Abstract
Metallothionein (MT) has been extensively investigated as a molecular marker of various types of cancer. In spite of the fact that numerous reviews have been published in this field, no meta-analytical approach has been performed. Therefore, results of to-date immunohistochemistry-based studies were summarized using meta-analysis in this review.
Web of science, PubMed, Embase and CENTRAL databases were searched (up to April 30, 2013) and the eligibility of individual studies and heterogeneity among the studies was assessed. Random and fixed effects model meta-analysis was employed depending on the heterogeneity, and publication bias was evaluated using funnel plots and Egger's tests.
A total of 77 studies were included with 8,015 tissue samples (4,631 cases and 3,384 controls). A significantly positive association between MT staining and tumors (vs. healthy tissues) was observed in head and neck (odds ratio, OR 9.95; 95% CI 5.82–17.03) and ovarian tumors (OR 7.83; 1.09–56.29), and a negative association was ascertained in liver tumors (OR 0.10; 0.03–0.30). No significant associations were identified in breast, colorectal, prostate, thyroid, stomach, bladder, kidney, gallbladder, and uterine cancers and in melanoma. While no associations were identified between MT and tumor staging, a positive association was identified with the tumor grade (OR 1.58; 1.08–2.30). In particular, strong associations were observed in breast, ovarian, uterine and prostate cancers. Borderline significant association of metastatic status and MT staining were determined (OR 1.59; 1.03–2.46), particularly in esophageal cancer. Additionally, a significant association between the patient prognosis and MT staining was also demonstrated (hazard ratio 2.04; 1.47–2.81). However, a high degree of inconsistence was observed in several tumor types, including colorectal, kidney and prostate cancer.
Despite the ambiguity in some tumor types, conclusive results are provided in the tumors of head and neck, ovary and liver and in relation to the tumor grade and patient survival.
Introduction
Metallothioneins (MTs) are cysteine-rich low-molecular-mass intracellular proteins occurring in a wide variety of eukaryotes and constituting the major fraction of intracellular protein thiols [1]. The MT gene family consists of four subfamilies designated as MT-1 through to MT-4 in mammals. MTs are involved in many physiological and pathophysiological processes such as intracellular storage, transport and metabolism of metal ions, whereas they regulate essential trace metal homeostasis and play a protective role in heavy metal detoxification reactions [2], [3]. They can protect cells against UV/ionic radiation [4], [5] as well as cytotoxic alkylating agents including chemotherapeutics [6]–[9], modulate oxygen free radicals and nitric oxide, and inhibit apoptosis [10]–[12].
MTs are usually expressed at low levels, but they are inducible [13]–[16]. The synthesis of MT was shown to be increased during oxidative stress [17], [18] to protect the cells against cytotoxicity [19], [20], radiation and DNA damage [21]–[23]. Many studies have shown an increased expression of MT in human tumors of breast, colon, kidney, liver, lung, nasopharynx, ovary, prostate, salivary gland, testes, thyroid and urinary bladder [14]. MT expression in tumor tissues is mainly correlated with the proliferative capacity of tumor cells [24]. However, there are few exceptional cases, e.g. down-regulation of MT in hepatocellular carcinoma [25]. Nevertheless, these case-control and cohort studies give us inconsistent results regarding the association of MTs and tumor histology, staging, grading, prognosis, and survival. Although there is a number of good systematic reviews [2], [3], [11], [13], [14], [26]–[28], [29,] with particular interest in breast tumors [30], [31], a meta-analytic approach has not been employed yet. Thus, the aim of this study is to evaluate the associations between immunohistochemical MT staining and clinicopathological conditions, tumor type, stage, grade, prognosis, and survival using the meta-analysis.
Materials and Methods
Literature search
Search was performed in Web of science (Science citation index expanded 1945 to April 2013), PubMed (Medline 1968 to April 2013) search engines and in bibliographies of cited references. The following keywords were used: histo* OR immunohisto* OR IHC; metallothionein; cancer OR tumor OR tumour OR neoplas*; melanoma. The date of publishing and language were not restricting
Selection criteria
Case-control and cohort studies regarding the associations between malignant neoplasms and metallothionein immunohistochemical staining were searched. Full text articles were included only. Following information were extracted from the studies: (1) MT level in malignant tumors and healthy/benign tissues, (2) MT level regarding the tumor stage, (3) tumor grade, (4) age and sex of patients, and (5) MT level and survival. The following data formats were accepted: (1) means, standard deviation and sample size, (2) sample size, means, P values and type of statistical test type (one- or two-tailed), and (3) sample size, P values, statistical test type and effect direction for continuous data and (1) odds ratios and 95% confidence intervals (CI), (2) 2×2 tables, and (3) Chi-squared and effect directions for dichotomous data. Continuous and dichotomous outcomes were combined. Cox proportional hazard model was used for survival meta-analysis. Univariate model of overall survival was used, hazard ratio and 95% CI was extracted from the studies. Studies with the sample size <6 participants and without histological verification of tumor were not included. If similar data was found in more than one study, studies with more extensive data set were used for the analysis. The eligibility of the studies for meta-analysis was evaluated by two authors (J.G. and M.R.).
Coding of categorical variables
Since different scales regarding MT IHC staining were used across the studies, the following rules were applied: (1) when MT staining was encoded as positive/negative, no change was applied; (2) when percentage data was included, staining >10% was considered positive and vice versa; (3) when no percentage data was identified and data were encoded by 0–2 or 0–3 points, 0–1 was considered negative. Because >2 categories are used in grading/staging scales, Grades 2–3 were grouped and compared with Grade 1; stages 3–4 and 1–2 were grouped in a similar way.
Statistical analysis
Odds ratios with 95% intervals were used as point estimates except for the survival analysis. For the survival analysis, hazard ratios with 95% confidence intervals were used. To assess heterogeneity across the studies, Higgins I2, describing the percentage of variability in point estimates was calculated [32]. The random effects model meta-analysis using the DerSimonian and Laird method was employed when a distinct heterogeneity was observed [33] (I2 more than 50.0%), otherwise, a fixed model was used. The model selection is based on a study by Borenstein et al. [34]. In that study, key assumptions of each model and mathematical bases are explained, and differences between the models are outlined; therefore, they will not be discussed in this paper. Subgroups were combined using the fixed effects. Within-subgroup estimates of tau-squared were not pooled. When the number of studies within the groups exceeded 4, the publication bias was evaluated using funnel plots and two-sided Egger's tests. Funnel plots of subgroups whose Egger's test p<0.05, are asymmetric. Comprehensive Meta-analysis Version 2 software (Biostat, Englewood, NJ) was used for the analysis.
Results and Discussion
Identification and characteristics of relevant studies
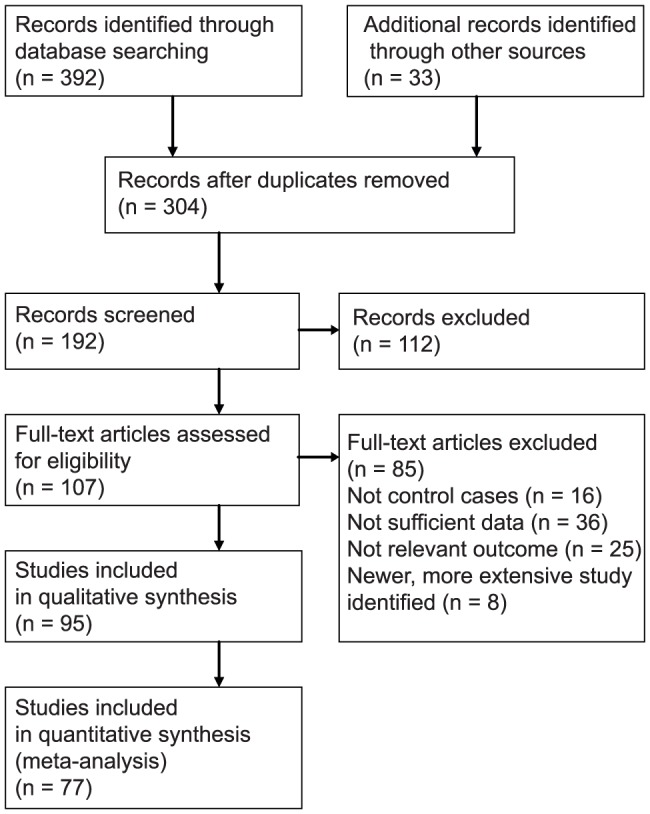
The acquisition process of studies is depicted in Fig. 1. A total of 77 articles were included in the final analysis after eliminating articles unsatisfying the selection criteria and duplicates. The set of 77 studies includes 8,015 tissue samples (4,631 cases and 3,384 controls). On average 95.2 patients were included per study, 1270 cases and controls were included in the largest study [35] and 12 cases and controls were included in the smallest study [36]. The date of publication ranged from 1987–2013 with the median of year 2002. In total, 51 North American and European studies, 20 Asian, 2 South American studies and one African study were included.
Figure 1. Flow chart showing the number of citations retrieved by database searching.
MT isoform characteristics
Monoclonal antibody clone E9 was used in a majority of studies; this antibody shows affinity for both MT-1 and 2 isoforms. Anti-MT3 antibody was only used by Sens et al. in bladder cancer [37]. Strong positive associations of MT3 staining with the tumor grade were determined. Therefore, with the exception of Sens et al., all following analyzes concern the MT-1 and 2 isoforms.
MT and patient characteristics
Patient age
First, the association of MT immunoreactivity and patient's age was analyzed. No significant association was determined using the fixed effects model meta-analysis (odds ratio, OR = 1.07; 95% confidence interval, CI, 0.48 to 0.628). No publication bias was identified (Egger's 2-tailed test p = 0.12). In total, 9 studies with 1,069 patients were included [25], [38]–[45]. The following tumors were included: bladder, breast, colorectal, hepatocellular, head and neck, and stomach.
This is a generally expected finding, no correlation of MT immunoreactivity and age was already reported in breast cancer patients [46]-[49]. No such age-dependent association is beneficial from the perspective of potential diagnostic use.
Patient gender
Consequently, the role of gender was analyzed. No publication bias was observed (p = 0.61) and no significant trend was detected using the fixed effect meta-analysis (OR = 0.99; 95% CI, 0.74 to 1.34). The analysis was performed on 9 studies reporting gender (944 patients). The following tumor types were included: bladder [44], colorectal [38], [39], [50], head and neck [41], [51], hepatocellular [25], and stomach [43]. Similarly as in the patients' age, no significance was expected. Such gender-independence is advantageous for appropriate tumor biomarker.
