Abstract
In Colombia, a laboratory-based surveillance of invasive Streptococcus pneumoniae isolates as part of SIREVA II PAHO has been conducted since 1994. This study describes the serotype distribution, antimicrobial resistance, and genetic relationships of pneumococcal isolates recovered in Colombia from 2005 to 2010. In this study, demographic data of invasive S. pneumoniae isolates were analyzed, and antimicrobial susceptibility patterns were determined. Pulse field gel electrophoresis (n = 629) and multilocus sequence typing (n = 10) were used to determine genetic relationship of isolates with minimal inhibitory concentration to penicillin ≥0.125 µg/mL. A total of 1775 isolates of S. pneumoniae were obtained. Fifteen serotypes accounted for 80.7% of isolates. Serotype 14 (23.1%) was the most frequent in the general population. Penicillin resistance was 30.7% in meningitis and 9.0% in non-meningitis. Clones Spain6BST90, Spain9VST156, Spain23FST81, and Colombia23FST338 were associated to isolates. Additionally, serotype 6A isolates were associated with ST460 and ST473, and 19A isolates with ST276, ST320, and ST1118. In conclusion, the surveillance program provided updated information of trends in serotype distribution, antimicrobial resistance and the circulation of clones in invasive pneumococcal diseases. These results could be helpful to understand the epidemiology of S. pneumoniae in Colombia, and provide a baseline to measure the impact of vaccine introduction.
Introduction
Streptococcus pneumoniae is an important cause of invasive pneumococcal disease (IPD), as pneumonia, meningitis, and sepsis [1]. At present, over 95 serotypes have been described [2]–[5], which present variation in IPD, age groups, virulence and geographical distribution [6]. Serotype distribution has changed in countries that have implemented pneumococcal conjugate vaccines (PCV), as PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F), and currently available vaccines, PCV10 (PVC7 serotypes plus serotypes 1, 5, 7F) and PCV13 (PCV10 serotypes plus serotypes 3, 6A, 19A). Although vaccine serotypes have declined after PCVs introduction, non-PCV serotypes have increased in IPD [7]–[9]. Additionally, for over three decades antibiotic-resistant strains of S. pneumoniae have increased worldwide [9], which has been related with the spread or emergence of clones [10]. In 2009, PCV7 universal vaccination started in children <2 years of age with a 2+1 dose schedule in 12 regions of Colombia, including Bogotá. Subsequently, PCV7 was switched to PCV10 in 2010, and extended to the whole country [11], [12].
A laboratory-based passive surveillance of invasive S. pneumoniae isolates as part of SIREVA II PAHO has been conducted in Colombia, since 1994 [13]. The aim of this study was to determine serotype distribution, antimicrobial susceptibility and genetic relationships in Colombian pneumococcal isolates recovered from 2005 to 2010.
Materials and Methods
S. pneumoniae Isolates
Invasive isolates studied were received at the Instituto Nacional de Salud in Colombia as part of a passive surveillance, through the IPD National Surveillance (SIREVA II– program) from January 2005 to December 2010. A case of IPD was defined as isolation of S. pneumoniae from a normally sterile body site such as blood, cerebrospinal fluid or other normally sterile body fluid. The isolates were identified by morphology, optochin sensitivity and bile solubility. The present study includes 395 invasive isolates from children <2 years, which were previously studied and published by Parra et al. [14].
Serotyping and Antibiotic Susceptibility Testing
Serotype was determined by Quellung reaction. Antimicrobial susceptibility tests were performed by Kirby-Bauer disk diffusion and the broth micro-dilution methods to penicillin (PEN), ceftriaxone (CRO), trimethoprim-sulfamethoxazole (SXT), chloramphenicol (CHL), tetracycline (TET), erythromycin (ERY) and vancomycin (V). The Clinical and Laboratory Standards Institute (CLSI) guidelines were used [15]. Resistance to penicillin and ceftriaxone was analyzed by diagnosis of meningitis and non-meningitis. Multi drug resistance (MDR) was defined as resistant to three or more antibiotic classes.
Molecular Characterization
Isolates with minimal inhibitory concentration (MIC) to penicillin ≥0.125 µg/mL, were characterized by pulsed-field gel electrophoresis (PFGE), according to a previous report [16]. R6 strain and representative strains of clones Spain9VST156, Spain23FST81, Spain6BST90 and Colombia23FST338 were used as reference. PFGE patters were analyzed with the Gelcompare II software (Copyright Applied Maths 1998–2005). A dendrogram was constructed by the unweighted-pair group method with arithmetic means (UPGMA), using the Dice similarity coefficient, an optimization value of 1.5% and a tolerance position of 1.3%. Clusters of PFGE patterns exhibiting similarity of >75% were designated by capital letters. Sequence type (ST) from representative isolates in each clonal group was determined according to Enright et al [17]. Isolates for MLST were selected according the PFGE clusters and serotype. Software available on the MLST Web site was used to analyze genetic profiles [18].
