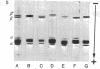
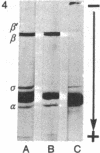
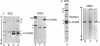Abstract
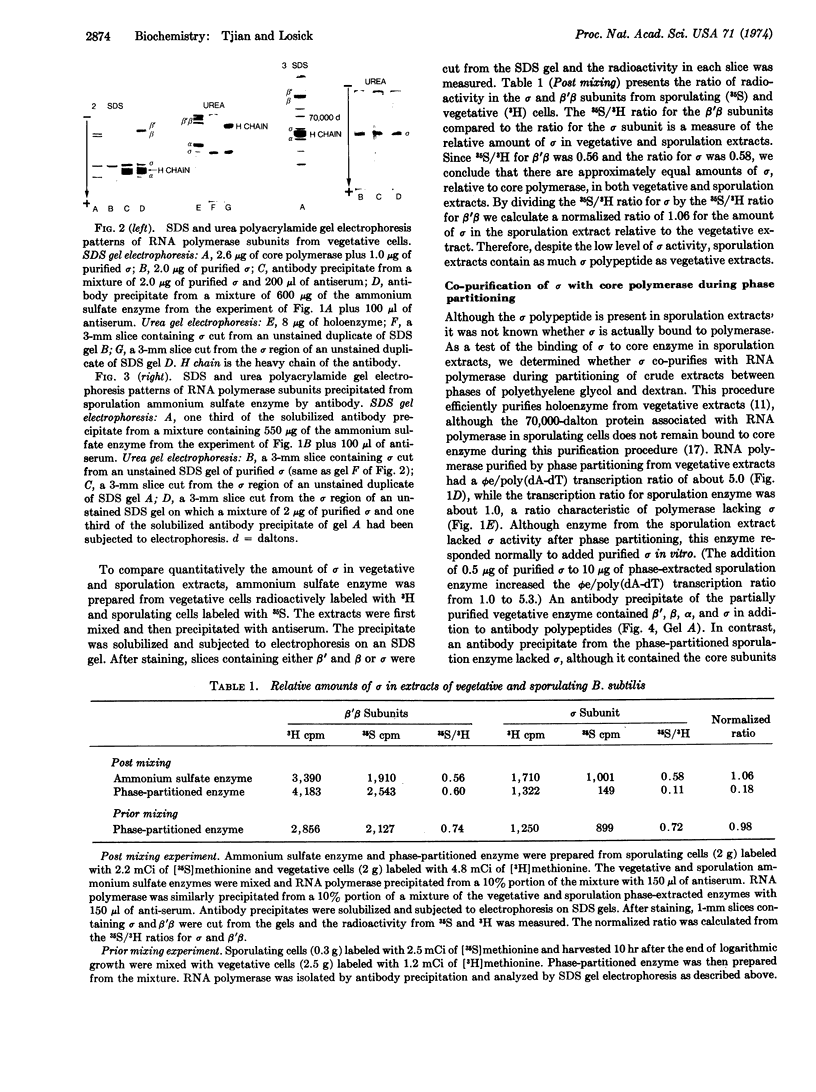
The activity of the σ subunit of Bacillus subtilis RNA polymerase decreases markedly during the first hours of sporulation [T.G. Linn et al. (1973) Proc. Nat. Acad. Sci. USA 70, 1865-1869]. We have prepared antibody against RNA polymerase holoenzyme to determine the fate of σ polypeptide during spore formation. This antiserum specifically and independently precipitates σ and core polymerase from crude extracts of B. subtilis as judged by both sodium dodecyl sulfate and urea gel electrophoresis of the precipitates. We report that crude extracts of sporulating cells lacking σ activity contain as much σ polypeptide as extracts of vegetative cells. However, σ polypeptide in extracts from sporulating cells is apparently only weakly associated with RNA polymerase, as indicated by the failure of σ to co-purify efficiently with core enzyme during phase partitioning.
The loss of σ activity and the weak binding of σ to core enzyme occurs normally in a mutant blocked at an intermediate stage of sporulation (SpoII-4Z) and in wild-type bacteria sporulating in 121B medium, Difco sporulation medium, or Sterlini-Mandelstam resuspension medium. In contrast, σ in two mutants (SpoOa-5NA and SpoOb-6Z) blocked at an early stage of spore formation remains active and tightly associated with RNA polymerase during stationary phase.
Keywords: antibody precipitation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Brevet J. Direct assay for sigma factor activity and demonstration of the loss of this activity during sporulation in Bacillus subtilis. Mol Gen Genet. 1974 Feb 6;128(3):223–221. doi: 10.1007/BF00267111. [DOI] [PubMed] [Google Scholar]
- Brevet J., Sonenshein A. L. Template specificity of ribonucleic acid polymerase in asporogenous mutants of Bacillus subtilis. J Bacteriol. 1972 Dec;112(3):1270–1274. doi: 10.1128/jb.112.3.1270-1274.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L., Linn T. G., Losick R. Isolation of a new RNA polymerase-binding protein from sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):490–494. doi: 10.1073/pnas.70.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H., Michel J., Cami B., Schaeffer P. Symposium on bacterial spores: II. Genetics of sporulation in Bacillus subtilis Marburg. J Appl Bacteriol. 1970 Mar;33(1):13–24. doi: 10.1111/j.1365-2672.1970.tb05230.x. [DOI] [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Orrego C., Kerjan P., Manca de Nadra M. C., Szulmajster J. Ribonucleic acid polymerase in a thermosensitive sporulation mutant (ts-4) of Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):636–647. doi: 10.1128/jb.116.2.636-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorenstein R. G., Losick R. Purification and properties of the sigma subunit of ribonucleic acid polymerase from vegetative Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6163–6169. [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]