Metallothionein as cancer biomarker
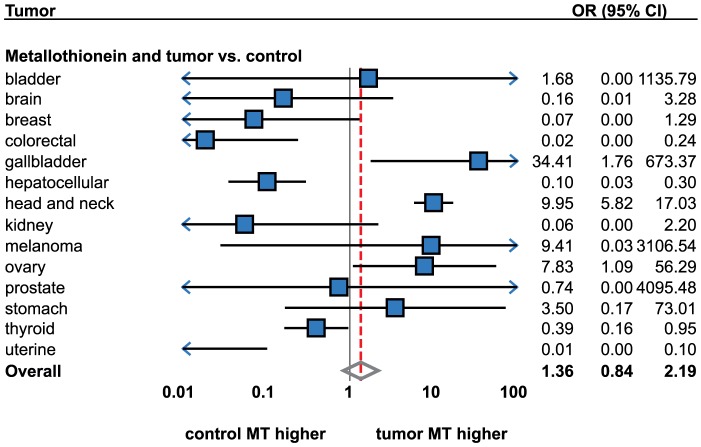
Consequently, the association of MT staining and tumor presence was analyzed. A total number of 31 studies were included (2,454 cases and healthy individuals). A high degree of heterogeneity was identified (Higgins I2 = 89.6%) when the meta-analysis was performed on all tumor types together (Fig. 2, Table 1). Therefore, no significant association between MT staining and tumor presence was identified using the random effects model (OR = 1.19; 95% CI, 0.47 to 3.01). No publication bias was identified. The high degree of heterogeneity between the studies calls for explanation. Therefore, a subgroup analysis by tumor types was performed.
Figure 2. Forrest plot showing associations between metallothionein staining and tumors (tumors versus healthy controls).
The result of meta-analysis for particular tumor types displayed instead of individual studies. For more detailed results see Table 1 and 2. Sorted alphabetically by tumor types. Forrest plot displayed as odds ratio and 95% confidence intervals. Red dashed line indicates the result for all tumor types together. OR, odds ratio; CI, confidence interval.
Table 1. Association of MT staining and clinicopatological factors. Tumor type not taken into account.
| Factor | Number of studies | Number of participants | OR/HR* (95% CI) | Heterogeneity | Publication bias | Model | |
| P-value | I2 (%) | P-value | |||||
| Age | 9 | 1069 | 1.07 (0.82–1.39) | 0.679 | 0.00 | 0.117 | Fixed effects |
| Gender | 9 | 944 | 0.99 (0.74–1.34) | 0.393 | 5.08 | 0.611 | Fixed effects |
| Tumor vs. control | 31 | 2454 | 1.19 (0.47–3.01) | 0.000 | 89.58 | 0.236 | Random effects |
| Tumor stage | 19 | 1237 | 1.15 (0.74–1.77) | 0.000 | 60.83 | 0.987 | Random effects |
| Tumor size | 6 | 1962 | 1.37 (0.45–4.13) | 0.000 | 90.88 | 0.194 | Random effects |
| Tumor grade | 33 | 2504 | 1.58 (1.08–2.30) | 0.000 | 66.57 | 0.886 | Random effects |
| Metastases, nodal + distant | 21 | 1987 | 1.59 (1.03–2.46) | 0.000 | 71.68 | 0.039 | Random effects |
| Metastases, distant | 6 | 741 | 1.56 (0.56–4.37) | 0.014 | 64.82 | 0.075 | Random effects |
| Metastases, nodal | 15 | 1246 | 1.62 (0.98–2.68) | 0.000 | 72.92 | 0.201 | Random effects |
| Overall survival | 10 | 2041 | 2.04 (1.47–2.81) | 0.000 | 97.69 | 0.572 | Random effects |
Heterogeneity of studies analyzed using Cochran's Q-test (p-value displayed) and using I2. Egger's two-tailed test used for publication bias analysis (p-value displayed). * effect measure is odds ratio, OR, except for survival analysis using hazard ratio, HR. CI, confidence interval
Head and neck cancer
Head and neck tumors were analyzed most extensively, five studies were identified (Table 2). Tumors in the following locations were included: oral cavity [41], tongue [52], pharynx [53], and larynx [54], [55]. Except for Sundelin et al., all studies showed a significant increase of MT levels in tumorous tissues (OR = 9.95; 95% CI, 5.82 to 17.03). The fixed effects model meta-analysis was used due to low heterogeneity, I2 = 34.5. This is in agreement with our study based on voltammetric MT detection [56].
Table 2. Association of MT staining and clinicopathological factors – individual tumor types taken into account.
| Tumor | Factor | Number of studies | OR/HR* (95% CI) | Heterogeneity | Model | |
| P-value | I2 (%) | |||||
| bladder | Tumor vs. control | 2 | 1.68 (0–1135.79) | 0.002 | 89.89 | Random effects |
| Tumor stage | 3 | 1.56 (0.77–3.16) | 0.354 | 3.64 | Fixed effects | |
| Tumor grade | 3 | 0.96 (0.21–4.33) | 0.027 | 72.24 | Random effects | |
| Metastases, nodal + distant | 3 | 1.78 (0.57–5.51) | 0.070 | 62.44 | Random effects | |
| Metastases, nodal | 2 | 1.96 (0.40–9.66) | 0.022 | 80.89 | Random effects | |
| Metastases, distant | 1 | 1.31 (0.21–8.27) | - | - | - | |
| Overall survival | 1 | 2.06 (1.26–3.37) | - | - | - | |
| breast | Tumor vs. control | 1 | 0.07 (0.00–1.29) | - | - | - |
| Tumor stage | 1 | 1.20 (0.59–2.43) | - | - | - | |
| Tumor size | 1 | 1.73 (0.68–4.40) | - | - | - | |
| Tumor grade | 5 | 1.85 (1.22–2.82) | 0.096 | 49.19 | Fixed effects | |
| Metastases, nodal + distant | 3 | 2.57 (0.59–11.26) | 0.001 | 86.55 | Random effects | |
| Metastases, nodal | 3 | 2.57 (0.59–11.26) | 0.001 | 86.55 | Random effects | |
| colorectal | Tumor vs. control | 1 | 0.02 (0.00–0.24) | - | - | - |
| Tumor stage | 5 | 1.27 (0.51–3.16) | 0.000 | 83.09 | Random effects | |
| Tumor size | 2 | 0.71 (0.32–1.56) | 0.299 | 7.15 | Fixed effects | |
| Tumor grade | 6 | 2.32 (0.86–6.29) | 0.000 | 77.64 | Random effects | |
| Metastases, nodal + distant | 6 | 1.11 (0.38–3.24) | 0.001 | 75.84 | Random effects | |
| Metastases, nodal | 4 | 1.12 (0.30–4.16) | 0.001 | 82.13 | Random effects | |
| Metastases, distant | 2 | 1.04 (0.07–15.17) | 0.050 | 73.87 | Random effects | |
| Overall survival | 1 | 1.05 (1.00–1.10) | - | - | - | |
| esophageal | Metastases, nodal + distant | 2 | 2.89 (1.17–7.15) | 0.464 | 0.00 | Fixed effects |
| Metastases, nodal | 1 | 2.30 (0.77–6.85) | Fixed effects | |||
| Metastases, distant | 1 | 4.78 (0.94–24.33) | - | - | - | |
| gallbladder | Tumor vs. control | 1 | 34.41 (1.76–673.37) | - | - | - |
| Tumor grade | 1 | 2.25 (0.37–13.71) | - | - | - | |
| hepatocellular | Tumor vs. control | 3 | 0.10 (0.03–0.30) | 0.548 | 0.00 | Fixed effects |
| Tumor stage | 1 | 3.53 (0.36–34.18) | - | |||
| Tumor size | 2 | 1.04 (0.12–9.42) | 0.023 | 80.53 | Random effects | |
| Tumor grade | 2 | 0.47 (0.28–0.80) | 0.832 | 0.00 | Fixed effects | |
| Metastases, nodal + distant | 1 | 0.62 (0.39–0.98) | - | - | - | |
| Metastases, distant | 1 | 0.62 (0.39–0.98) | - | - | - | |
| head and neck | Tumor vs. control | 5 | 9.95 (5.82–17.03) | 0.191 | 34.56 | Fixed effects |
| Tumor stage | 2 | 2.02 (0.36–11.27) | 0.123 | 58.04 | Random effects | |
| Tumor grade | 4 | 0.72 (0.22–2.40) | 0.091 | 53.68 | Random effects | |
| Metastases, nodal + distant | 2 | 3.49 (0.25–48.04) | 0.036 | 77.24 | Random effects | |
| Metastases, nodal | 2 | 3.49 (0.25–48.04) | 0.036 | 77.24 | Random effects | |
| Overall survival | 2 | 3.14 (1.61–6.15) | 0.826 | 0.00 | Fixed effects | |
| kidney | Tumor vs. control | 2 | 0.06 (0.00–2.20) | 0.043 | 75.47 | Random effects |
| Tumor stage | 2 | 0.38 (0.07–2.13) | 0.086 | 66.00 | Random effects | |
| Tumor grade | 2 | 1.74 (0.66–4.59) | 0.001 | 90.91 | Fixed effects | |
| lung | Tumor grade | 1 | 0.92 (0.05–18.12) | - | - | - |
| Overall survival | 2 | 1.03 (0.63–1.67) | 0.622 | 0.00 | Fixed effects | |
| lung, adenoca. | Tumor grade | 1 | 5.77 (0.29–116.67) | - | - | - |
| lung, squamous cell | Tumor grade | 1 | 0.15 (0.01–2.81) | - | - | - |
| melanoma | Tumor vs. control | 2 | 9.41 (0.03–3106.54) | 0.000 | 93.39 | Random effects |
| Tumor size | 1 | 4.85 (3.51–6.70) | - | - | - | |
| Metastases, nodal + distant | 2 | 2.47 (1.30–4.70) | 0.336 | 0.00 | Fixed effects | |
| Metastases, nodal | 1 | 2.23 (1.14–4.39) | - | - | - | |
| Metastases, distant | 1 | 6.63 (0.81–54.61) | - | - | - | |
| Overall survival | 1 | 7.16 (4.71–10.89) | - | - | - | |
| ovary | Tumor vs. control | 4 | 7.83 (1.09–56.29) | 0.003 | 78.82 | Random effects |
| Tumor grade | 2 | 3.08 (1.38–6.88) | 0.616 | 0.00 | Fixed effects | |
| Overall survival | 1 | 1.58 (1.01–2.47) | - | - | - | |
| prostate | Tumor vs. control | 2 | 0.74 (0–4095.48) | 0.000 | 95.32 | Random effects |
| Tumor grade | 4 | 2.09 (0.86–5.07) | 0.365 | 5.64 | Fixed effects | |
| Gleason Grade | 2 | 1.40 (0.47–4.14) | 0.238 | 28.06 | Fixed effects | |
| Overall survival | 1 | 1.86 (1.79–1.94) | - | - | - | |
| stomach | Tumor vs. control | 3 | 3.50 (0.17–73.01) | 0.000 | 95.71 | Random effects |
| Tumor stage | 1 | 0.73 (0.33-1.63) | - | - | - | |
| Tumor grade | 1 | 1.45 (0.67–3.14) | - | - | - | |
| Metastases, nodal + distant | 1 | 0.59 (0.27–1.28) | - | - | - | |
| Metastases, nodal | 1 | 0.59 (0.27–1.28) | - | - | - | |
| Overall survival | 1 | 4.23 (1.8–9.94) | - | - | - | |
| testes | Tumor stage | 2 | 0.33 (0.10–1.03) | 0.301 | 6.54 | Fixed effects |
| thyroid | Tumor vs. control | 3 | 0.39 (0.16–0.95) | 0.182 | 41.39 | Fixed effects |
| thyroid, follicular | Tumor vs. control | 3 | 2.27 (1.11–4.63) | 0.212 | 35.59 | Fixed effects |
| thyroid, medullar | Tumor vs. control | 1 | 0.11 (0.03–0.39) | - | - | - |
| thyroid, papillary | Tumor vs. control | 3 | 0.23 (0.02–2.85) | 0.026 | 72.64 | Random effects |
| uterine | Tumor vs. control | 1 | 0.01 (0.00–0.10) | - | - | - |
| Tumor stage | 2 | 1.53 (0.61–3.86) | 0.538 | 0.00 | Fixed effects | |
| Tumor grade | 2 | 2.81 (1.17–6.8) | 0.569 | 0.00 | Fixed effects | |
| Metastases, nodal + distant | 1 | 1.01 (0.27–3.79) | - | - | - | |
| Metastases, nodal | 1 | 1.01 (0.27–3.79) | - | - | - | |
Heterogeneity of studies analyzed using Cochran's Q-test (p-value displayed) and using I2. Egger's two-tailed test used for publication bias analysis (p-value displayed). * effect measure is odds ratio, OR, except for survival analysis using hazard ratio, HR. No heterogeneity test and no model indicated when 1 study per factor analyzed. CI, confidence interval.