Statistical Analysis
Analysis data of serotypes and IPD were stratified by age groups (<2, 2 to <5, 5 to 14, 15 to 29, 30 to 49, 50 to 64 and ≥65 years). Data were analyzed using Microsoft Excel™ and Statistical Package for Social Sciences (SPSS) software® (version 18). Statistical significance differences were assessed using Chi-square test with a significance level of <0.05.
Results
A total of 1775 S. pneumoniae invasive isolates were collected by 26 Public Health Laboratories and the Capital District. Ten Political Administrative Divisions of Colombia provided 95.4% of isolates (Figure S1). Among 1775 isolates, 59.4% were from male patients. Isolates were recovered more frequently in children <2 years (30.8%) of age and adults ≥50 (22.8%) (Table 1).
Table 1. General distribution of S. pneumoniae isolates by age group recovered in Colombia from 2005 to 2010.
| Year | Age group in years | |||||||
| <2 | 2 to <5 | 5 to 14 | 15 to 29 | 30 to 49 | 50 to 64 | ≥65 | Total | |
| n (%) | ||||||||
| 2005 | 85(30.6) | 30(10.8) | 29(10.4) | 28(10.1) | 46(16.5) | 23(8.3) | 37(13.3) | 278 |
| 2006 | 104(35.3) | 34(11.5) | 33(11.2) | 23(7.8) | 43(14.6) | 26(8.8) | 32(10.8) | 295 |
| 2007 | 110(32.8) | 37(11.0) | 32(9.6) | 28(8.4) | 48(14.3) | 39(11.6) | 41(12.2) | 335 |
| 2008 | 100(38.8) | 34(13.2) | 23(8.9) | 17(6.6) | 36(14.0) | 24(9.3) | 24(9.3) | 258 |
| 2009 | 68(25.9) | 35(13.3) | 39(14.8) | 21(8.0) | 38(14.4) | 31(11.8) | 31(11.8) | 263 |
| 2010 | 80(23.1) | 46(13.3) | 40(11.6) | 38(11.0) | 45(13.0) | 47(13.6) | 50(14.5) | 346 |
| Total | 547(30.8) | 216(12.2) | 196(11.0) | 155(8.7) | 256(14.4) | 190(10.7) | 215(12.1) | 1775 |
Isolates were recovered from blood (70.2%), cerebrospinal fluid (23.8%) and others (6.0%). Pneumonia (37.6%), meningitis (26%), and sepsis (24%) were the most common diagnoses. Pneumonia was the most frequent diagnosis in all age groups. In the <2 years group meningitis (31.8%) was the second diagnosis more frequent, while in the 2 to <5 and ≥50 age groups, sepsis was the second more important diagnosis with a percentage of 20.8% and 30.1%, respectively (Table 2).
Table 2. General distribution of S. pneumoniae isolates by diagnosis and age group.
| Diagnosis | Age group | Total | ||||||
| <2 | 2 to <5 | 5 to 14 | 15 to 29 | 30 to 49 | 50 to 64 | ≥65 | ||
| Pneumonia | 192(35.1) | 107(49.5) | 65(33.2) | 55(35.5) | 91(35.5) | 63(33.2) | 95(44.2) | 668(37.6) |
| Meningitis | 174(31.8) | 23(10.6) | 60(30.6) | 51(32.9) | 81(31.6) | 47(24.7) | 27(12.6) | 463(26.1) |
| Sepsis | 114(20.8) | 60(27.8) | 43(21.9) | 35(22.6) | 57(22.3) | 56(29.5) | 66(30.7) | 431(24.3) |
| Others | 67(12.2) | 26(12.0) | 28(14.3) | 14(9.0) | 27(10.5) | 24(12.6) | 27(12.6) | 213(12.0) |
| Total | 547 | 216 | 196 | 155 | 256 | 190 | 215 | 1775 |
Others included febrile syndrome n = 88 (5%), bacteremia n = 30 (1.7%), and without a specific pneumococcal diagnosis, but recovered by blood culture n = 99 (5.6%).
