Abstract
The structural heterogeneity of rabbit lung collagen was examined by extracting labeled collagen from short-term cultures of lung minces with 1 M NaCl-50 mM Tris·HCl (pH 7.4), 0.5 M acetic acid, or 0.4 ionic strength phosphate buffer. The extracted collagens were purified by carboxymethyl-cellulose chromatography, and their cyanogen bromide peptides were mapped by ion exchange chromatography and acrylamide gels. Rabbit skin α1(I) and α2 chains and rabbit sternal cartilage α1(II) chains were used as markers.
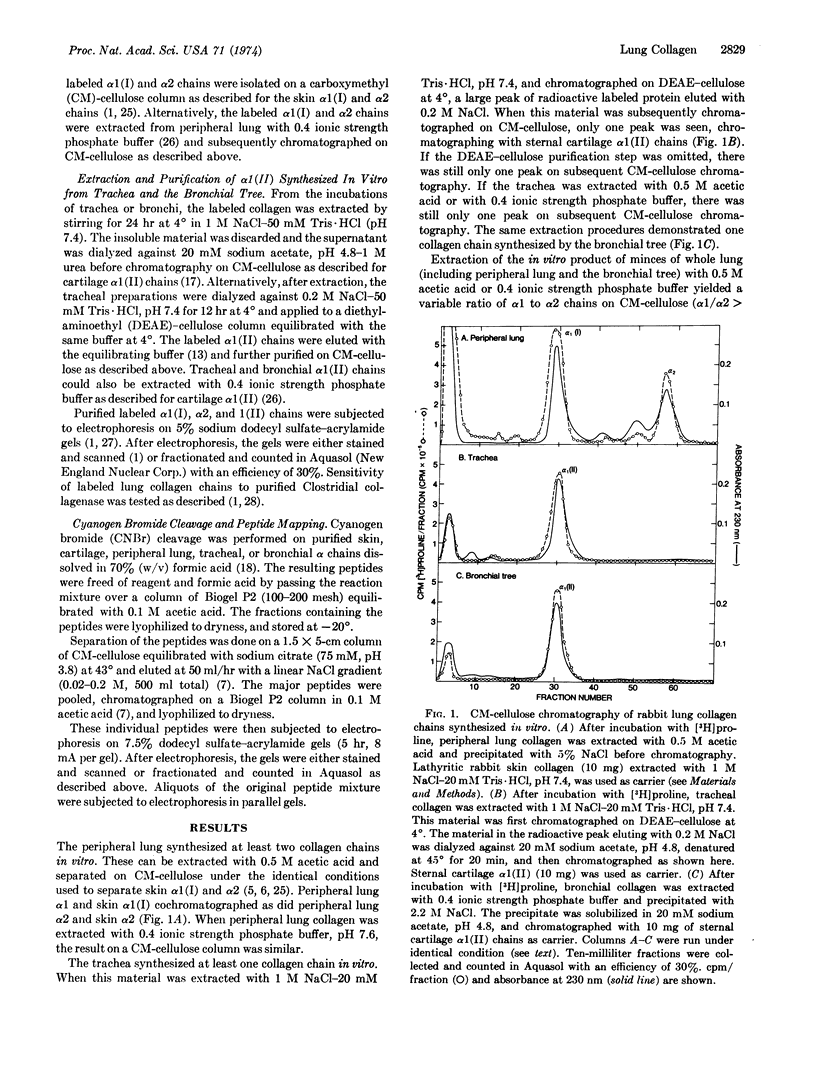
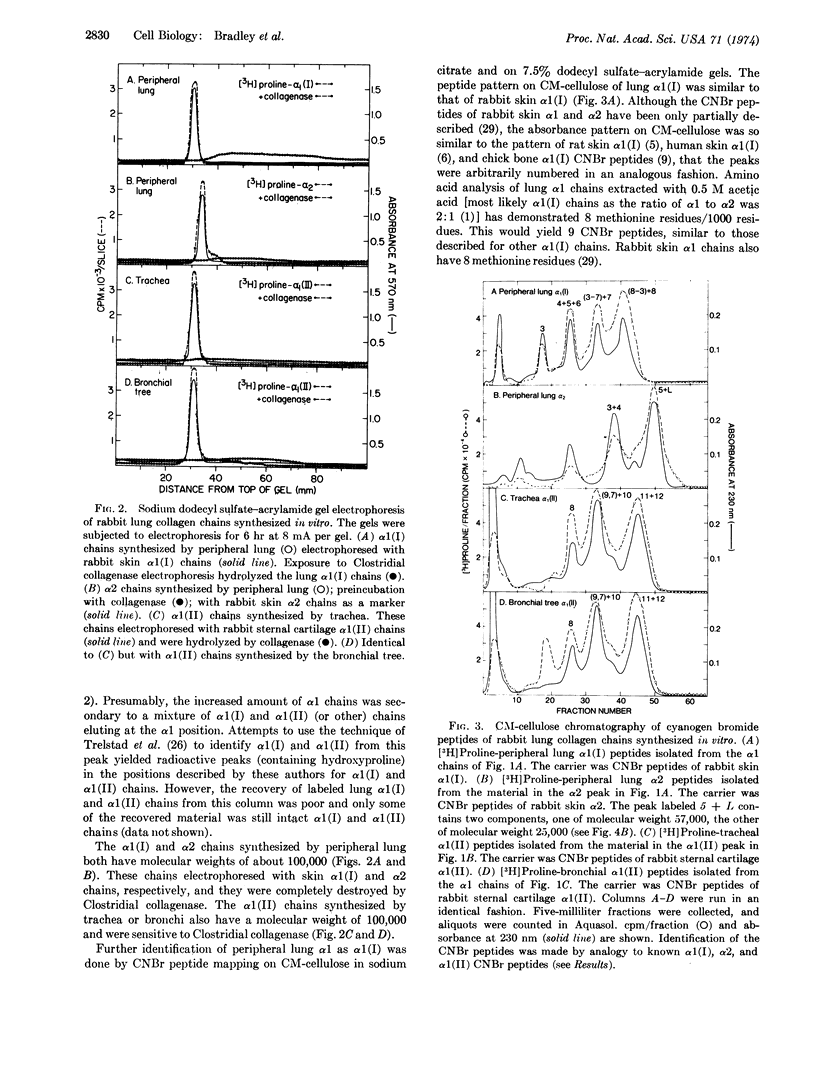
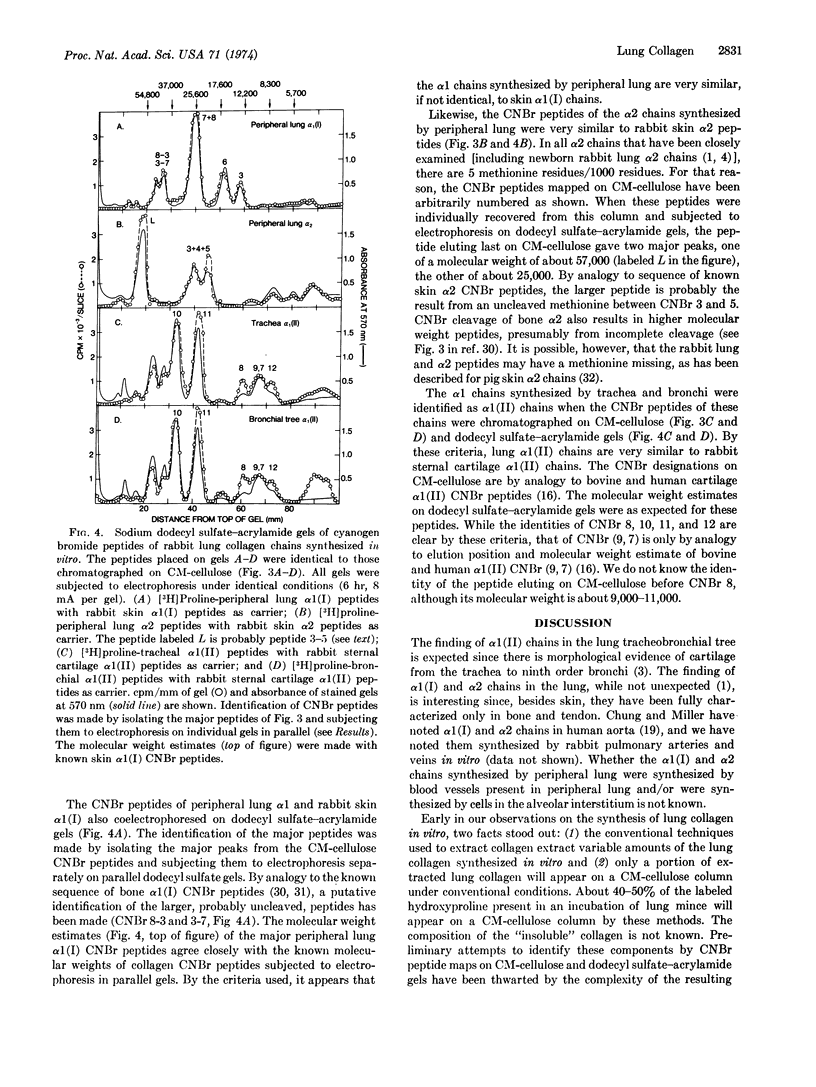
The peripheral lung, containing alveoli, small blood vessels, and small airways, synthesized α1(I) and α2 chains. The trachea and the bronchial tree (first through seventh order branches) both synthesized α1(II) chains. Lung α1(I), α2, and α1(II) chains all have a molecular weight of about 100,000 and are all sensitive to Clostridial collagenase.
The extraction and purification methods used isolate only 50% of the collagen synthesized by these structures in vitro. Once all collagen types in lung can be described and quantitated, it should be possible to utilize lung collagen types as biochemical markers to study normal lung development and to define the lung fibrotic diseases.