Ovarian cancer
A total of four studies were identified [57]–[60]. All studies except for Tan et al. reported a significant increase [59]. Using the random effects meta-analysis, significantly higher MT staining was identified in tumors as compared with healthy tissues (OR = 7.83; 95% CI, 1.09 to 56.30). These results are in agreement with Murphy et al. using Hg-binding assay and with Germain et al. [61], [62].
Thyroid tumors
Three studies were identified [63]–[65]. The following histological types were analyzed: papillary, follicular and medullar. When the histological type was not considered as a unit of analysis, non-significant differences were determined when compared with non-malignant tissues by the random effects model meta-analysis.
The subgroup meta-analysis by histological types revealed the sources of heterogeneous results; significantly higher MT levels were determined in follicular cancer (OR = 2.27; 95% CI, 1.11 to 4.62) using the fixed effects, no change in papillary cancer and a significant decrease in medullar cancer (OR = 0.10; 95% CI, 0.03 to 0.39). However, medullar cancer was only dealt with in one study [64]. Apart from IHC technique, contradictory results were demonstrated by mRNA expression analysis, decreased MT expression was demonstrated in papillary cancer and no expression change was demonstrated in follicular cancer [66].
Prostate cancer
No significant associations between MT staining and tumor presence were determined using the random effects model. Results observed in this tumor were contradictory. While one study showed significantly lower MT in the tumorous tissue [67], the other identified a significantly increased MT level [68]. The study by Wei et al., however, used benign prostatic hyperplasia as a control instead of healthy tissue [67].
While the alteration of zinc-metallothionein metabolism is an early sign of prostate cancer progression [69], the benign tissue is not an adequate biological sample for such analyzes. In addition, a study based on radioimmunoanalysis revealed a non-significantly decreased MT level in the tumorous tissue [70]. Although prostate cancer is unique regarding the zinc and MT metabolism [71], [72], no conclusive findings were provided by this meta-analysis and more studies are therefore needed.
Hepatocellular cancer
Three studies were identified. Although lower MT levels in the tumorous tissue were reported in all studies [73]–[75], only Lu et al. reported a significant decrease [75]. Despite this fact, the fixed model analysis revealed significantly lower MT levels (OR = 0.10; 95% CI, 0.04 to 0.30). Nevertheless, Lu et al. used specifically the MT1F isoform antibody instead of nonselective MT1-2, which was used by a majority of study groups.
The decreased MT level in the hepatocellular tumor is a well-established finding. Apart from immunohistochemistry, decreased expression of MT1F [75], MT1G [76], [77] and MT1X [25] was determined. ELISA-based detection also showed a decrease [78]. No change in MT levels was determined in one HPLC-based study [79]
Stomach cancer
Contradictory results were evident in three identified studies. While two research groups reported increased levels in tumorous tissues [80], [81], the study by Tuccari et al. demonstrated a significant increase in MT levels [43]. While all groups used the E9 antibody clone and included both early and advanced tumors, these contradictory results call for a further explanation by another study. However, contradictory results were shown using other approaches. Apart from IHC analyses, inconclusive results are provided also in gene expression- and radioimmunoanalysis-based methods. Elevation of MT1, 2, and 3 mRNA was determined in an RT-PCR-based study [80] and decrease of MT1 and 2 protein was determined in a radioimmunoanalysis-based study [82].
Bladder cancer
Two studies regarding bladder cancer were identified [83], [84]. Using the random effects model, no significant difference was identified. Contradictory results of studies were observed, while a positive association was demonstrated by Zhou et al. and a negative trend was reported by Saika et al. This contradiction may perhaps be explained by the use of benign tissues as controls instead of healthy individuals by Zhou et al. In addition to MT1 and 2 isoforms, Sens et al. demonstrated MT3 to be significantly up-regulated in tumorous tissues, and suggested its use as a potential biomarker for bladder cancer [37].
Kidney cancer
Two studies were identified [83], [85]. Although both of them show a decrease, the level of significance is achieved in one study only [85]. As a result, no significance is observed using the random effects model meta-analysis. While both IHC studies used the E9 antibody clone, differential affinity to MT-1 and 2 is not expected. Additionally to IHC determination, down-regulated MT1A, 1F, 1H, and 1G and up-regulated MT2A expression were observed also in other studies [14], [86], [87]. On a protein level, decreased MT levels were determined in the HPLC-based study [88].
Melanoma
MT levels in melanoma tissues were analyzed in two studies [89], [90]. Of those, a significant increase is presented only by Zelger et al. [90] and so a non-significant change in MT levels is observed using the random effects model.
Other tumor types
As compared with the previous chapter, only one study per tumor type was identified for several tumor types. Therefore, no meta-analytical approach was used. This applied to the following tumor types: breast, colon and rectum, gallbladder and uterine corpus.
Compared to non-malignant tissues, significantly higher MT staining was identified in gallbladder cancer [91], a significant decrease in colorectal [92] and cervical [93] tumors and no associations were observed in ductal breast tumors [94] and glioblastomas [95]. Immunohistochemical-based studies of other tumors were not identified.
While El Sharkarvy described non-significant changes in breast cancer tissues, other studies regarding breast cancer described intensive staining in the ductal type, while small or no staining was observed in lobular and papillary cancer [14], [46], [96]. There are number of reviews regarding MT expression in tumorous tissues. Pedersen, for instance, points to the issue of discrepancy between the MT expression in various tumors and the lack of overall consensus regarding the precise role of MT in human neoplasms [26]. According to these researchers, the enhanced expression is associated with the rapid proliferation or regeneration of normal cells and even with the aggressiveness and drug resistance of neoplasms [26]. This applies to the following tumors: kidney, breast, lung, nasopharynx, salivary gland, ovary, testes, urinary bladder, leukemia, and non-Hodgkin's lymphoma [14], [27], [97]. However, this meta-analysis only shows an agreement in ovarian and nasopharyngeal cancer while the rest of tumors did not exhibit any alterations of MT levels. On the other hand, the decrease of MT expression is associated with the poor prognosis namely of prostatic, hepatic, thyroid, brain and testicular tumors [14], [25], . An agreement is found in hepatic cancer only by this analysis.
Apart from differences between the individual tumor types, contradictory results were found even within particular histological types. According to the meta-analysis, such contradictions were observed in prostate, melanoma, and stomach tumors. While the high MT levels are associated with rapid proliferation and drug resistance and the low levels are associated with poor prognosis, it is likely that the MT levels change during the tumor progression. It is known that the zinc and metallothionein metabolism is being altered during early stages of tumorigenesis in prostate cancer, [69], [103], [104]; a similar tendency is expected in other histological types. Thus, a careful selection of controls is crucial; benign controls are therefore not an optimal biological material to demonstrate changes in MT/zinc levels. Additionally, it was reported that healthy tissues adjacent to a tumor vary distinctly as compared with the healthy tissues (i.e. tissues of patients without tumors) regarding zinc and MT levels. Considering the fact that MT is tightly related to oxidative stress buffering [11], the increase in oxidative stress affects the MT expression. Therefore, radiation, cytostatic drug therapy, or long-term stress must be taken into account when evaluating the MT levels.
Tumor stage
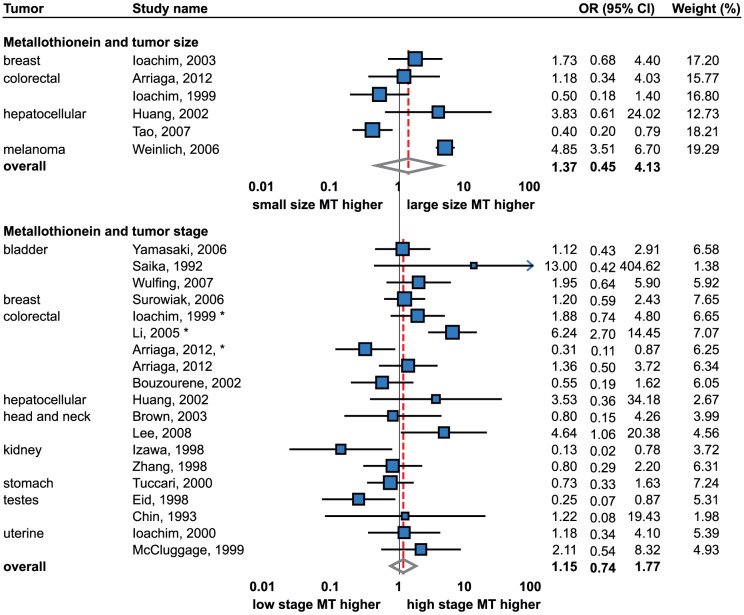
Although there are outlines that poor prognosis is associated with the higher MT expression in some tumors, for example breast tumors [30], [40], [49], no significant associations were determined by this meta-analysis (OR = 1.15; 95% CI, 0.74 to 1.77). No publication bias was observed (Egger's 2-tailed p = 0.99) and a vast majority of studies did not reveal any significant trends. In total, 1,237 samples were analyzed (Fig. 3).