S. pneumoniae Serotype Distribution
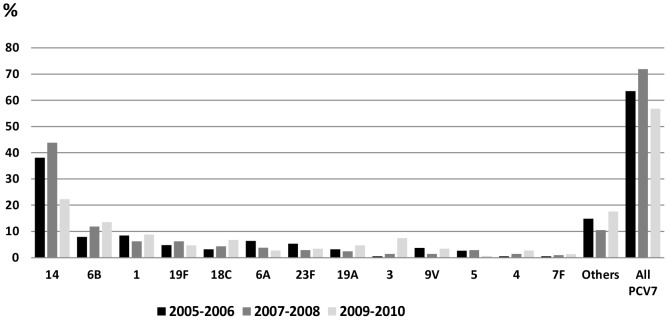
A total of fifty-six serotypes were identified, and fifteen serotypes accounted for 80.7% of the total isolates: 14 (23.1%), 1 (12.2%), 6B (7%), 3 (5.5%), 23F (4.8%), 19F (4.7%), 6A (4.1%), 5 (3.5%), 19A (3.2%), 18C (2.7%), 9V (2.7%), 7F (2.0%),12F (1.9%), 4 (1.9%), and 16F (1.6%). The other serotypes presented a proportion of 19.3% (Table S1). Figure 1 shows the frequency of these serotypes in 2005–2006, 2007–2008 and 2009–2010. Among serotypes that presented a significant decrease from 2007–2008 to 2009–2010 were serotype 14, which changed from 27.7% to 18.2% (p<0.001) and serotype 5 from 4.0% to 1.5% (p<0.001). In this same period serotypes 3 and 19A increased from 4.4% to 7.2% (p = 0.01) and 2.9% to 4.9% (p = 0.03), respectively. The other serotypes changed from 17.8% in 2005–2006 to 21.5% in 2009–2010 (p = 0.05) (Figure 1).
Figure 1. Annual frequency of serotypes recovered in Colombia from 2005 to 2010.
*In other were included 41 serotypes with a frequency less than 1.1%. The number and frequency of all isolates are shown in the Table S1.
Serotype 14 was the most prevalent in <2 and 2 to <5 age groups. Serotype 1 was mainly observed in the 2 to 5 (15.7%), 5 to 14 (23.5%), 15 to 29 (15.2%) 30 to 49 age groups. Serotype 3 was the most frequent in adults ≥50 (11.9%) (Table 3). Rank order of the most common serotypes from patients with pneumonia were 14 (28.3%), 1 (15.1%), 3 (6.0%), 6B (5.4%), 5 (5.1%) and 19A (3.6%); whereas, in meningitis the most frequent serotypes were 14 (15.3%), 6B (10.2%), 23F (8.0%), 19F (7.6%), 1 (6.7%) and 6A (5.8%) (Table S2).
Table 3. General distribution of serotypes by age group recovered in Colombia, 2005–2010.
| Serotypes | Age groups in years | Total | ||||||
| <2 | 2 to <5 | 5 to 14 | 15 to 29 | 30 to 49 | 50 to 64 | ≥65 | ||
| 14 | 197(36.0) | 87(40.3) | 25(12.8) | 25(16.1) | 35(13.7) | 19(10.0) | 22(10.2) | 410 |
| 1 | 42(7.7) | 34(15.7) | 46(23.5) | 24(15.5) | 34(13.3) | 23(12.1) | 13(6.0) | 216 |
| 6B | 60(11.0) | 11(5.1) | 12(6.1) | 8(5.2) | 12(4.7) | 7(3.7) | 14(6.5) | 124 |
| 3 | 15(2.7) | 6(2.8) | 9(4.6) | 7(4.5) | 12(4.7) | 21(11.1) | 27(12.6) | 97 |
| 23F | 21(3.8) | 12(5.6) | 10(5.1) | 10(6.5) | 12(4.7) | 9(4.7) | 12(5.6) | 86 |
| 19F | 29(5.3) | 7(3.2) | 11(5.6) | 7(4.5) | 13(5.1) | 10(5.3) | 6(2.8) | 83 |
| 6A | 24(4.4) | 5(2.3) | 13(6.6) | 4(2.6) | 7(2.7) | 7(3.7) | 12(5.6) | 72 |
| 5 | 12(2.2) | 6(2.8) | 5(2.6) | 6(3.9) | 12(4.7) | 9(4.7) | 12(5.6) | 62 |
| 19A | 18(3.3) | 18(8.3) | 3(1.5) | 2(1.3) | 8(3.1) | 3(1.6) | 4(1.9) | 56 |
| 18C | 25(4.6) | 5(2.3) | 6(3.1) | 4(2.6) | 4(1.6) | 2(1.1) | 2(0.9) | 48 |
| 9V | 15(2.7) | 4(1.9) | 7(3.6) | 4(2.6) | 8(3.1) | 4(2.1) | 6(2.8) | 48 |
| 7F | 5(0.9) | 2(0.9) | 2(1.0) | 7(4.5) | 9(3.5) | 3(1.6) | 7(3.3) | 35 |
| 12F | 5(0.9) | 0(0.0) | 0(0.0) | 1(0.6) | 9(3.5) | 11(5.8) | 8(3.7) | 34 |
| 4 | 8(1.5) | 0(0.0) | 0(0.0) | 4(2.6) | 7(2.7) | 7(3.7) | 7(3.5) | 33 |
| 16F | 5(0.9) | 4(1.9) | 2(1.0) | 3(1.9) | 4(1.6) | 5(2.8) | 6(2.7) | 29 |
| Others* | 66(12.1) | 15(6.9) | 45(23.0) | 39(25.2) | 70(27.3) | 50(26.3) | 57(26.4) | 342 |
| Total | 547(30.8) | 216(12.2) | 196(11.0) | 155(8.7) | 256(14.4) | 190(10.8) | 215(12.1) | 1775 |
Others included a total of 41 serotypes, frequency less to 1.1%.
