, Nini L, Irvine TS, Dagnino L. Essential role of integrin-linked kinase in regulation of phagocytosis in keratinocytes. FASEB J. 2012;26:4218–29. doi: 10.1096/fj.12-207852. [DOI] [PubMed] [Google Scholar]
- 77.Yu J, Shi R, Zhang D, Wang E, Qiu X. Expression of integrin-linked kinase in lung squamous cell carcinoma and adenocarcinoma: correlation with E-cadherin expression, tumor microvessel density and clinical outcome. Virchows Arch. 2011;458:99–107. doi: 10.1007/s00428-010-1016-3. [DOI] [PubMed] [Google Scholar]
- 78.Zhao D, Tang XF, Yang K, Liu JY, Ma XR. Over-expression of integrin-linked kinase correlates with aberrant expression of Snail, E-cadherin and N-cadherin in oral squamous cell carcinoma: implications in tumor progression and metastasis. Clin Exp Metastasis. 2012;29:957–69. doi: 10.1007/s10585-012-9485-1. [DOI] [PubMed] [Google Scholar]
- 79.Younes MN, Yigitbasi OG, Yazici YD, Jasser SA, Bucana CD, El-Naggar AK, Mills GB, Myers JN. Effects of the integrin-linked kinase inhibitor QLT0267 on squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2007;133:15–23. doi: 10.1001/archotol.133.1.15. [DOI] [PubMed] [Google Scholar]
- 80.White SJ, McLean WH. Kindler surprise: mutations in a novel actin-associated protein cause Kindler syndrome. J Dermatol Sci. 2005;38:169–75. doi: 10.1016/j.jdermsci.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 81.Siegel DH, Ashton GH, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC, South AP, Smith FJ, Prescott AR, Wessagowit V, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–87. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashton GH, McLean WH, South AP, Oyama N, Smith FJ, Al-Suwaid R, Al-Ismaily A, Atherton DJ, Harwood CA, Leigh IM, et al. Recurrent mutations in kindlin-1, a novel keratinocyte focal contact protein, in the autosomal recessive skin fragility and photosensitivity disorder, Kindler syndrome. J Invest Dermatol. 2004;122:78–83. doi: 10.1046/j.0022-202X.2003.22136.x. [DOI] [PubMed] [Google Scholar]
- 83.Montanez E, Ussar S, Schifferer M, Bösl M, Zent R, Moser M, Fässler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, Fässler R. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herz C, Aumailley M, Schulte C, Schlötzer-Schrehardt U, Bruckner-Tuderman L, Has C. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem. 2006;281:36082–90. doi: 10.1074/jbc.M606259200. [DOI] [PubMed] [Google Scholar]
- 86.Lai-Cheong JE, Ussar S, Arita K, Hart IR, McGrath JA. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol. 2008;128:2156–65. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Has C, Herz C, Zimina E, Qu HY, He Y, Zhang ZG, Wen TT, Gache Y, Aumailley M, Bruckner-Tuderman L. Kindlin-1 Is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am J Pathol. 2009;175:1442–52. doi: 10.2353/ajpath.2009.090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piccinni E, Di Zenzo G, Maurelli R, Dellambra E, Teson M, Has C, Zambruno G, Castiglia D. Induction of senescence pathways in Kindler syndrome primary keratinocytes. Br J Dermatol. 2013;168:1019–26. doi: 10.1111/bjd.12184. [DOI] [PubMed] [Google Scholar]
- 89.He Y, Esser P, Heinemann A, Bruckner-Tuderman L, Has C. Kindlin-1 and -2 have overlapping functions in epithelial cells implications for phenotype modification. Am J Pathol. 2011;178:975–82. doi: 10.1016/j.ajpath.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qu H, Wen T, Pesch M, Aumailley M. Partial loss of epithelial phenotype in kindlin-1-deficient keratinocytes. Am J Pathol. 2012;180:1581–92. doi: 10.1016/j.ajpath.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest. 2003;111:1683–90. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamill KJ, Hopkinson SB, Skalli O, Jones JC. Actinin-4 in keratinocytes regulates motility via an effect on lamellipodia stability and matrix adhesions. FASEB J. 2013;27:546–56. doi: 10.1096/fj.12-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA, Filamin A. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103:19836–41. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tu CL, Chang W, Bikle DD. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. J Invest Dermatol. 2011;131:1119–28. doi: 10.1038/jid.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moik DV, Janbandhu VC, Fässler R. Loss of migfilin expression has no overt consequences on murine development and homeostasis. J Cell Sci. 2011;124:414–21. doi: 10.1242/jcs.075960. [DOI] [PubMed] [Google Scholar]
- 96.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–5. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 97.Faoro L, Singleton PA, Cervantes GM, Lennon FE, Choong NW, Kanteti R, Ferguson BD, Husain AN, Tretiakova MS, Ramnath N, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem. 2010;285:18575–85. doi: 10.1074/jbc.M109.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crowe DL, Ohannessian A. Recruitment of focal adhesion kinase and paxillin to beta1 integrin promotes cancer cell migration via mitogen activated protein kinase activation. BMC Cancer. 2004;4:18. doi: 10.1186/1471-2407-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fässler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118:2913–21. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 101.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr., Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–62. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stanchi F, Bordoy R, Kudlacek O, Braun A, Pfeifer A, Moser M, Fässler R. Consequences of loss of PINCH2 expression in mice. J Cell Sci. 2005;118:5899–910. doi: 10.1242/jcs.02686. [DOI] [PubMed] [Google Scholar]