Figure 3. Forrest plot of studies reporting the association of metallothionein staining and tumor size and stage.
Random effects model used for both outcomes, Relative weight of individual studies displayed in %. * indicates studies using Dukes staging system. For more detailed results see Table 1 and 2. Sorted alphabetically by tumor types. Forrest plot displayed as odds ratio and 95% confidence intervals. Red dashed line indicates the result for all studies together. OR, odds ratio; CI, confidence interval.
Subsequently, the perspective of individual tumor types was taken into account. A colorectal cancer pT staging of two included studies did not reveal any significance using the meta-analysis [38], [105]; however, conflicting results were determined in colorectal cancer evaluating the Dukes staging. While a negative association (i.e. lower MT staining in higher stages) was revealed by one study [38], a positive association was determined by another study [106] and non-significant association was determined in another study [39]. Rather confusing was a study using radioimmunoanalysis with a positive association of MT staining and Dukes stage but no association to TNM staging [82].
Using the meta-analysis, no association of MT staining and tumor stage was observed in bladder [44], [83], [107], endometrial [108], [109], testicular [6], [98], kidney [110], [111], and head and neck cancer [41], [51]. There are also studies regarding tumors of breast [45], liver [74], and stomach [43] showing no significant association between MT staining and the tumor stage (Table 2). Nevertheless, each tumor was represented only by one study and no meta-analytical approach was applied. Therefore, more studies are needed.
Tumor size
The association of MT staining and tumor size was a further subject of the meta-analysis. Six studies were included (1,962 samples); no association was observed using the random effects model and no publication bias was determined (Fig. 3).
When individual tumor types were evaluated separately, no significant trends were observed in colorectal cancer using the fixed effects and in hepatocellular cancer using the random effects model. Breast cancers and melanomas were identified in one study, with the only positive association found in melanoma. Additionally to IHC, no correlation of MT immunopositivity and tumor size was described by other researchers either [40], [46]–[49], [112]. Thus, according to our results and previous studies, MT staining is considered tumor size independent.
Tumor grade
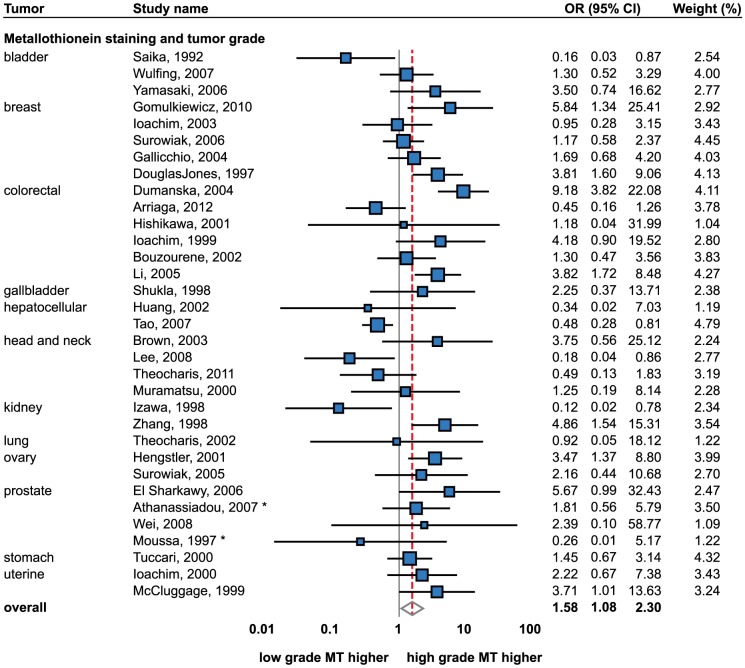
The association of MT staining with the histological grade was studied most extensively; a total of 32 studies (2,504 samples) were identified (Fig. 4). Although a relatively high degree of heterogeneity between the studies was observed (I2 = 67.2%), still a significant positive association (i.e. higher MT in higher-grade tumors) was detected using the random effects model (OR = 1.61; 1.107–2.35). No publication bias was observed (p = p = 0.74).
Figure 4. Forrest plot of studies reporting the association of metallothionein staining and tumor grading.
Random effects model used. Relative weight of individual studies displayed in %. For more detailed results see Table 1 and 2. * indicates studies using Gleason grading. Sorted alphabetically by tumor types. Forrest plot displayed as odds ratio and 95% confidence intervals. Red dashed line indicates the result for all studies together. OR, odds ratio; CI, confidence interval.
Consequently, this association was analyzed in particular tumor types. Colorectal cancer was studied most with six studies being included [38], [39], [50], [105], [106], [113]. A positive trend was observed in only two studies [106], [113]. Thus, the random effects meta-analysis revealed no association between IHC staining of MT and the tumor grade.
Contrarily, a strong positive association was determined in breast tumors using the fixed effects model (OR = 1.85; 95% CI, 1.22–2.82). A total number of five studies of this tumor type were found [40], [45], [114]–[116] and through the established positive association, a significant trend was observed in two studies only [114], [116]. These IHC-based results are in agreement with findings based on other techniques, a similarly positive association was observed by numerous authors [46], [47], [49], [112], [117]–[119].
Four studies regarding head and neck tumors were included [41], [42], [51], [120]. However, a significant trend was observed only in one study [41]. Thus, no significant association was observed the using random effects model.
Prostate cancer is particularly interesting from the perspective of MT immunostaining. Four studies were included in total [67], [121]–[123]. Athanassiadou and Moussa used Gleason scale as a measure of tumor grading. When the grading scale was not taken into account, no trend was identified using the fixed effects model. However, the analysis suggests, that only Gleason scale shows no association; by contrast, the tumor grading was positively associated with MT staining (OR = 4.65; 1.01–21.52) using the fixed effects.
Using the fixed effects model, a positive association with the tumor grade was also determined in two studies of ovarian (OR = 3.08; 1.38–6.88) [124], [125] and two studies of endometrial cancers (OR = 2.82; 1.17–6.80). However, no MT-grading association was determined in ovarian cancer using Hg-binding assay [61].
On the other hand, a negative association of tumor staging was observed in hepatocellular cancer using the fixed effects, (OR = 0.43; 0.28–0.80). Thus, HCC is considered as the only histological type showing lower MT staining in higher tumor grades. However, only two studies were included and this is why the finding is still of a limited predictive value [25], [74].
Inconsistent results were observed in bladder and kidney cancers. While a non-significant association was identified in both tumor types, both positive [111] and negative [110] associations was determined in the case of renal cancer. In terms of bladder cancer, a negative association was demonstrated by Saika et al. [83], while non-significant trends were observed by other two studies [44], [107]. Additionally, a decreased MT3 gene expression was associated with higher-grade tumors [37].
Stomach [43], lung [126] and gallbladder [91] tumors were represented by only one study each, but non-significant associations between the tumor grade and MT staining were determined in all these studies. Lung tumors were studied by their histological type; however, non-significant associations were described either in adenocarcinomas or squamous cell carcinomas [126]. Other histological types were not included.
Taking into account the described relations of MT immunostaining with the increased proliferation and cytostatic resistance [14], [27], [97], it is not surprising that the associations of MT levels with tumor grading were studied to high extent. Meta-analysis results indicate a positive association. However, the results of this meta-analysis also indicate that the MT-grading associations are tumor specific. While a positive association was determined in most tumors, a negative trend was determined in hepatocellular cancer and inconsistent results were found in colorectal, bladder and kidney tumors. While a more or less distinct pattern is evident in all tumors, data are still lacking to prove whether the general positive association of MT staining and tumor grade may be generalized for these tumor types. While the low MT levels are associated with a worse prognosis and the high MT levels with increased proliferation [26], the MT levels vary during the disease progression. It is likely that the heterogeneity observed in some tumor types is a consequence of this phenomenon. Therefore, a precise understanding of MT level fluctuation in individual tumor types during the progression of disease is needed. Understanding the temporal changes will provide better comprehension of cellular tumor mechanisms and may predict a possible development of cytostatic resistance.
Lymph node and metastatic status
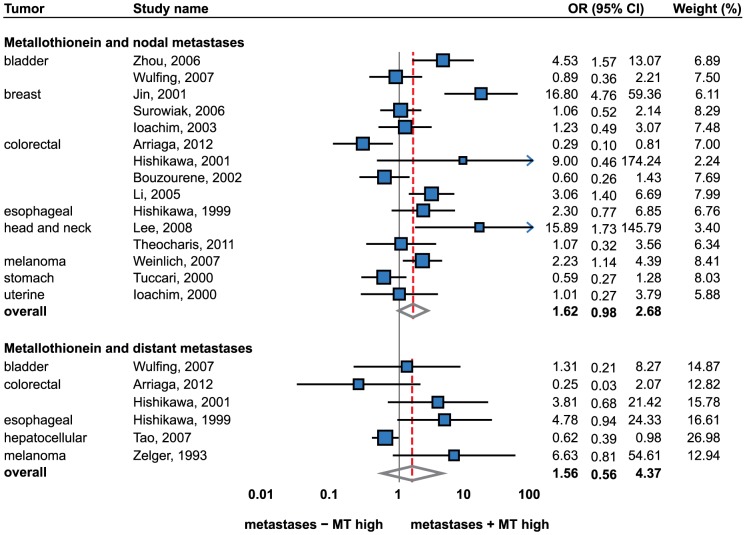
Consequently, the association of the metastatic status and MT staining was taken as a subject of the meta-analysis. Included were 15 studies comparing nodal metastases and 6 studies comparing distant metastases (Fig. 5). Using the random effects model meta-analysis, a positive association of the metastatic status and MT staining was observed (OR = 1.59; 95% CI, 1.03–2.46). However, a high degree of heterogeneity between the studies (I2 = 71.68%) and publication bias were observed (p = 0.04). Nevertheless, when nodal and distant metastases were analyzed separately, no significant trends were observed either in nodal or distant metastases.
Figure 5. Forrest plot of studies reporting the association of metallothionein staining and nodal and distant metastases.
Random effects model used for both outcomes, Relative weight of individual studies displayed in %. For more detailed results see Table 1 and 2. Sorted alphabetically by tumor types. Forrest plot displayed as odds ratio and 95% confidence intervals. Red dashed line indicates the result for all studies together. OR, odds ratio; CI, confidence interval.