Isolate distribution in children <2 years old presented a frequency of 38.8% in 2008, and it decreased to 25.9% and 23.1% in 2009 and 2010, respectively (Table 1). Additionally, in this group the frequency of PCV7 serotypes changed from 71.9% in 2007–2008 to 56.8% in 2009–2010 (p<0.001). This change was mainly due to a decrease in serotype 14, which changed from 43.8% (2007–2008) to 22.3% (2009–2010) (p<0.001). PCV10 and PCV13 frequency serotypes were 75.7% and 86.1%, respectively. Serotypes 1 and 3 increased from 6.2% to 8.8% (p = 0.18), and from 1% to 7.4% (p<0.001), respectively (Figure 2).
Figure 2. Serotypes frequency in children <2 years old, 2005–2010.
Penicillin and Ceftriaxone Susceptibility
Penicillin resistance was 30.7% (MIC50/90 0.03/2.0 µg/mL) in meningitis, and 9.0% in non-meningitis (intermediate 8.6% and high 0.4%; MIC50/90 of 0.03/2.0 µg/mL). Resistance to ceftriaxone was 15.7% in meningitis (intermediate 12.5% and high 3.2%) and 8.5% in non-meningitis (Table S3). Serotypes related with resistance to penicillin were 14 (60.0%), 23F (10.0%), 6B (6.5%), 19A (6.5%), 9V (4.2%), 6A (3.5%), 19F (3.5%) and other serotypes (n = 13) (5.8%).
Susceptibility to other Antimicrobials
Isolates showed resistance to SXT (43.8%), TET (17.1%), ERY (6.9%) and CHL (3.7%). All isolates were susceptible to vancomycin (Table S3). Resistance to ERY increased from 7.6% (2009) to 9.8% (2010) (p = 0.17), associated to serotypes 6A (21.1%), 19A (20.3%), 6B (19.5%), 14 (13.0%) and other serotypes (n = 12) (26.1%). The overall rate of MDR was 7.3% (n = 130 isolates), 9.7% in meningitis and 6.4% in non-meningitis. The most common MDR patterns were PEN-SXT-TET (16.2%), followed by PEN-CRO-SXT-ERY-TET (13%) and CHL-SXT-TET (10.8%).
Molecular Characterization
From a total of 629 isolates (MIC PEN ≥0.125 µg/mL) typed by PFGE, 85.4% belonged to one of 11 clusters (A–K) (Table 4 and figure S2). Cluster A, grouped mainly isolates 23F related to the Spain23FST81 clone, and serotypes 19A and 19F. Except for the 19A isolate, all isolates were MDR. Cluster B presented relation to Spain9VST156 clone, which grouped serotypes 9V, 14, 19A and 23F and MDR was found in 18 (4.8%) isolates. Cluster C related to Colombia23FST338 was associated mainly with isolates serotype 23F. This cluster also associated serotype 19A (a single locus variant of ST338), 19F, 23A, 23B, and MDR was observed in 21.2% (n = 7) of isolates. In cluster D, only serotype 6B isolates associated with Spain6BST90 clone were observed, which presented MDR of 78.3%. In clusters E, F and G, formed by serotypes 19A, related to ST320, ST1118 and ST276, respectively, all isolates associated to ST320 and ST276 were MDR, and in isolates grouped to ST1118 MDR was not observed. Clusters H and I included serotype 6A isolates. Cluster H, conformed by PEN and ERY resistant isolates were related to ST473, and cluster I, PEN and SXT resistant isolates associated to ST460. Clusters J and K grouped mainly with isolates serotype 14 (Table 4).
