Abstract
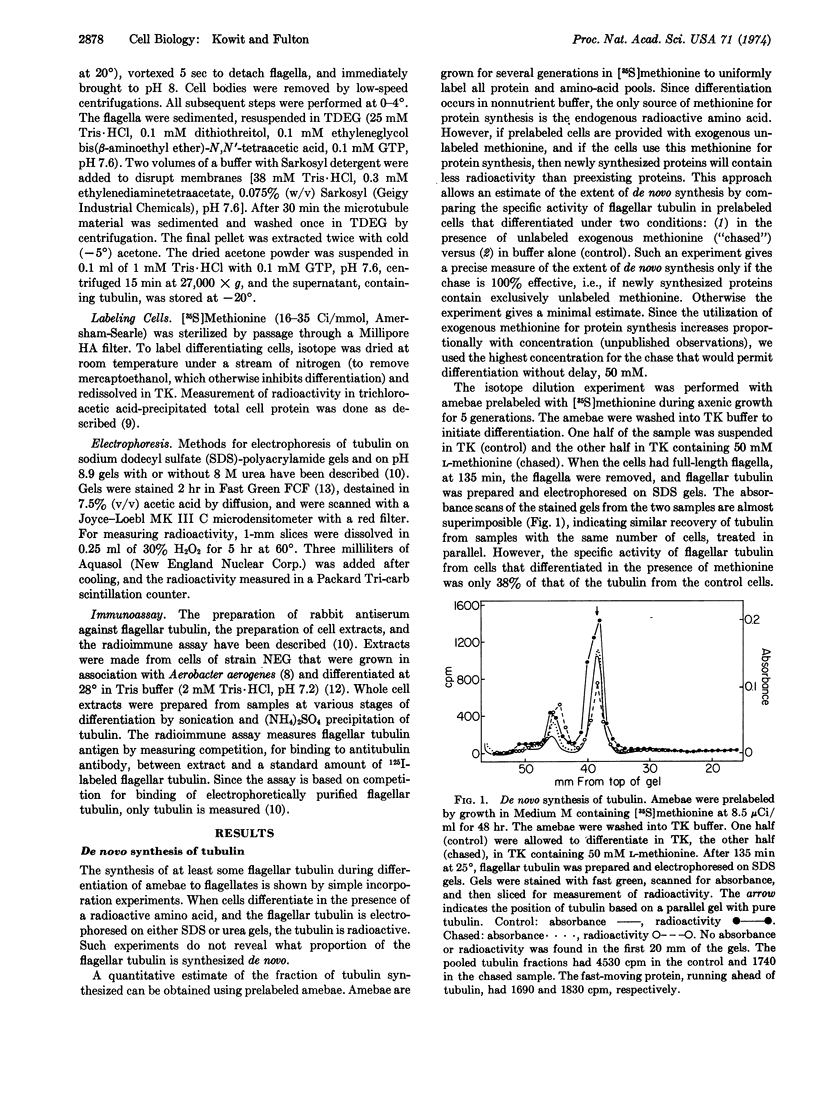
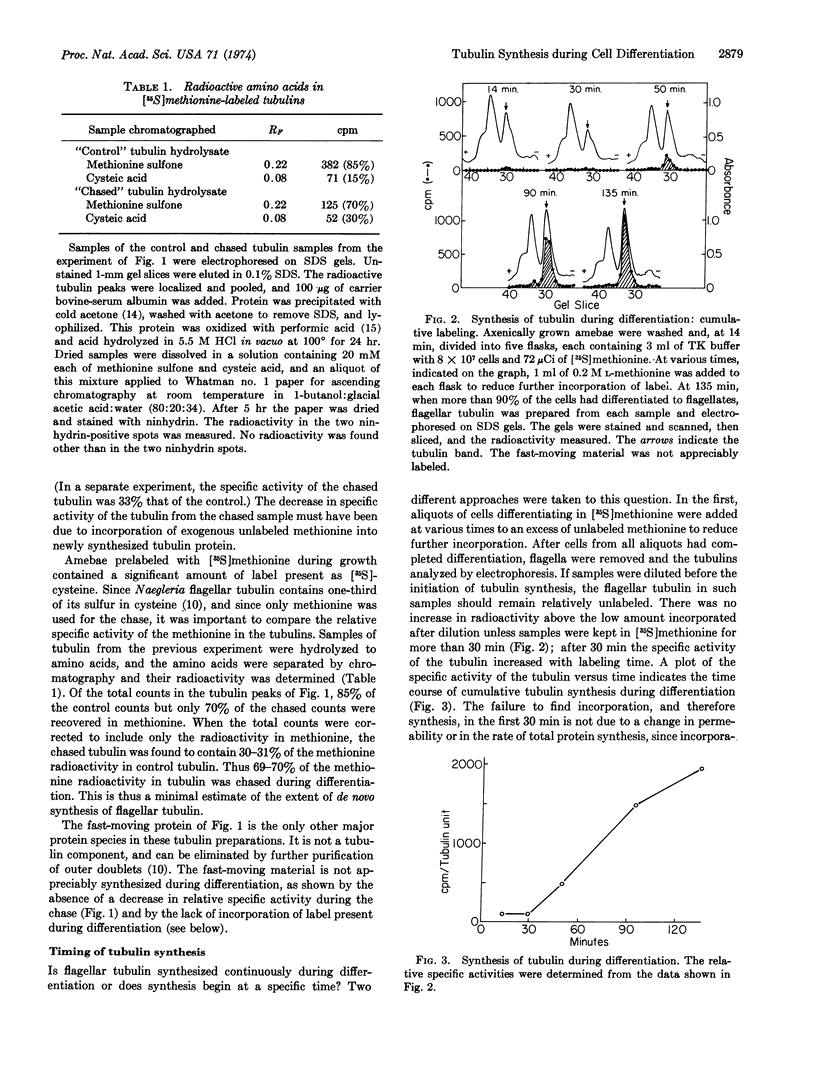
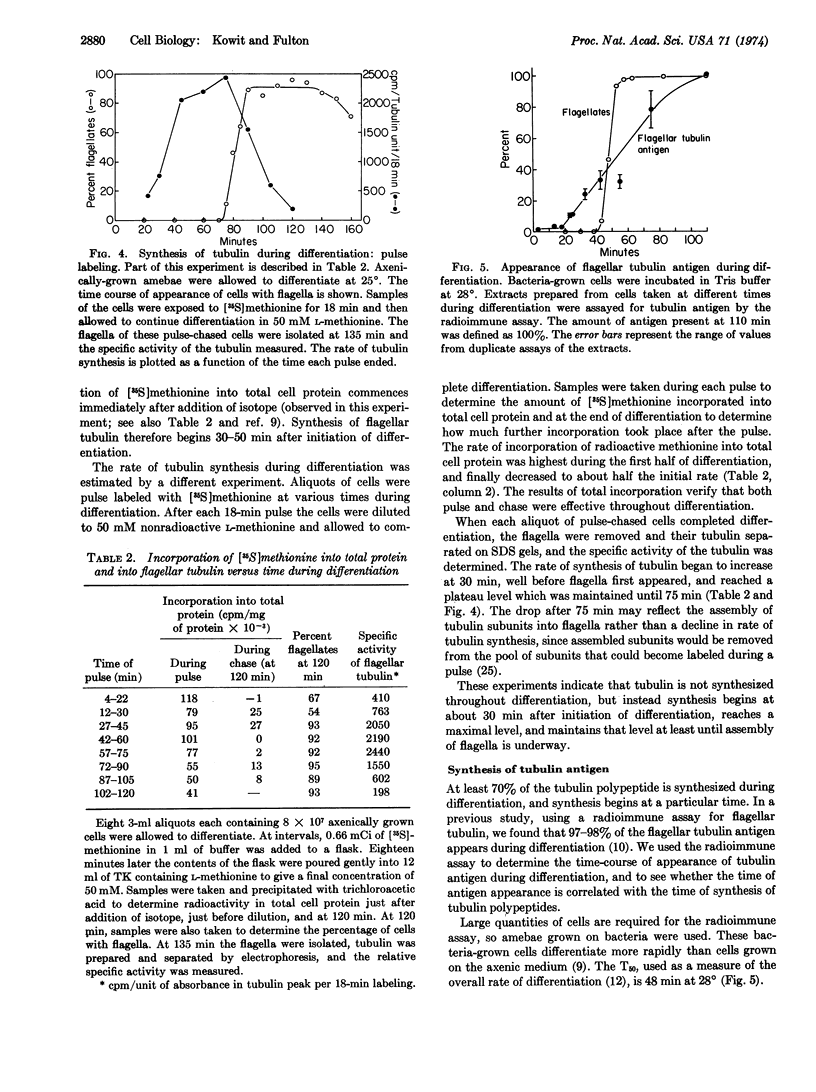
Amebae of Naegleria gruberi differentiate into flagellates when transferred from growth medium to nonnutrient buffer. Experiments were performed to determine whether the tubulin that forms the flagellar microtubules pre-exists in amebae or is synthesized during differentiation. Amebae prelabeled uniformly with [35S]methionine were allowed to differentiate in the presence and in the absence of exogenous unlabeled methionine. In the presence of unlabeled methionine the flagellar tubulin contained only 30% as much [35S]methionine as in its absence. Thus at least 70% of the tubulin was synthesized de novo. Isotope dilution and pulse experiments showed that flagellar tubulin synthesis began one-third of the way through differentiation, before any morphological change had occurred. Flagellar tubulin antigen, as measured using a specific antiserum, also began to increase one-third of the way through differentiation and increased 35- to 