Consequently, individual tumor type was considered as a unit of the analysis. A significant association was observed in esophageal cancer (OR = 2.89; 1.17 to 7.15) [127], melanoma (OR = 2.47; 1.30 to 4.70) [90], [128] and hepatocellular cancer (OR = 0.62; 0.39 to 0.98) [25]. Notably, only one study per tumor type was included regarding esophageal and hepatocellular cancers.
Tumors of colon and rectum brought ambiguous results. While no association was identified by the meta-analysis, still one study demonstrated a negative association [38], two studies demonstrated a positive association [50], [106] and one study did not reveal a significant trend [105]. The remaining tumor types, including bladder [84], [107], breast [40], [45], [119], head and neck [41], [42], stomach [43], and uterine cancers [108] indicated no significant association between MT immunostaining and the presence of tumor metastases. However, it must be taken into account that the power of the meta-analysis for individual tumor types is limited due to the limited number of studies. Hence, by combining the tumor types the power of the analysis increases and the combined result indicates a significant positive association. However, there is still a lack of IHC data to make conclusive remarks on all tumor types.
Apart from the immunohistochemical analysis, no correlation between the lymph node status and the metastatic potential was described in breast cancer by several investigators [46]–[49], [112]. A significant association between the lymph node status and metastases was observed in one study only [129]. Schmid et al. suggest a higher probability of MT-positive tumors to develop metastases [130]. With regard to non-small cell lung cancer, no difference in the expression of all functional MT-1 and 2 isoforms was determined in another study [131].
Survival analysis
The association of MT immunostaining and patient prognosis was evaluated by several researchers. However, various approaches were used. Univariate Cox proportional hazard model was included in the analysis. Unless noted otherwise, overall survival was used. Additionally, odds ratios, which reported the associations of MT staining with cancer-specific deaths, were also included. Median survival-based data were not evaluable by the meta-analysis and thus were not included.
Firstly, studies describing Cox proportional hazard model were analyzed. A total of 10 studies were included dealing with bladder, colorectal, head and neck, lung, melanoma, ovarian, prostate and stomach tumors (Fig. 6). A significant positive association (i.e. up-regulated MT associated with a worse prognosis) was determined using the random effects model (HR = 2.04; 95% CI, 1.47 to 2.81). No publication bias was determined (p = 0.57).
Figure 6. Forrest plot of studies reporting the association of metallothionein staining and the overall survival.
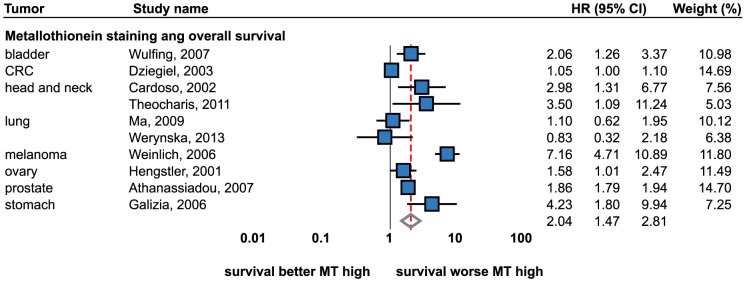
Analysis based on Cox-proportional hazard model. Random effects model used. Relative weight of individual studies displayed in %. For more detailed results see Table 1 and 2. Sorted alphabetically by tumor types. Forrest plot displayed as hazard ratio and 95% confidence intervals. Red dashed line indicates the result for all studies together. HR, hazard ratio; CI, confidence interval.
The significant positive association was determined in head and neck cancers (HR = 3.14; 95% CI, 1.61 to 6.15, fixed effects) [42], [132] while no association was determined in lung tumors [131], [133]. Both small cell and non-small cell tumors were included. Other tumor types were included in one study. The significant positive association was determined in the cancers of stomach [81], head and neck [42], prostate [121], bladder [107], head and neck [132], ovary [124], colon and rectum [134], and melanoma [35]. No association was identified in kidney tumors [135]. In addition to IHC analyses, poorer prognosis was associated with the higher MT1F and MT2A gene expression in non-small cell lung cancer in a qRT-PCR based study [131] and with higher MT protein levels in colorectal cancer in a radioimmunoanalysis-based study [82].
In addition to Cox model, retrospective studies reporting the following survival-related outcomes were analyzed: cancer-specific death, disease-free survival, short-term survival. While different outcomes were analyzed, the meta-analytical approach was limited in these studies. High MT expression was associated with poorer survival (OR = 3.42; 1.06 to 11.04) in melanoma patients, while an inverse effect was identified in patients with colon and rectum tumors. In those patients, lower MT levels were associated with a worse prognosis (OR = 0.41; 0.18 to 0.95). Univariable analysis of cancer-specific death was a subject of four studies. The following tumor types were included: breast [45], esophagus [136], head and neck [51], and stomach [43]. Neither any of the studies nor the result of the meta-analysis showed an association between cancer-specific death and MT staining. According to Joseph et al., no association was observed between MT staining and short-term survival [137]. Disease-free survival was analyzed in one study [36], which demonstrated a positive association in gastrointestinal stromal tumors (OR = 6.12; 1.12 to 33.52) and no association in leiomyosarcomas.
Conclusions
The associations of immunohistochemical MT staining and various clinicopathological conditions of patients with tumors were analyzed using the meta-analytical approach. To date, it is the first meta-analysis regarding MT in pathological conditions. This meta-analysis was conducted only in studies based on immunohistochemical detection due to indubitable advantages of this method, which clearly identifies tumorous tissues. Therefore, effects of adjacent tissues on gene expression measurements are eliminated. In addition, the largest number of studies was based on immunohistochemistry. Therefore, the IHC-based results are of great statistical power.
More intensive MT staining in tumorous tissues compared to healthy tissues was observed in head and neck and ovarian tumors and a negative association was determined in liver tumors. No significant associations were identified in breast, colorectal, prostate, thyroid, stomach, bladder, kidney, gallbladder, and uterine cancers and in melanoma. However, more studies are needed in tumors showing insignificant results to confirm or disprove finding that the MT level remains unchanged. Most “insignificant” tumors is often represented by only two studies, which are often conflicting.
While no associations were identified between MT and tumor staging, a positive association was identified with the tumor grade. In particular, strong associations were observed in breast, ovarian, uterine and prostate cancers. Conversely, a negative association between MT staining and hepatocellular tumors was determined in this analysis. Borderline significant association of metastatic status and MT staining was determined in all tumors, in esophageal cancer in particular. Significant association between patient's prognosis and MT staining was also demonstrated.
However, this study has several limitations. Despite the mentioned advantages of immunohistochemistry, the semi-quantitative analysis is to a certain extent always subjective. Moreover, antibodies show affinity to a broad spectrum of MT1 and 2 isoforms. Nevertheless, gene expression-based studies indicate that MT level alterations are confined to certain isoforms. Such differences between isoforms may cause between-study discrepancies, which was evident in several tumor types, including colorectal, kidney, and prostate cancers. Therefore, techniques capable to distinguish MT isoforms on the protein level are desirable, e.g. capillary-based electrophoresis [138]. Despite this fact, such heterogeneity might also be due to MT fluctuation during the development of cancers, high levels being associated with the high rate of proliferation and cytostatic resistance, and low levels being associated with poorer prognoses. Therefore, precise understanding of MT level fluctuation in individual tumor types during the progression of disease is needed. Understanding the temporal changes will provide better comprehension of cellular tumor mechanisms and may predict possible development of cytostatic resistance. Moreover, the association of MT staining was investigated by researchers in certain tumor types more than in others. Therefore, the overall results (i.e. without taking into account the specific type of tumor) are biased toward more published tumor types and a cautious interpretation of the overall results is therefore necessary.
Despite these drawbacks, conclusive results are provided in some tumor types and in relation to tumor grade and patient survival by this meta-analysis. It is however still necessary to clarify the ambiguity of the association between MT staining and colorectal, lung, kidney, and prostate tumors.
Supporting Information
Prisma 2009 checklist.
(DOC)
Prisma 2009 flow diagram showing the number of citations retrieved by database searching.