Table 4. Serotype distribution and genetic association in isolates with MIC values ≥0.125 (n = 629).
| PFGE patterns | Clone and ST | Serotypes | ||||||||
| 14 | 23F | 6B | 19A | 9V | 6A | 19F | Other*** | Total | ||
| A | Spain23FST81 | 0 | 12 | 0 | 1 | 0 | 0 | 1 | 0 | 14 |
| B | Spain9VST156 | 348 | 1 | 0 | 2* | 30 | 0 | 0 | 0 | 381 |
| C | Colombia23FST338 | 0 | 27 | 0 | 1 | 0 | 0 | 3 | 2 | 33 |
| D | Spain6BST90 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 23 |
| E | ST320 | 0 | 0 | 0 | 8 | 0 | 0 | 2 | 0 | 10 |
| F | ST1118 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 1 | 7 |
| G | ST276 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 10 |
| H | ST473 | 1 | 0 | 0 | 0 | 0 | 15 | 0 | 1 | 17 |
| I | ST460 | 2 | 0 | 0 | 0 | 0 | 10 | 0 | 3 | 15 |
| J | – | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| K | – | 13 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 18 |
| NR** | – | 12 | 10 | 18 | 9 | 2 | 7 | 13 | 21 | 92 |
| Total | 385 | 50 | 41 | 37 | 32 | 32 | 20 | 32 | 629 | |
One isolate was confirmed as ST156.
NR: unrelated isolates in specific cluster groups among isolates into dendrogram.
Other: the dendrogram shows these serotypes.
Note: the dendrogram of isolates is shown in the figure S2.
Discussion
This study described serotype distribution, antimicrobial resistance and circulation of clones associated with penicillin non-susceptibility, in invasive S. pneumoniae isolates recovered in Colombia from 2005 to 2010. The result showed that pneumonia and meningitis were the most frequent IPD. Both diagnoses have been reported as the most frequent in Colombia [13], as well as in Latin America and the Caribbean region, with an annual burden of 327,000 pneumonia cases, nearly 4,000 cases of meningitis and 1,229 cases of pneumococcal sepsis [19]. Even though sepsis was the second most common diagnosis in 2–6 and ≥50 years age patient, this could be due to variations in clinical definition of syndromes informed in the pneumococcal surveillance, where pneumococcal community-acquired pneumonia and meningitis are leading causes of sepsis [20], [21].
In general, serotypes 14, 1, 6B, 23F, 19F and 6A were the most frequent, similar to a study published previously in Colombia [13] and worldwide [22], [23]. Serotype 14 was the most prevalent in the national surveillance, and it was the main cause of IPD in children <5 years age. In a systematic review, realized to estimate the global and regional distributions of serotypes causing IPD in children <5 years of age, serotype 14 was the most common serotype accounting for 12%–29% of IPD worldwide [23]. Serotype 1 was a frequent cause of IPD in older children; this serotype has a lower propensity to cause infections in the older age groups [24]. An increase in serotype 3 frequency was observed, which was mainly found in the ≥50 age group. This serotype has been associated with a relative risk of infection among middle-aged people up to a peak in older adults [25].
Serotype 14 continues as the main cause of IPD and most isolates were associated with Spain9VST156 clone, similar to a previous report [13]. This clone is resistant to PEN and SXT, and has become widely disseminated among invasive isolates [26]. Furthermore, serotype 19A and 19F variants were observed, which have already been identified in others studies, suggesting a high tendency of this clonal cluster to undergo capsular switching events [26]. Isolates serotype 23F were related genetically to Spain23FST81 clone; this clone presents resistance pattern to PEN, CHL, TET, and many isolates have additional resistance to fluoroquinolones and macrolides [27], [28]. This clone displays a high genetic variability and some isolates have switched their capsular type to 9N, 19A, 19F, 14, 15B, 3 and 6A over the last decades [29], [30]. Additionally, 23F isolates were associated to Colombia23FST338, a clone initially identified in Colombian isolates [31], and also have been reported in other countries [32]–[34]. MDR serotype 6B isolates were related to Spain6BST90 clone. These international clones continue circulating in Colombia and were associated with resistance to antimicrobials. The implementation of PCV may reduce the prevalence of these clones, leading to the spreading of non-vaccine clones or capsular variants of international drug-resistant clones [34], [35], and continuous surveillance will be necessary to follow future changes.
The incidence and prevalence of IPD cases caused by serotype 19A increased substantially in the post-PCV7 introduction era and has been associated with MDR [36]. During the surveillance period, serotype 19A increased from 1.5% to 4.9%. In Latin American and Caribbean countries, serotype19A remains a less common agent of IPD than other serotypes and a significant increase in frequency was noted only in Argentina and Colombia [37]. Serotype 19A isolates were found related to the ST230 and ST320. The ST230 represents the Denmark14-ST230 clone and is an important cause for the maintenance of antimicrobial resistance. This clone was reported in the preceding years to PCV7 introduction in Portugal [38] and has been detected in several countries, such as France and Spain [39], [40]. ST320 has become one of the major STs among 19A S. pneumoniae isolates in USA and Canada after the introduction of PCV7 [41] and is prevalent in several Asian countries [42]. Among serotype 6A isolates, ST473 was identified. The ST473 is a penicillin-non-susceptible and erythromycin-resistant clone which has been identified among serotypes 6A and 6C invasive and noninvasive isolates [43], [44].