55-fold during the course of differentiation. These experiments demonstrate that most if not all of the flagellar tubulin is synthesized de novo during differentiation.
Keywords: microtubules, isotope dilution, gel electrophoresis, radioimmune assay
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auclair W., Siegel B. W. Cilia regeneration in the sea urchin embryo: evidence for a pool of ciliary proteins. Science. 1966 Nov 18;154(3751):913–915. doi: 10.1126/science.154.3751.913. [DOI] [PubMed] [Google Scholar]
- Behnke O., Forer A. Evidence for four classes of microtubules in individual cells. J Cell Sci. 1967 Jun;2(2):169–192. doi: 10.1242/jcs.2.2.169. [DOI] [PubMed] [Google Scholar]
- Coyne B., Rosenbaum J. L. Flagellar elongation and shortening in chlamydomonas. II. Re-utilization of flagellar proteins. J Cell Biol. 1970 Dec;47(3):777–781. doi: 10.1083/jcb.47.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton C., Dingle A. D. Appearance of the flagellate phenotype in populations of Naegleria amebae. Dev Biol. 1967 Feb;15(2):165–191. doi: 10.1016/0012-1606(67)90012-7. [DOI] [PubMed] [Google Scholar]
- Fulton C., Dingle A. D. Basal bodies, but not centrioles, in Naegleria. J Cell Biol. 1971 Dec;51(3):826–836. doi: 10.1083/jcb.51.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. MOLECULAR FACETS OF MITOTIC REGULATION, I. SYNTHESIS OF THYMIDINE KINASE. Proc Natl Acad Sci U S A. 1963 May;49(5):648–654. doi: 10.1073/pnas.49.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Purification and properties of flagellar outer doublet tubulin from Naegleria gruberi and a radioimmune assay for tubulin. J Biol Chem. 1974 Jun 10;249(11):3638–3646. [PubMed] [Google Scholar]
- Linck R. W. Chemical and structural differences between cilia and flagella from the lamellibranch mollusc, Aequipecten irradians. J Cell Sci. 1973 May;12(3):951–981. doi: 10.1242/jcs.12.3.951. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTTER W. J., WESSELLS N. K., GROBSTEIN C. CONTROL OF SPECIFIC SYNTHESIS IN THE DEVELOPING PANCREAS. Natl Cancer Inst Monogr. 1964 Apr;13:51–65. [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