(DOC)
Funding Statement
Financial support from Central European Institute of Technology (CEITEC CZ.1.05/1.1.00/02.0068) and Internal Grant Agency of Ministry of Health of the Czech Republic (IGA MH NT14337-3/2013) is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nordberg M, Nordberg GF (2000) Toxicological aspects of metallothionein. Cellular and Molecular Biology 46: 451–463. [PubMed] [Google Scholar]
- 2. Miles AT, Hawksworth GM, Beattie JH, Rodilla V (2000) Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Critical Reviews in Biochemistry and Molecular Biology 35: 35–70. [DOI] [PubMed] [Google Scholar]
- 3. Krizkova S, Ryvolova M, Hrabeta J, Adam V, Stiborova M, et al. (2012) Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metabolism Reviews 44: 287–301. [DOI] [PubMed] [Google Scholar]
- 4. Hanada K, Sawamura D, Tamai K, Baba T, Hashimoto I, et al. (1998) Novel function of metallothionein in photoprotection: Metallothionein-null mouse exhibits reduced tolerance against ultraviolet B injury in the skin. Journal of Investigative Dermatology 111: 582–585. [DOI] [PubMed] [Google Scholar]
- 5. Reeve VE, Nishimura N, Bosnic M, Michalska AE, Choo KHA (2000) Lack of metallothionein-I and -II exacerbates the immunosuppressive effect of ultraviolet B radiation and cis-urocanic acid in mice. Immunology 100: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chin JL, Banerjee D, Kadhim SA, Kontozoglou TE, Chauvin PJ, et al. (1993) Metallothionein in testicular germ-cell tumors and drug-resistance - clinical correlation. Cancer 72: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 7. Fuertes MA, Alonso C, Perez JM (2003) Biochemical modulation of cisplatin mechanisms of action: Enhancement of antitumor activity and circumvention of drug resistance. Chemical Reviews 103: 645–662. [DOI] [PubMed] [Google Scholar]
- 8. Sunada F, Itabashi M, Ohkura H, Okumura T (2005) p53 negativity, CDC25B positivity, and metallothionein negativity are predictors of a response of esophageal squamous cell carcinoma to chemoradiotherapy. World Journal of Gastroenterology 11: 5696–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, et al. (1988) Overexpression of metallothionein confers resistance to anticancer drugs. Science 241: 1813–1815. [DOI] [PubMed] [Google Scholar]
- 10. Kumar SD, Vijaya M, Samy RP, Dheen ST, Ren MQ, et al. (2012) Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radical Biology and Medicine 53: 1595–1606. [DOI] [PubMed] [Google Scholar]
- 11. Babula P, Masarik M, Adam V, Eckschlager T, Stiborova M, et al. (2012) Mammalian metallothioneins: properties and functions. Metallomics 4: 739–750. [DOI] [PubMed] [Google Scholar]
- 12. Tsangaris GT, Tzortzatou-Stathopoulou F (1998) Metallothionein expression prevents apoptosis: A study with antisense phosphorothioate oligodeoxynucleotides in a human T cell line. Anticancer Research 18: 2423–2433. [PubMed] [Google Scholar]
- 13. Eckschlager T, Adam V, Hrabeta J, Figova K, Kizek R (2009) Metallothioneins and Cancer. Current Protein and Peptide Science 10: 360–375. [DOI] [PubMed] [Google Scholar]
- 14. Cherian MG, Jayasurya A, Bay BH (2003) Metallothioneins in human tumors and potential roles in carcinogenesis. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis 533: 201–209. [DOI] [PubMed] [Google Scholar]
- 15. Ebadi M, Leuschen MP, ElRefaey H, Hamada FM, Rojas P (1996) The antioxidant properties of zinc and metallothionein. Neurochemistry International 29: 159–166. [DOI] [PubMed] [Google Scholar]
- 16. Kang YJ (2006) Metallothionein redox cycle and function. Experimental Biology and Medicine 231: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 17. Sato M, Bremner I (1993) Oxygen free-radicals and metallothionein Free Radical Biology and Medicine. 14: 325–337. [DOI] [PubMed] [Google Scholar]
- 18. Iszard MB, Liu J, Klassen CD (1995) Effect of several metallothionein inducers on oxidative stress defense mechanisms in rats. Toxicology 104: 25–33. [DOI] [PubMed] [Google Scholar]
- 19. Aschner M, Conklin DR, Yao CP, Allen JW, Tan KH (1998) Induction of astrocyte metallothioneins (MTs) by zinc confers resistance against the acute cytotoxic effects of methylmercury on cell swelling, Na+ uptake, and K+ release. Brain Research 813: 254–261. [DOI] [PubMed] [Google Scholar]
- 20. Namdarghanbari M, Wobig W, Krezoski S, Tabatabai N, Petering D (2011) Mammalian metallothionein in toxicology, cancer, and cancer chemotherapy. Journal of Biological Inorganic Chemistry 16: 1087–1101. [DOI] [PubMed] [Google Scholar]
- 21. Cai L, Koropatnick J, Cherian MG (1995) Metallothionein protects DNA from copper-induced but not iron-induced cleavage in-vitro. Chemico-Biological Interactions 96: 143–155. [DOI] [PubMed] [Google Scholar]
- 22. Shibuya K, Nishimura N, Suzuki JS, Tohyama C, Naganuma A, et al. (2008) Role of metallothionein as a protective factor against radiation carcinogenesis. Journal of Toxicological Sciences 33: 651–655. [DOI] [PubMed] [Google Scholar]
- 23. Schwarz MA, Lazo JS, Yalowich JC, Allen WP, Whitmore M, et al. (1995) Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric-oxide. Proceedings of the National Academy of Sciences of the United States of America 92: 4452–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondo Y, Rusnak JM, Hoyt DG, Settineri CE, Pitt BR, et al. (1997) Enhanced apoptosis in metallothionein null cells. Molecular Pharmacology 52: 195–201. [DOI] [PubMed] [Google Scholar]
- 25. Tao X, Zheng JM, Xu AM, Chen XF, Zhang SH (2007) Downregulated expression of metallothionein and its clinicopathological significance in hepatocellular carcinoma. Hepatology Research 37: 820–827. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen MO, Larsen A, Stoltenberg M, Penkowa M (2009) The role of metallothionein in oncogenesis and cancer prognosis. Progress in Histochemistry and Cytochemistry 44: 29–64. [DOI] [PubMed] [Google Scholar]
- 27. Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP (2004) Metallothionein expression in human neoplasia. Histopathology 45: 103–118. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi S (2012) Molecular functions of metallothionein and its role in hematological malignancies. Journal of Hematology & Oncology 5. [DOI] [PMC free article] [PubMed]
- 29. Gumulec J, Masarik M, Krizkova S, Hlavna M, Babula P, et al. (2012) Evaluation of alpha-methylacyl-CoA racemase, metallothionein and prostate specific antigen as prostate cancer prognostic markers. Neoplasma 59: 191–200. [DOI] [PubMed] [Google Scholar]
- 30. Jin RX, Huang JX, Tan PH, Bay BH (2004) Clinicopathological significance of metallothioneins in breast cancer. Pathology & Oncology Research 10: 74–79. [DOI] [PubMed] [Google Scholar]
- 31. Bay BH, Jin RX, Jayasurya A (2001) Analysis of metallothionein expression in human cancers. Acta Histochemica et Cytochemica 34: 171–176. [Google Scholar]
- 32. Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 33. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 34. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 35. Weinlich G, Eisendle K, Hassler E, Baltaci M, Fritsch PO, et al. (2006) Metallothionein - overexpression as a highly significant prognostic factor in melanoma: a prospective study on 1270 patients. British Journal of Cancer 94: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez-Gutierrez S, Gonzalez-Campora R, Amerigo-Navarro J, Beato-Moreno A, Sanchez-Leon M, et al. (2007) Expression of P-glycoprotein and metallothionein in gastrointestinal stromal tumor and leiomyosarcomas. Clinical implications. Pathology & Oncology Research 13: 203–208. [DOI] [PubMed] [Google Scholar]
- 37. Sens MA, Somji S, Lamm DL, Garrett SH, Slovinsky F, et al. (2000) Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environmental Health Perspectives 108: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arriaga JM, Levy EM, Bravo AI, Bayo SM, Amat M, et al. (2012) Metallothionein expression in colorectal cancer: relevance of different isoforms for tumor progression and patient survival. Human Pathology 43: 197–208. [DOI] [PubMed] [Google Scholar]
- 39. Ioachim EE, Goussia AC, Agnantis NJ, Machera M, Tsianos EV, et al. (1999) Prognostic evaluation of metallothionein expression in human colorectal neoplasms. Journal of Clinical Pathology 52: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ioachim E, Tsanou E, Briasoulis E, Batsis C, Karavasilis V, et al. (2003) Clinicopathological study of the expression of hsp27, pS2, cathepsin D and metallothionein in primary invasive breast cancer. Breast 12: 111–119. [DOI] [PubMed] [Google Scholar]
- 41. Lee SS, Yang SF, Ho YC, Tsai CH, Chang YC (2008) The upregulation of metallothionein-1 expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Oncology 44: 180–186. [DOI] [PubMed] [Google Scholar]
- 42. Theocharis S, Klijanienko J, Giaginis C, Rodriguez J, Jouffroy T, et al. (2011) Metallothionein expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patient survival. Histopathology 59: 514–525. [DOI] [PubMed] [Google Scholar]
- 43. Tuccari G, Giuffre G, Arena F, Barresi G (2000) Immunohistochemical detection of metallothionein in carcinomatous and normal human gastric mucosa. Histology and Histopathology 15: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 44. Yamasaki Y, Smith C, Weisz D, van Huizen I, Xuan J, et al. (2006) Metallothionein expression as prognostic factor for transitional cell carcinoma of bladder. Urology 67: 530–535. [DOI] [PubMed] [Google Scholar]
- 45. Surowiak P, Materna V, Gyorffy B, Matkowski R, Wojnar A, et al. (2006) Multivariate analysis of oestrogen receptor alpha, pS2, metallothionein and CD24 expression in invasive breast cancers. British Journal of Cancer 95: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fresno M, Wu WY, Rodriguez JM, Nadji M (1993) Localization of metallothionein in breast carcinomas - an immunohistochemical study. Virchows Archiv A, Pathological Anatomy and Histopathology 423: 215–219. [DOI] [PubMed] [Google Scholar]
- 47. Oyama T, Takei H, Hikino T, Iino Y, Nakajima T (1996) Immunohistochemical expression of metallothionein in invasive breast cancer in relation to proliferative activity, histology and prognosis. Oncology 53: 112–117. [DOI] [PubMed] [Google Scholar]
- 48. Goulding H, Jasani B, Pereira H, Reid A, Galea M, et al. (1995) Metallothionein expression in human breast-cancer. British Journal of Cancer 72: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin RX, Chow VTK, Tan PH, Dheen ST, Duan W, et al. (2002) Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis 23: 81–86. [DOI] [PubMed] [Google Scholar]
- 50. Hishikawa Y, Kohno H, Ueda S, Kimoto T, Dhar DK, et al. (2001) Expression of metallothionein in colorectal cancers and synchronous liver metastases. Oncology 61: 162–167. [DOI] [PubMed] [Google Scholar]