Antimicrobial resistance in S. pneumoniae has been one of the main worldwide problems, especially penicillin and erythromycin non-susceptible isolates [9]. The rates of penicillin-resistant pneumococcal meningitis and non-meningitis in Colombia were lower than other Latin American countries such as Bolivia, Ecuador, Mexico, Peru and Venezuela [45]. In Colombia, antimicrobial resistance has been associated with the presence of multi-resistant clones [13]. The increase of non-PCV7 serotypes and clonal spread of serotype 19A (ST 230 and ST 320) and 6A (ST473) strains would be the main reasons for prevalence of erythromycin resistance in Colombia.
In 2009, PCV7 vaccination started in children less than 2 years of age in 12 regions of Colombia [11], [12]. A significant decline in the frequency of PCV7 vaccine serotypes in <2 years age group was observed, this could be associated with the PCV7 introduction in Colombia, similar to other countries where routine PCV7 use has resulted in a significant decrease or a near elimination of PCV7 serotypes in IPD for children [46], [47].
The limitations in this study were related to the fact that 81.3% of S. pneumoniae were sent from three Political Administrative Divisions of Colombia; therefore, the data may not reflect a national status of serotype distribution, antimicrobial resistance and genetic relations of isolates. However, the results may reflect an overall trend in the changes of S. pneumoniae in Colombia. Additionally, the molecular characterization was based on non-susceptible isolates and not on the total of isolates, which provide an incomplete clonal structure. Finally, serotype 6D is not differentiated from serotype 6B; thus, serotype 6D isolates could be included among serotype 6B isolates.
In conclusion, the surveillance program has been essential in assessing changes in antimicrobial resistance, serotypes and clones in resistant isolates. Continuous monitoring of IPD is necessary to measure the impact of PCV10/13 introduction and also to understand the Colombian epidemiology of S. pneumonia.
Supporting Information
Distribution of S. pneumoniae recovered from political administrative division of Colombia.
(TIF)
Genetic relationships dendrogram of Streptococcus pneumoniae isolates by PFGE.
(PDF)
General frequency of serotypes by years of surveillance.
(XLS)
Serotype distribution by diagnosis.
(XLS)
Susceptibility and minimum inhibitory concentrations in S. pneumoniae isolates in invasive pneumococcal diseases during 2005 and 2010.
(DOC)
Acknowledgments
The authors thank the Hospitals and regional laboratories for their cooperation in sending the isolates as part of the SIREVA II network. We Also thank Carolina Duarte for technical support, Elizabeth Castañeda (PhD and Emeritus Researcher, Instituto Nacional de Salud, Bogotá, Colombia) and Clara Ines Agudelo for their critical review of the manuscript. Additionally, the authors are grateful to the reviewers and editor for their comments, which were useful and constructive to improve the manuscript.
Funding Statement
The Sources of founding were obtainded from SIREVAII-PAHO and Instituto National de Salud of Colombia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lynch JP, Zhanel GG (2010) Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med 16: 217–25. [DOI] [PubMed] [Google Scholar]
- 2. Calix JJ, Nahm MH (2010) A new pneumococcal serotype, 11E, has a variably inactivated wcjE Gene. J Infect Dis 202: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calix JJ, Porambo RJ, Brady AM, Larson TR, et al. (2012) Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: Discovery of a new pneumococcal serotype. J Biol Chem 287: 27885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko KS, Baek JY, Song JH (2013) Capsular Gene Sequences and Genotypes of “Serotype 6E” Streptococcus pneumoniae Isolates. J Clin Microbiol 51: 3395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliver MB, van der Linden MP, Küntzel SA, Saad JS, Nahm MH (2013) Discovery of Streptococcus pneumoniae serotype 6 variants with glycosyltransferases synthesizing two differing repeating units. J Biol Chem 288: 25976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hausdorff WP, Feikin DR, Klugman KP (2005) Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 5: 83–93. [DOI] [PubMed] [Google Scholar]
- 7. Tan TQ (2012) Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev 25: 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberger DM, Malley R, Lipsitch M (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378: 1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reinert RR (2009) The antimicrobial resistance profile of Streptococcus pneumoniae . Clin Microbiol Infect 15 Suppl 37–11. [DOI] [PubMed] [Google Scholar]
- 10. Henriques-Normark B, Blomberg C, Dagerhamn J, Battig P, Normark S (2008) The rise and fall of bacterial clones: Streptococcus pneumoniae . Nat Rev Microbiol 6: 827–837. [DOI] [PubMed] [Google Scholar]
- 11.Ministerio de la Protección Social. Acuerdo Número 406 de 2009. Available: www.dssa.gov.co/index.php/./doc./460-acuerdo-406-neumococo. Accessed 13 November 2012.