- 103.Braun A, Bordoy R, Stanchi F, Moser M, Kostka GG, Ehler E, Brandau O, Fässler R. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp Cell Res. 2003;284:237–48. doi: 10.1016/S0014-4827(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 104.Ogbureke KUE, Nikitakis NG, Warburton G, Ord RA, Sauk JJ, Waller JL, Fisher LW. Up-regulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 2007;43:920–32. doi: 10.1016/j.oraloncology.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Ogbureke KUE, Weinberger PM, Looney SW, Li L, Fisher LW. Expressions of matrix metalloproteinase-9 (MMP-9), dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at histologically negative surgical margins may predict recurrence of oral squamous cell carcinoma. Oncotarget. 2012;3:286–98. doi: 10.18632/oncotarget.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol. 1997;136:1349–61. doi: 10.1083/jcb.136.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang MK, Liao YC, Kuwabara Y, Lo SH. Inactivation of tensin3 in mice results in growth retardation and postnatal lethality. Dev Biol. 2005;279:368–77. doi: 10.1016/j.ydbio.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 108.Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, Steinbach PJ, Tosato G, Hunter K, Lowy DR. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009;16:246–58. doi: 10.1016/j.ccr.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leccia MT, van der Gaag EJ, Jalbert NL, Byers HR. Zyxin redistributes without upregulation in migrating human keratinocytes during wound healing. J Invest Dermatol. 1999;113:651–7. doi: 10.1046/j.1523-1747.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- 110.Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler FB, et al. Targeted disruption of the murine zyxin gene. Mol Cell Biol. 2003;23:70–9. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 112.Scaringi C, Minniti G, Caporello P, Enrici RM. Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. Anticancer Res. 2012;32:4213–23. [PubMed] [Google Scholar]
- 113.Vermorken JB, Guigay J, Mesia R, Trigo JM, Keilholz U, Kerber A, Bethe U, Picard M, Brummendorf TH. Phase I/II trial of cilengitide with cetuximab, cisplatin and 5-fluorouracil in recurrent and/or metastatic squamous cell cancer of the head and neck: findings of the phase I part. Br J Cancer. 2011;104:1691–6. doi: 10.1038/bjc.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 115.Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has anti-tumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–35. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 116.Bao W, Strömblad S. Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J Cell Biol. 2004;167:745–56. doi: 10.1083/jcb.200404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 118.O’Day S, Pavlick A, Loquai C, Lawson D, Gutzmer R, Richards J, Schadendorf D, Thompson JA, Gonzalez R, Trefzer U, et al. CNTO 95 Investigators A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer. 2011;105:346–52. doi: 10.1038/bjc.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson DW, Jr., Cormier JN, Zhao N, Uhlar CM, Revicki DA, Cella D. Health-related quality of life among patients with metastatic melanoma: results from an international phase 2 multicenter study. Melanoma Res. 2012;22:54–62. doi: 10.1097/CMR.0b013e32834d3da0. [DOI] [PubMed] [Google Scholar]
- 120.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. 2012;30:1527–33. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 121.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roth JM, Caunt M, Cretu A, Akalu A, Policarpio D, Li X, Gagne P, Formenti S, Brooks PC. Inhibition of experimental metastasis by targeting the HUIV26 cryptic epitope in collagen. Am J Pathol. 2006;168:1576–86. doi: 10.2353/ajpath.2006.050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ng B, Zakrzewski J, Warycha M, Christos PJ, Bajorin DF, Shapiro RL, Berman RS, Pavlick AC, Polsky D, Mazumdar M, et al. Shedding of distinct cryptic collagen epitope (HU177) in sera of melanoma patients. Clin Cancer Res. 2008;14:6253–8. doi: 10.1158/1078-0432.CCR-07-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamilton HK, Rose AE, Christos PJ, Shapiro RL, Berman RS, Mazumdar M, Ma MW, Krich D, Liebes L, Brooks PC, et al. Increased shedding of HU177 correlates with worse prognosis in primary melanoma. J Transl Med. 2010;8:19. doi: 10.1186/1479-5876-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–88. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–52. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khavari PA. Modelling cancer in human skin tissue. Nat Rev Cancer. 2006;6:270–80. doi: 10.1038/nrc1838. [DOI] [PubMed] [Google Scholar]
- 128.Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–5. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, Fang M, Tao S, Green CL, Khavari PA. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med. 2002;8:1105–14. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- 130.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–13. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 131.DiPersio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, Hynes RO. alpha3beta1 and alpha6beta4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci. 2000;113:3051–62. doi: 10.1242/jcs.113.17.3051. [DOI] [PubMed] [Google Scholar]
- 132.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 133.Niculescu C, Ganguli-Indra G, Pfister V, Dupé V, Messaddeq N, De Arcangelis A, Georges-Labouesse E. Conditional ablation of integrin alpha-6 in mouse epidermis leads to skin fragility and inflammation. Eur J Cell Biol. 2011;90:270–7. doi: 10.1016/j.ejcb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Pöschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–23. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 135.Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci. 2000;20:1822–30. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–13. doi: 10.1016/S0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr., Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–15. doi: 10.1128/MCB.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bengtsson T, Aszodi A, Nicolae C, Hunziker EB, Lundgren-Akerlund E, Fässler R. Loss of alpha10beta1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J Cell Sci. 2005;118:929–36. doi: 10.1242/jcs.01678. [DOI] [PubMed] [Google Scholar]
- 139.Popova SN, Barczyk M, Tiger CF, Beertsen W, Zigrino P, Aszodi A, Miosge N, Forsberg E, Gullberg D. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27:4306–16. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–15. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira M, Fujiwara H, Morita K, Watt FM. An activating beta1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 2009;69:1334–42. doi: 10.1158/0008-5472.CAN-08-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, et al. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–74. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 143.Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional beta4-integrin knockout mice. J Cell Sci. 2005;118:1045–60. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- 144.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr., Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–8. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, Fässler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–74. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 146.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]