- 51. Brown JJ, Xu H, Nishitani J, Mohammed H, Osborne R, et al. (2003) Potential biomarkers for head and neck squamous cell carcinoma. Laryngoscope 113: 393–400. [DOI] [PubMed] [Google Scholar]
- 52. Sundelin K, Jadner M, Norberg-Spaak L, Davidsson A, Hellquist HB (1997) Metallothionein and Fas (CD95) are expressed in squamous cell carcinoma of the tongue. European Journal of Cancer 33: 1860–1864. [DOI] [PubMed] [Google Scholar]
- 53.Dutsch-Wicherek M, Lazar A, Tomaszewska R, Kazmierczak W, Wicherek L (2013) Analysis of metallothionein and vimentin immunoreactivity in pharyngeal squamous cell carcinoma and its microenvironment. Cell & Tissue Research. [DOI] [PMC free article] [PubMed]
- 54. Ioachim E, Assimakopoulos D, Peschos D, Zissi A, Skevas A, et al. (1999) Immunohistochemical expression of metallothionein in benign premalignant and malignant epithelium of the larynx: Correlation with p53 and proliferative cell nuclear antigen. Pathology Research and Practice 195: 809–814. [DOI] [PubMed] [Google Scholar]
- 55. Pastuszewski W, Dziegiel P, Krecicki T, Podhorska-Okolow M, Ciesielska U, et al. (2007) Prognostic significance of metallothionein, p53 protein and Ki-67 antigen expression in laryngeal cancer. Anticancer Research 27: 335–342. [PubMed] [Google Scholar]
- 56. Sochor J, Hynek D, Krejcova L, Fabrik I, Krizkova S, et al. (2012) Study of Metallothionein Role in Spinocellular Carcinoma Tissues of Head and Neck Tumours using Brdicka Reaction. International Journal of Electrochemical Science 7: 2136–2152. [Google Scholar]
- 57. McCluggage WG, Strand K, Abdulkadir A (2002) Immunohistochemical localization of metallothionein in benign and malignant epithelial ovarian tumors. International Journal of Gynecological Cancer 12: 62–65. [DOI] [PubMed] [Google Scholar]
- 58. Ozer H, Yenicesu G, Arici S, Cetin M, Tuncer E, et al. (2012) Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagnostic Pathology 7: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan Y, Sinniah R, Bay BH, Singh G (1999) Metallothionein expression and nuclear size-in benign, borderline, and malignant serous ovarian tumours. Journal of Pathology 189: 60–65. [DOI] [PubMed] [Google Scholar]
- 60. Zagorianakou N, Stefanou D, Makrydimas G, Zagorianakou P, Briasoulis E, et al. (2006) Clinicopathological study of metallothionein immunohistochemical expression, in benign, borderline and malignant ovarian epithelial tumors. Histology and Histopathology 21: 341–347. [DOI] [PubMed] [Google Scholar]
- 61. Murphy D, McGown AT, Crowther D, Mander A, Fox BW (1991) Metallothionein levels in ovarian-tumors before and after chemotherapy. British Journal of Cancer 63: 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Germain I, Tetu B, Brisson J, Mondor M, Cherian MG (1996) Markers of chemoresistance in ovarian carcinomas: An immunohistochemical study of 86 cases. International Journal of Gynecological Pathology 15: 54–62. [DOI] [PubMed] [Google Scholar]
- 63. Schmid KW, Greeff M, Hittmair A, Totsch M, Ofner D, et al. (1994) Metallothionein expression in normal, hyperplastic, and neoplastic thyroid follicular and parafollicular C-cells using monoclonal antimetallothionein antibody-E9. Endocrine Pathology 5: 114–122. [DOI] [PubMed] [Google Scholar]
- 64. Krolicka A, Kobierzycki C, Pula B, Podhorska-Okolow M, Piotrowska A, et al. (2010) Comparison of Metallothionein (MT) and Ki-67 Antigen Expression in Benign and Malignant Thyroid Tumours. Anticancer Research 30: 4945–4949. [PubMed] [Google Scholar]
- 65. Nartey N, Cherian MG, Banerjee D (1987) Immunohistochemical localization of metallothionein in human thyroid-tumors. American Journal of Pathology 129: 177–182. [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrario C, Lavagni P, Gariboldi M, Miranda C, Losa M, et al. (2008) Metallothionein 1G acts as an oncosupressor in papillary thyroid carcinoma. Laboratory Investigation 88: 474–481. [DOI] [PubMed] [Google Scholar]
- 67.Wei H, Desouki MM, Lin S, Xiao D, Franklin RB, et al. (2008) Differential expression of metallothioneins (MTs) 1, 2, and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues. Molecular Cancer 7. [DOI] [PMC free article] [PubMed]
- 68. Yamasaki M, Nomura T, Sato F, Mimata H (2007) Metallothionein is up-regulated under hypoxia and promotes the survival of human prostate cancer cells. Oncology Reports 18: 1145–1153. [PubMed] [Google Scholar]
- 69. Franklin R, Feng P, Milon B, Desouki M, Singh K, et al. (2005) hZIP 1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Molecular Cancer 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suzuki T, Yamanaka H, Tamura Y, Nakajima K, Kanatani K, et al. (1992) Metallothionein of prostatic tissues and fluids in rats and humans. Tohoku Journal of Experimental Medicine 166: 251–257. [DOI] [PubMed] [Google Scholar]
- 71. Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, et al. (2011) Insight to Physiology and Pathology of Zinc(II) Ions and Their Actions in Breast and Prostate Carcinoma. Current Medicinal Chemistry 18: 5041–5051. [DOI] [PubMed] [Google Scholar]
- 72. Costello LC, Franklin RB (2012) Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Review of Anticancer Therapy 12: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, et al. (2000) Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. Journal of Hepatology 33: 415–422. [DOI] [PubMed] [Google Scholar]
- 74. Huang GW, Yang LY (2002) Metallothionein expression in hepatocellular carcinoma. World Journal of Gastroenterology 8: 650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lu DD, Chen YC, Zhang XR, Cao XR, Jiang HY, et al. (2003) The relationship between metallothionein-1F (MT1F) gene and hepatocellular carcinoma. Yale Journal of Biology and Medicine 76: 55–62. [PMC free article] [PubMed] [Google Scholar]
- 76. Kanda M, Nomoto S, Okamura Y, Nishikawa Y, Sugimoto H, et al. (2009) Detection of metallothionein 1G as a methylated tumor suppressor gene in human hepatocellular carcinoma using a novel method of double combination array analysis. International Journal of Oncology 35: 477–483. [DOI] [PubMed] [Google Scholar]
- 77. Chan KYY, Lai PBS, Squire JA, Beheshti B, Wong NLY, et al. (2006) Positional expression profiling indicates candidate genes in deletion hotspots of hepatocellular carcinoma. Modern Pathology 19: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 78. Nakayama A, Fukuda H, Ebara M, Hamasaki H, Nakajima K, et al. (2002) A new diagnostic method for chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma based on serum metallothionein, copper, and zinc levels. Biological & Pharmaceutical Bulletin 25: 426–431. [DOI] [PubMed] [Google Scholar]
- 79. Kubo S, Fukuda H, Ebara M, Ikota N, Saisho H, et al. (2005) Evaluation of distribution patterns for copper and zinc in metallothionein and superoxide dismutase in chronic liver diseases and hepatocellular carcinoma using high-performance liquid chromatography (HPLC). Biological & Pharmaceutical Bulletin 28: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 80. Ebert MPA, Gunther T, Hoffmann J, Yu J, Miehlke S, et al. (2000) Expression of metallothionein II in intestinal metaplasia, dysplasia, and gastric cancer. Cancer Research 60: 1995–2001. [PubMed] [Google Scholar]
- 81. Galizia G, Ferraraccio F, Lieto E, Orditura M, Castellano P, et al. (2006) p27 downregulation and metallothionein overexpression in gastric cancer patients are associated with a poor survival rate. Journal of Surgical Oncology 93: 241–252. [DOI] [PubMed] [Google Scholar]
- 82. Janssen AML, van Duijn W, Oostendorp-van de Ruit MM, Kruidenier L, Bosman CB, et al. (2000) Metallothionein in human gastrointestinal cancer. Journal of Pathology 192: 293–300. [DOI] [PubMed] [Google Scholar]
- 83. Saika T, Tsushima T, Nasu Y, Akebi N, Noda M, et al. (1992) Histopathological study of metallothionein in bladder cancer and renal cell carcinoma. Nihon Hinyokika Gakkai Zasshi 83: 636–642. [DOI] [PubMed] [Google Scholar]
- 84. Zhou XD, Sens DA, Sens MA, Namburi V, Singh RK, et al. (2006) Metallothionein-1 and-2 expression in cadmium- or arsenic-derived human malignant urothelial cells and tumor heterotransplants and as a prognostic indicator in human bladder cancer. Toxicological Sciences 91: 467–475. [DOI] [PubMed] [Google Scholar]
- 85. Ishii K, Usui S, Yamamoto H, Sugimura Y, Tatematsu M, et al. (2001) Decreases of metallothionein and aminopeptidase N in renal cancer tissues. Journal of Biochemistry 129: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nguyen A, Jing Z, Mahoney PS, Davis R, Sikka SC, et al. (2000) In vivo gene expression profile analysis of metallothionein in renal cell carcinoma. Cancer Letters 160: 133–140. [DOI] [PubMed] [Google Scholar]
- 87. Hoey JG, Garrett SH, Sens MA, Todd JH, Sens DA (1997) Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicology Letters 92: 149–160. [DOI] [PubMed] [Google Scholar]
- 88. Hellemans G, Soumillion A, Proost P, Van Damme J, Van Poppel H, et al. (1999) Metallothioneins in human kidneys and associated tumors. Nephron 83: 331–340. [DOI] [PubMed] [Google Scholar]
- 89. Sugita K, Yamamoto O, Asahi M (2001) Immunohistochemical analysis of metallothionein expression in malignant melanoma in Japanese patients. American Journal of Dermatopathology 23: 29–35. [DOI] [PubMed] [Google Scholar]
- 90. Zelger B, Hittmair A, Schir M, Ofner C, Ofner D, et al. (1993) Immunohistochemically demonstrated metallothionein expression in malignant-melanoma. Histopathology 23: 257–264. [DOI] [PubMed] [Google Scholar]
- 91. Shukla VK, Aryya NC, Pitale A, Pandey M, Dixit VK, et al. (1998) Metallothionein expression in carcinoma of the gallbladder. Histopathology 33: 154–157. [DOI] [PubMed] [Google Scholar]
- 92. Bruewer M, Schmid KW, Krieglstein CF, Senninger N, Schuermann G (2002) Metallothionein: Early marker in the carcinogenesis of ulcerative colitis-associated colorectal carcinoma. World Journal of Surgery 26: 726–731. [DOI] [PubMed] [Google Scholar]
- 93. McCluggage WG, Maxwell P, Bharucha H (1998) Immunohistochemical detection of metallothionein and MIB1 in uterine cervical squamous lesions. International Journal of Gynecological Pathology 17: 29–35. [DOI] [PubMed] [Google Scholar]
- 94. El Sharkarvy SL, Farrag ARH (2008) Mean nuclear area and metallothionein expression in ductal breast tumors: Correlation with estrogen receptor status. Applied Immunohistochemistry & Molecular Morphology 16: 108–112. [DOI] [PubMed] [Google Scholar]
- 95. Tews DS, Nissen A, Kulgen C, Gaumann AKA (2000) Drug resistance-associated factors in primary and secondary glioblastomas and their precursor tumors. Journal of Neuro-Oncology 50: 227–237. [DOI] [PubMed] [Google Scholar]