- 12.Ministerio de Salud y Protección Social. Jornada de Vacunación en Las Americas 2012. Available: http://www.minsalud.gov.co/Lineamientos/Documento_Marco_Jornada_de_Vacunacion_de_las_Am%C3%A9ricas_abril2012.pdf. Accessed 13 November 2012.
- 13. Agudelo CI, Moreno J, Sanabria OM, Ovalle MV, Di Fabio JL, et al. (2006) Streptococcus pneumoniae: evolución de los serotipos y los patrones de susceptibilidad antimicrobiana en aislamientos invasores en 11 años de vigilancia en Colombia (1994–2004). Biomédica 26: 234–49. [PubMed] [Google Scholar]
- 14. Parra EL, De La Hoz F, Díaz PL, Sanabria O, Realpe ME, et al. (2013) Changes in Streptococcus pneumoniae serotype distribution in invasive disease and nasopharyngeal carriage after the heptavalent pneumococcal conjugate vaccine introduction in Bogotá, Colombia. Vaccine 31: 4033–8. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Laboratory Standards Institute (2010) Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement. CLSI document M100-S21. Wayne, PA: CLSI.
- 16. Vela MC, Fonseca N, Di Fabio JL, Castañeda E, et al. (2001) Presence of international multiresistant clones of Streptococcus pneumoniae in Colombia. Microb Drug Resist. 7: 153–64. [DOI] [PubMed] [Google Scholar]
- 17. Enright M, Spratt BG (1998) A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144: 3049–60. [DOI] [PubMed] [Google Scholar]
- 18.Aanensen DM, Spratt BG (2005) The multilocus sequence typing network: mlst.net Nucleic Acids Res. Web Server issue: W728–33. Available: http://spneumoniae.mlst.net/. Accessed 15 July 2013. [DOI] [PMC free article] [PubMed]
- 19.Constenla D, Gomez E, de la Hoz FP, O’Loughlin R, Sinha A, et al. (2007) The burden of pneumococcal disease and cost-effectiveness of a pneumococcal vaccine in Latin America and the Caribbean: a review of the evidence and a preliminary economic analysis. Report. Washington, DC: A collaborative project of: The Albert B. Sabin Vaccine Institute, Pan American Health Organization, GAVI’s Pneumococcal Accelerated Development and Introduction Plan at Johns Hopkins (PneumoADIP) Centers for Disease Control and Prevention. Available: http://www.ispch.cl/sites/default/files/document1.pdf). Accessed 05 September 2013.
- 20. Schaaf B, Kruse J, Rupp J, Reinert RR, Droemann D, et al. (2007) Sepsis severity predicts outcome in community-acquired pneumococcal pneumonia. Eur Respir J 30: 517–24. [DOI] [PubMed] [Google Scholar]
- 21. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J (2006) Attenuated cerebrospinal fluid leukocyte count and sepsis in adults with pneumococcal meningitis: a prospective cohort study. BMC Infect Dis 6: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hausdorff WP, Bryant J, Paradiso PR, Siber GR (2000) Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 30: 100–21. [DOI] [PubMed] [Google Scholar]
- 23.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Hance F, et al.. (2010) Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 7. doi:pii: e1000348. [DOI] [PMC free article] [PubMed]
- 24. Horácio AN, Diamantino-Miranda J, Aguiar SI, Ramirez M, Melo-Cristino J (2012) Serotype changes in adult invasive pneumococcal infections in Portugal did not reduce the high fraction of potentially vaccine preventable infections. Vaccine 30: 218–224. [DOI] [PubMed] [Google Scholar]
- 25. Scott JA, Hall AJ, Dagan R, Dixon JM, Eykyn SJ, et al. (1996) Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis 22: 973–81. [DOI] [PubMed] [Google Scholar]
- 26. Sjöström K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, et al. (2007) Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci USA 104: 12907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pletz MW, McGee L, Jorgensen J, Beall B, Facklam RR, et al. (2004) Levofloxacin-resistant invasive Streptococcus pneumoniae in the United States: evidence for clonal spread and the impact of conjugate pneumococcal vaccine. Antimicrob Agents Chemother 48: 3491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reinert RR, Ringelstein A, van der Linden M, Cil MY, Al-Lahham A, et al. (2005) Molecular epidemiology of macrolide-resistant Streptococcus pneumoniae isolates in Europe. J Clin Microbiol 43: 1294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiller NL, Eutsey RA, Powell E, Earl JP, Janto B, et al. (2011) Differences in genotype and virulence among four multidrug-resistant Streptococcus pneumoniae isolates belonging to the PMEN1 clone. PLoS One. 6: e28850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baek JY, Ko KS, Kim SH, Kang CI, Chung DR, et al. (2011) Comparison of genotypes of Streptococcus pneumoniae serotypes 6A and 6B before and after the introduction of PCV7 vaccination in Korea. Diagn Microbiol Infect Dis. 69: 370–5. [DOI] [PubMed] [Google Scholar]
