Abstract
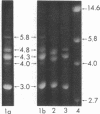
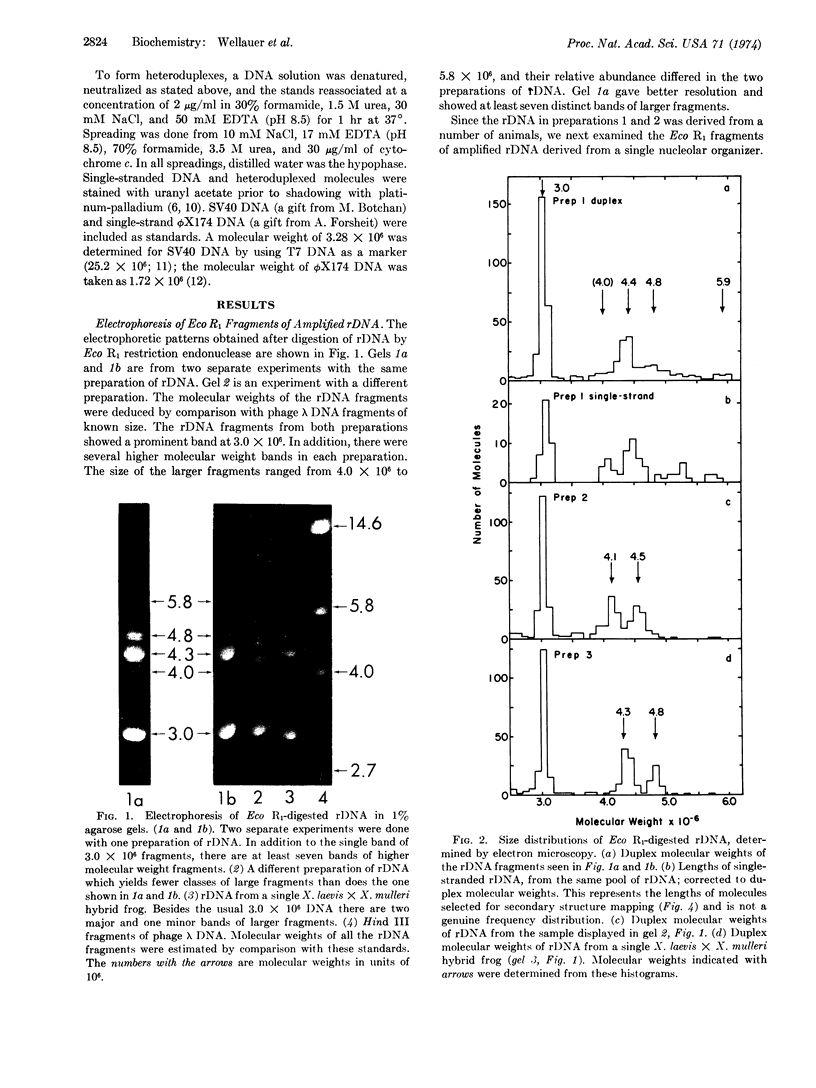
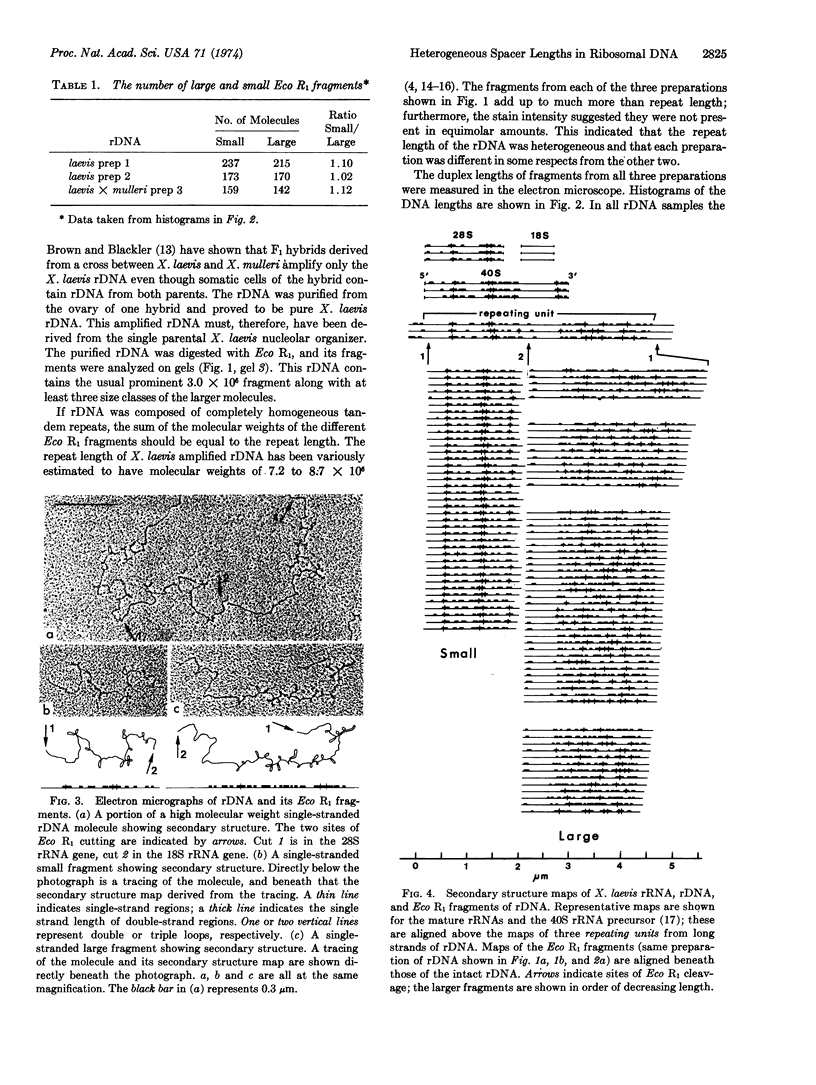
Eco R1 restriction endonuclease makes two cuts in each repeating unit of amplified ribosomal DNA (rDNA) from Xenopus laevis. The locations of these cuts have been established by comparison of the secondary structure of single-stranded Eco R1 fragments (as visualized in the electron microscope) with that of X. laevis rRNAs and of long strands of uncut rDNA. Of the two classes of fragments generated, the smaller one contains 90% of the 28S rRNA gene, has a duplex molecular weight of 3.0 × 106 and is homogeneous in size. The larger class of molecules contains 80% of the 18S rRNA gene and all of the nontranscribed spacer. These latter fragments are heterogeneous with molecular weights ranging from 4.0 to 5.9 × 106. The distribution of sizes within the large class of fragments varies among different preparations of rDNA, and heterogeneity is present in the DNA amplified from the rDNA of a single nucleolar organizer. The heterogeneity is located in the nontranscribed spacer region which is variable in length. This has been demonstrated by the formation of deletion loops in heteroduplexes made between larger fragments of different lengths.
Keywords: Eco R1 restriction endonuclease, gel electrophoresis, electron microscopy, secondary structure maps, genes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. D., Blackler A. W. Gene amplification proceeds by a chromosome copy mechanism. J Mol Biol. 1972 Jan 14;63(1):75–83. doi: 10.1016/0022-2836(72)90522-0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. The structure and evolution of ribosomal and 5S DNAs in Xenopus laevis and Xenopus mulleri. Cold Spring Harb Symp Quant Biol. 1974;38:501–505. doi: 10.1101/sqb.1974.038.01.054. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol. 1972 Jan 14;63(1):57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Extrachromosomal nucleolar genes in amphibian oocytes. Genetics. 1969;61(1 Suppl):133–143. [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of ribosomal RNA and its precursors as determined by electron microscopy. Cold Spring Harb Symp Quant Biol. 1974;38:525–535. doi: 10.1101/sqb.1974.038.01.057. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Brown D. D. Denaturation map of the ribosomal DNA of Xenopus laevis. J Mol Biol. 1971 Sep 14;60(2):235–247. doi: 10.1016/0022-2836(71)90290-7. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]