Keywords: protein synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P. Comparative sequence studies of rat skin and tendon collagen. I. Evidence for incomplete hydroxylation of individual prolyl residues in the normal proteins. Biochemistry. 1967 Oct;6(10):3082–3093. doi: 10.1021/bi00862a015. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Nesse R. The comparative biochemistry of collagen: the structure of rabbit skin colllagen and its relevance to immunochemical studies of collagen. Arch Biochem Biophys. 1970 Jun;138(2):443–450. doi: 10.1016/0003-9861(70)90367-x. [DOI] [PubMed] [Google Scholar]
- Bradley K. H., McConnell S. D., Crystal R. G. Lung collagen composition and synthesis. Characterization and changes with age. J Biol Chem. 1974 May 10;249(9):2674–2683. [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Click E. M., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha 1 and alpha 2 chains of human skin collagen. Biochemistry. 1970 Nov 24;9(24):4699–4706. doi: 10.1021/bi00826a012. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. Isolation and characterization of cyanogen bromide peptides from the collagen of bovine articular cartilage. Biochem J. 1973 Aug;133(4):615–622. doi: 10.1042/bj1330615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Kefalides N. A., Prockop D. J. The biosynthesis of basement membrane collagen in embryonic chick lens. II. Synthesis of a precursor form by matrix-free cells and a time-dependent conversion to chains in intact lens. J Biol Chem. 1972 Jun 10;247(11):3545–3551. [PubMed] [Google Scholar]
- Grant M. E., Schofield J. D., Kefalides N. A., Prockop D. J. The biosynthesis of basement membrane collagen in embryonic chick lens. 3. Intracellular formation of the triple helix and the formation of aggregates through disulfide bonds. J Biol Chem. 1973 Nov 10;248(21):7432–7437. [PubMed] [Google Scholar]
- Heinrich W., Lange P. M., Stirtz T., Iancu C., Heidemann E. Isolation and characterization of the large cyanogen bromide peptides from the alpha1- and alpha2-chains of pig skin collagen. FEBS Lett. 1971 Jul 15;16(1):63–67. doi: 10.1016/0014-5793(71)80687-7. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Trelstad R. L., Gross J. The collagen of chick embryonic notochord. Biochem Biophys Res Commun. 1973 Jul 2;53(1):39–45. doi: 10.1016/0006-291x(73)91397-1. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Trelstad R. L., Toole B. P., Gross J. The collagen of osteogenic cartilage in the embryonic chick. Biochem Biophys Res Commun. 1973 Jun 8;52(3):870–876. doi: 10.1016/0006-291x(73)91018-8. [DOI] [PubMed] [Google Scholar]
- Low F. N. Extracellular components of the pulmonary alveolar wall. Arch Intern Med. 1971 May;127(5):847–852. [PubMed] [Google Scholar]
- Miller E. J., Epstein E. H., Jr, Piez K. A. Identification of three genetically distinct collagens by cyanogen bromide cleavage of insoluble human skin and cartilage collagen. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1024–1029. doi: 10.1016/0006-291x(71)90006-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lane J. M., Piez K. A. Isolation and characterization of the peptides derived from the alpha-1 chain of chick bone collagen after cyanogen bromide cleavage. Biochemistry. 1969 Jan;8(1):30–39. doi: 10.1021/bi00829a006. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Woodall D. L., Vail M. S. Biosynthesis of cartilage collagen. Use of pulse labeling to order the cyanogen bromide peptides in the alpha L(II) chain. J Biol Chem. 1973 Mar 10;248(5):1666–1671. [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Nold J. G., Kang A. H., Gross J. Collagen molecules: distribution of alpha chains. Science. 1970 Dec 4;170(3962):1096–1098. doi: 10.1126/science.170.3962.1096. [DOI] [PubMed] [Google Scholar]
- Obrink B. A study of the interactions between monomeric tropocollagen and glycosaminoglycans. Eur J Biochem. 1973 Mar 1;33(2):387–400. doi: 10.1111/j.1432-1033.1973.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Byers P. H., Martin G. R. Production of procollagen by human fibroblasts in culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3260–3262. doi: 10.1073/pnas.69.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Igarashi S., Gross J. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970 Dec 8;9(25):4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Toole B. P., Gross J. Collagen heterogeneity. High resolution separation of native ( 1(I) 2 2 and ( 1(II) 3 and their component chains. J Biol Chem. 1972 Oct 25;247(20):6469–6473. [PubMed] [Google Scholar]
- Vuust J., Piez K. A. A kinetic study of collagen biosynthesis. J Biol Chem. 1972 Feb 10;247(3):856–862. [PubMed] [Google Scholar]
- Vuust J., Piez K. A. Biosynthesis of the alpha chains of collagen studied by pulse-labeling in culture. J Biol Chem. 1970 Nov 25;245(22):6201–6207. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