- 31. Sá-Leão R, Tomasz A, Sanches IS, Brito-Avô A, Vilhelmsson SE, et al. (2000) Carriage of internationally spread clones of Streptococcus pneumoniae with unusual drug resistance patterns in children attending day care centers in Lisbon, Portugal. J Infect Dis 182: 1153–60. [DOI] [PubMed] [Google Scholar]
- 32. Hsieh YC, Huang YC, Lin HC, Ho YH, Chang KY, et al. (2009) Characterization of invasive isolates of Streptococcus pneumoniae among Taiwanese children. Clin Microbiol Infect 15: 991–6. [DOI] [PubMed] [Google Scholar]
- 33. Serrano I, Melo-Cristino J, Carriço JA, Ramirez M (2005) Characterization of the genetic lineages responsible for pneumococcal invasive disease in Portugal. J Clin Microbiol 43: 1706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gherardi G, D’Ambrosio F, Visaggio D, Dicuonzo G, Del Grosso M, et al. (2012) Serotype and clonal evolution of penicillin-nonsusceptible invasive Streptococcus pneumoniae in the 7-valent pneumococcal conjugate vaccine era in Italy. Antimicrob Agents Chemother 56: 4965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gherardi G, Brueggemann AB, Pai R, Crook DW, Beall B (2007) Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 3: e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reinert R, Jacobs MR, Kaplan SL (2010) Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine 28: 4249–59. [DOI] [PubMed] [Google Scholar]
- 37. Castañeda E, Agudelo CI, De Antonio R, Rosselli D, Calderón C, et al. (2012) Streptococcus pneumoniae serotype 19A in Latin America and the Caribbean: a systematic review and meta-analysis, 1990–2010. BMC Infect Dis 12: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aguiar SI, Pinto FR, Nunes S, Serrano I, Melo-Cristino J, et al. (2010) Denmark14–230 clone as an increasing cause of pneumococcal infection in Portugal within a background of diverse serotype 19A lineages. J Clin Microbiol 48: 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahjoub-Messai F, Doit C, Koeck JL, Billard T, Evrard B, et al. (2009) Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal Vaccination for French children. J Clin Microbiol 47: 837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muñoz-Almagro C, Ciruela P, Esteva C, Marco F, Navarro M, et al. (2011) Serotypes and clones causing invasive pneumococcal disease before the use of new conjugate vaccines in Catalonia, Spain. J Infect. 63: 151–62. [DOI] [PubMed] [Google Scholar]
- 41. Pillai DR, Shahinas D, Buzina A, Pollock RA, Lau R, et al. (2009) Genome-wide dissection of globally emergent multi-drug resistant serotype 19A Streptococcus pneumoniae . BMC Genomics 10: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin J, Baek JY, Kim SH, Song JH, Ko KS (2011) Predominance of ST320 among Streptococcus pneumoniae serotype 19A isolates from 10 Asian countries. J Antimicrob Chemother 66: 1001–4. [DOI] [PubMed] [Google Scholar]
- 43. Rolo D, Fenoll A, Ardanuy C, Calatayud L, Cubero M, et al. (2011) Trends of invasive serotype 6C pneumococci in Spain: emergence of a new lineage. J Antimicrob Chemother 66: 1712–8. [DOI] [PubMed] [Google Scholar]
- 44. Carvalho Mda G, Pimenta FC, Gertz RE Jr, Joshi HH, Trujillo AA, et al. (2009) PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J Clin Microbiol 47: 554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonias y Meningitis Bacterianas. Informe Regional de SIREVA II, (2012). Available: http://new.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953&lang=. Accessed, 02 September 2013.
- 46. Leal J, Vanderkooi OG, Church DL, Macdonald J, Tyrrell GJ, et al. (2012) Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J 31: e169–75. [DOI] [PubMed] [Google Scholar]
- 47. Grall N, Hurmic O, Al Nakib M, Longo M, Poyart C, et al. (2011) Epidemiology of Streptococcus pneumonia in France before introduction of the PCV-13 vaccine. Eur J Clin Microbiol Infect Dis 30: 1511–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of S. pneumoniae recovered from political administrative division of Colombia.
(TIF)
Genetic relationships dendrogram of Streptococcus pneumoniae isolates by PFGE.
(PDF)
General frequency of serotypes by years of surveillance.
(XLS)
Serotype distribution by diagnosis.
(XLS)
Susceptibility and minimum inhibitory concentrations in S. pneumoniae isolates in invasive pneumococcal diseases during 2005 and 2010.
(DOC)