- 96. Bier B, Douglasjones A, Totsch M, Dockhorndworniczak B, Bocker W, et al. (1994) Immunohistochemical demonstration of metallothionein in normal human breast-tissue and benign and malignant breast-lesions. Breast Cancer Research and Treatment 30: 213–221. [DOI] [PubMed] [Google Scholar]
- 97. Thirumoorthy N, Kumar KTM, Sundar AS, Panayappan L, Chatterjee M (2007) Metallothionein: An overview. World Journal of Gastroenterology 13: 993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Eid H, Geczi L, Bodrogi I, Institoris E, Bak M (1998) Do metallothioneins affect the response to treatment in testis cancers? Journal of Cancer Research and Clinical Oncology 124: 31–36. [DOI] [PubMed] [Google Scholar]
- 99. Meijer C, Timmer A, De Vries EGE, Groten JP, Knol A, et al. (2000) Role of metallothionein in cisplatin sensitivity of germ-cell tumours. International Journal of Cancer 85: 777–781. [DOI] [PubMed] [Google Scholar]
- 100. Huang Y, De La Chapelle A, Pellegata NS (2003) Hypermethylation, but not LOH, is associated with the low expression of MT1G and CRABP1 in papillary thyroid carcinoma. International Journal of Cancer 104: 735–744. [DOI] [PubMed] [Google Scholar]
- 101. Dziegiel P, Jelen M, Muszczynska B, Maciejczyk A, Szulc A, et al. (2004) Role of metallothionein expression in non-small cell lung carcinomas. Roczniki Akademii Medycznej w Bialymstoku 49 Suppl 143–45. [PubMed] [Google Scholar]
- 102. Endo T, Yoshikawa M, Ebara M, Kato K, Sunaga M, et al. (2004) Immunohistochemical metallothionein expression in hepatocellular carcinoma: relation to tumor progression and chemoresistance to platinum agents. Journal of Gastroenterology 39: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 103. Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O (2010) Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods 52: 316–321. [DOI] [PubMed] [Google Scholar]
- 104. Cortesi M, Fridman E, Volkov A, Shilstein SS, Chechik R, et al. (2008) Clinical assessment of the cancer diagnostic value of prostatic Zinc: A comprehensive needle-biopsy study. Prostate 68: 994–1006. [DOI] [PubMed] [Google Scholar]
- 105. Bouzourene H, Chaubert P, Gebhard S, Boman FT, Coucke P (2002) Role of metallothioneins in irradiated human rectal carcinoma. Cancer 95: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 106. Li SY, Yu B, An P, Zuo FY, Cai HY (2005) Expression of metallothionein and FasL in colorectal cancer and its clinical significance. Zhonghua Wai Ke Za Zhi (Chinese Journal of Surgery) 43: 1118–1120. [PubMed] [Google Scholar]
- 107. Wulfing C, van Ahlen H, Eltze E, Piechota H, Hertle L, et al. (2007) Metallothionein in bladder cancer: correlation of overexpression with poor outcome after chemotherapy. World Journal of Urology 25: 199–205. [DOI] [PubMed] [Google Scholar]
- 108. Ioachim EE, Kitsiou E, Carassavoglou C, Stefanaki S, Agnantis NJ (2000) Immunohistochemical localization of metallothionein in endometrial lesions. Journal of Pathology 191: 269–273. [DOI] [PubMed] [Google Scholar]
- 109. McCluggage WG, Maxwell P, Hamilton PW, Jasani B (1999) High metallothionein expression is associated with features predictive of aggressive behaviour in endometrial carcinoma. Histopathology 34: 51–55. [DOI] [PubMed] [Google Scholar]
- 110. Izawa JI, Moussa M, Cherian MG, Doig G, Chin JL (1998) Metallothionein expression in renal cancer. Urology 52: 767–772. [DOI] [PubMed] [Google Scholar]
- 111. Zhang XH, Takenaka I (1998) Incidence of apoptosis and metallothionein expression in renal cell carcinoma. British Journal of Urology 81: 9–13. [DOI] [PubMed] [Google Scholar]
- 112. Bay BH, Jin RX, Huang JX, Tan PH (2006) Metallothionein as a prognostic biomarker in breast cancer. Experimental Biology and Medicine 231: 1516–1521. [DOI] [PubMed] [Google Scholar]
- 113. Dumanska M, Dziegiel P, Sopel M, Wojnar A, Zabel M (2004) Evaluation of apoptosis, proliferation intensity and metallothionein (MT) expression in comparison with selected clinicopathological variables in primary adenocarcinomas of the large intestine. Folia Morphologica (Poland) 63: 107–110. [PubMed] [Google Scholar]
- 114. DouglasJones AG, Navabi H, Morgan JM, Jasani B (1997) Immunoreactive p53 and metallothionein expression in duct carcinoma in situ of the breast - No correlation. Virchows Archiv 430: 373–379. [DOI] [PubMed] [Google Scholar]
- 115. Gallicchio L, Flaws JA, Sexton M, Ioffe OB (2004) Cigarette smoking and metallothionein expression in invasive breast carcinomas. Toxicology Letters 152: 245–253. [DOI] [PubMed] [Google Scholar]
- 116. Gomulkiewicz A, Podhorska-Okolow M, Szulc R, Smorag Z, Wojnar A, et al. (2010) Correlation between metallothionein (MT) expression and selected prognostic factors in ductal breast cancers. Folia Histochemica et Cytobiologica 48: 242–248. [DOI] [PubMed] [Google Scholar]
- 117. Haerslev T, Jacobsen GK, Zedeler K (1995) The prognostic-significance of immunohistochemically detectable metallothionein in primary breast carcinomas. APMIS 103: 279–285. [DOI] [PubMed] [Google Scholar]
- 118. Zhang R, Zhang H, Wei H, Luo X (2000) Expression of metallothionein in invasive ductal breast cancer in relation to prognosis. Journal of Environmental Pathology Toxicology and Oncology 19: 95–97. [PubMed] [Google Scholar]
- 119. Jin RX, Bay BH, Chow VTK, Tan PH (2001) Metallothionein 1F mRNA expression correlates with histological grade in breast carcinoma. Breast Cancer Research and Treatment 66: 265–272. [DOI] [PubMed] [Google Scholar]
- 120. Muramatsu Y, Hasegawa Y, Fukano H, Ogawa T, Namuba M, et al. (2000) Metallothionein immunoreactivity in head and neck carcinomas; Special reference to clinical behaviors and chemotherapy responses. Anticancer Research 20: 257–264. [PubMed] [Google Scholar]
- 121. Athanassiadou P, Bantis A, Gonidi M, Athanassiades P, Agelonidou E, et al. (2007) The expression of metallothioneins on imprint smears of prostate carcinoma: Correlation with clinicopathologic parameters and tumor proliferative capacity. Tumori 93: 189–194. [DOI] [PubMed] [Google Scholar]
- 122. Moussa M, Kloth D, Peers G, Cherian MG, Frei JV, et al. (1997) Metallothionein expression in prostatic carcinoma: correlation with Gleason grade, pathologic stage, DNA content and serum level of prostate-specific antigen. Clinical and Investigative Medicine-Medecine Clinique Et Experimentale 20: 371–380. [PubMed] [Google Scholar]
- 123. El Sharkawy SL, Abbas NF, Badawi MA, El Shaer MA (2006) Metallothionein isoform II expression in hyperplastic, dysplastic and neoplastic prostatic lesions. Journal of Clinical Pathology 59: 1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hengstler JG, Pilch H, Schmidt M, Dahlenburg H, Sagemuller J, et al. (2001) Metallothionein expression in ovarian cancer in relation to histopathological parameters and molecular markers of prognosis. International Journal of Cancer 95: 121–127. [DOI] [PubMed] [Google Scholar]
- 125. Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, et al. (2005) Augmented expression of metallothionein and glutathione S-transferase pi as unfavourable prognostic factors in cisplatin-treated ovarian cancer patients. Virchows Archiv 447: 626–633. [DOI] [PubMed] [Google Scholar]
- 126. Theocharis S, Karkantaris C, Philipides T, Agapitos E, Gika A, et al. (2002) Expression of metallothionein in lung carcinoma: correlation with histological type and. Histopathology 40: 143–151. [DOI] [PubMed] [Google Scholar]
- 127. Hishikawa Y, Koji T, Dhar DK, Kinugasa S, Yamaguchi M, et al. (1999) Metallothionein expression correlates with metastatic and proliferative potential in squamous cell carcinoma of the oesophagus. British Journal of Cancer 81: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weinlich G, Topar G, Eisendle K, Fritsch PO, Zelger B (2007) Comparison of metallothionein-overexpression with sentinel lymph node biopsy as prognostic factors in melanoma. Journal of the European Academy of Dermatology and Venereology 21: 669–677. [DOI] [PubMed] [Google Scholar]
- 129. Haerslev T, Jacobsen K, Nedergaard L, Zedeler K (1994) Immunohistochemical detection of metallothionein in primary breast carcinomas and their axillary lymph-node metastases. Pathology Research and Practice 190: 675–681. [DOI] [PubMed] [Google Scholar]
- 130. Schmid KW, Ellis IO, Gee JMW, Darke BM, Lees WE, et al. (1993) Presence and possible significance of immunocytochemically demonstrable metallothionein over-expression in primary invasive ductal carcinoma of the breast. Virchows Archiv A, Pathological Anatomy and Histopathology 422: 153–159. [DOI] [PubMed] [Google Scholar]
- 131. Werynska B, Pula B, Muszczynska-Bernhard B, Gomulkiewicz A, Piotrowska A, et al. (2013) Metallothionein 1F and 2A overexpression predicts poor outcome of non-small cell lung cancer patients. Experimental and Molecular Pathology 94: 301–308. [DOI] [PubMed] [Google Scholar]
- 132. Cardoso SV, Barbosa HM, Candellori IM, Loyola AM, Aguiar MCF (2002) Prognostic impact of metallothionein on oral squamous cell carcinoma. Virchows Archiv 441: 174–178. [DOI] [PubMed] [Google Scholar]
- 133. Ma HQ, Sun HH, Huang FX, Li J, Cao XL, et al. (2009) Expression of ERCC1, Bcl-2, MT and their Clinical Significance in Advanced Non-small-cell Lung Cancer Treated With Cisplatin-based Chemotherapy. Latin American Journal of Pharmacy 28: 827–834. [Google Scholar]
- 134. Dziegiel P, Forgacz J, Suder E, Surowiak P, Kornafel J, et al. (2003) Prognostic significance of metallothionein expression in correlation with Ki-67 expression in adenocarcinomas of large intestine. Histology and Histopathology 18: 401–407. [DOI] [PubMed] [Google Scholar]
- 135.Mitropoulos D, Kyroudi-Voulgari A, Theocharis S, Serafetinides E, Moraitis E, et al. (2005) Prognostic significance of metallothionein expression in renal cell carcinoma. World Journal of Surgical Oncology 3. [DOI] [PMC free article] [PubMed]
- 136. Aloia TA, Harpole DH, Reed CE, Allegra C, Moore MBH, et al. (2001) Tumor marker expression is predictive of survival in patients with esophageal cancer. Annals of Thoracic Surgery 72: 859–866. [DOI] [PubMed] [Google Scholar]
- 137. Joseph MG, Banerjee D, Kocha W, Feld R, Stitt LW, et al. (2001) Metallothionein expression in patients with small cell carcinoma of the lung - Correlation with other molecular markers and clinical outcome. Cancer 92: 836–842. [PubMed] [Google Scholar]
- 138. Ryvolova M, Adam V, Kizek R (2012) Analysis of metallothionein by capillary electrophoresis. Journal of Chromatography A 1226: 31–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prisma 2009 checklist.
(DOC)
Prisma 2009 flow diagram showing the number of citations retrieved by database searching.
(DOC)