Abstract
The laminin receptor (LamR) is a cell surface receptor for extracellular matrix laminin, whereas the same protein within the cell interacts with ribosomes, nuclear proteins and cytoskeletal fibers. LamR has been shown to be a receptor for several bacteria and viruses. Furthermore, LamR interacts with both cellular and infectious forms of the prion protein, PrPC and PrPSc. Indeed, LamR is a receptor for PrPC. Whether LamR interacts with PrPSc exclusively in a capacity of the PrP receptor, or LamR specifically recognizes prion determinants of PrPSc, is unclear. In order to explore whether LamR has a propensity to interact with prions and amyloids, we examined LamR interaction with the yeast prion-forming protein, Sup35. Sup35 is a translation termination factor with no homology or functional relationship to PrP. Plasmids expressing LamR or LamR fused with the green fluorescent protein (GFP) were transformed into yeast strain variants differing by the presence or absence of the prion conformation of Sup35, respectively [PSI +] and [psi −]. Analyses by immunoprecipitation, centrifugal fractionation and fluorescent microscopy reveal interaction between LamR and Sup35 in [PSI +] strains. The presence of [PSI +] promotes LamR co-precipitation with Sup35 as well as LamR aggregation. In [PSI +] cells, LamR tagged with GFP or mCherry forms bright fluorescent aggregates that co-localize with visible [PSI +] foci. The yeast prion model will facilitate studying the interaction of LamR with amyloidogenic prions in a safe and easily manipulated system that may lead to a better understanding and treatment of amyloid diseases.
Introduction
The laminin receptor-1 (LamR) is a multifunctional protein required for cell viability [1], [2], [3]. Originally isolated by its ability to bind laminin-1 [4], [5], [6], LamR has since been identified as a highly conserved ribosomal protein that has evolved extra-ribosomal functions in multicellular organisms [7].
As a cell surface receptor, LamR functions in cell migration [8], through interaction with and remodeling of the extracellular matrix [9]. In tumor cells these functions have been associated with increased invasiveness and metastasis [9], [10], [11], [12]. The role of LamR in cellular translation and proliferation [13] may account for the upregulation of LamR expression in tumor cells [10], [11]. LamR has also been identified as a receptor for bacterial [14], [15] and viral infections [16], [17], [18], [19]. For a review of LamR and associated pathologies see [20].
The LamR is also a receptor for cellular [21], [22] and infectious [23], [24] prion proteins, PrPC and PrPSc, respectively. The LamR was identified as an interacting partner with the human prion protein, PrP, in a yeast two-hybrid screen [22]. Function of LamR as a cell surface receptor for the cellular prion protein, PrPC, has been shown by co-localization of LamR and PrPC on the surface of mouse neuronal cells, as well as by the ability of LamR antibody to block exogenous PrPC cellular binding and subsequent internalization [21]. Physiological significance of LamR interaction with PrPSc has been demonstrated by the prevention of prion propagation in neuronal cells by incubation with LamR antibody or by transfection of LamR antisense RNA and siRNA [25]. Cultures of intestinal enterocytes have been shown to internalize bovine spongiform encephalopathy prions through binding to cell surface LamR [24], indicating that LamR may be involved in the initial stage of prion infections. Similarly, co-localization of LamR with scrapie and chronic wasting disease prions has also been demonstrated [23].
PrPSc-associated prion disease belongs to the broader class of pathologies known as amyloidoses, among which are Alzheimer’s and Parkinson’s diseases. Prions propagate by conversion of soluble prionogenic PrPC proteins into the aggregated PrPSc form in a concentration- and nucleation-dependent manner, similar to the process of amyloidosis. Upon conversion to the prion state, the proteins adopt a cross-beta-sheet-rich structure, typical of amyloids. Purified recombinant PrP proteins can polymerize to amyloid fibers, which are resistant to SDS denaturation and bind to the amyloid-binding fluorophore thioflavin T and Congo Red dye [26]. However, the relationship between PrP amyloid forming in vitro, and known as PrPRes, and prion infectivity is not completely defined. Infectious PrPSc and recombinant PrPRes fibrils have been shown to differ structurally and to have different seeding specificities [27].
Starting from 1994, several prions and prion-like proteins have been identified in yeast Saccharomyces cerevisiae (reviewed in [28], [29]). While the physiological importance of the ability to form self-propagating structures is a subject of debate, the occurrence of prion proteins is evolutionally conserved, and, for several yeast prions, numerous prion-bearing strains were isolated from nature [29], [30], [31]. Prionogenic proteins do not share amino acid sequence or functional homology. The tendency to form amyloid structure appears to be dependent upon amino acid composition: abundance of polar residues and paucity of hydrophobic and charged residues [31]. Yeast prions show typical amyloidogenic properties (reviewed in [29], [32], [33]).
Sup35, a yeast prion-forming protein that has been extensively studied, is a translational termination factor (eRF3) in its soluble form [34], [35]. However, when aggregated as a prion [36], [37], [PSI+], Sup35 is unavailable to terminate protein synthesis. Under this condition, protein termination is suppressed as ribosomes occasionally read through stop codons. Introduction of a stop codon mutation within a gene encoding the metabolic enzyme, ADE1 [38], engendered a model system for studying yeast prionogenesis. The model utilizes the different phenotypes of prion positive [PSI +] vs prion negative [psi −] strains [39], [40]. This model has already produced many valuable studies into the nature of prion propagation and amyloidogenesis [31], [41], [42], [43], [44]. Furthermore, a yeast [PSI+]-based model was used for drug-screening: compounds isolated for their ability to affect yeast prions in this system, have also been demonstrated to be effective against PrP prions in mammalian cell assays [45].
In this study, we utilize the yeast Sup35/[PSI+] prion model system to investigate the putative propensity of LamR to interact with prionogenic proteins by examining the association of LamR with non-PrP prions. Yeast plasmids expressing LamR were introduced into Saccromyces cerivceae [PSI +] and [psi −] strains. Evidence from immunoprecipitation, high-speed centrifugation assays and fluorescent microscopy reveal an interaction between LamR and Sup35 in [PSI +] cells, indicating that LamR interacts with Sup35-based prion protein.
Materials and Methods
Yeast Strains
Yeast strains used are derivatives of 74-D694 (MATa ade1-14 his3- Δ200 ura3-52 leu2-3, 112 trp1-289) [psi −][PIN+] [39]. [PSI+][PIN+] are strong and weak [PSI +] isolates (L1762 and L1759, respectively) obtained by overexpression of the Sup35 prion domain [40]. Isolates [psi −][pin −] (L1951) and strong (L1763) and weak (L1759) [PSI+][pin −] were obtained from [psi −][PIN+] (L1749), strong [PSI+][PIN+] (L1762) and weak [PSI+][PIN+] (1758), respectively, by curing [PIN+] upon growing yeast on media containing 5 mM GuHCl [46], [47].
Plasmids and Transformation
The centromeric pRS400 series plasmids are the backbone for plasmids used in this study [48]. The URA3 pRS416-based pCUP1-GFP encodes GFP expressed under the control of the copper inducible CUP1 promoter, and pCUP1-SUP35::GFP encodes the SUP35 ORF fused to the N-terminus of GFP [49]. These plasmids were a gift from S. Lindquist to I. Derkatch.
PCR fragments, synthesized from a human LamR expression vector [50], were generated to construct pCUP1-LamR and pCUP1-LamR::GFP; 5′ primer: BamHI GGATCC ATGTCCGGAGCCCTTGATGTCC; 3′ primers: SacII CCGCGG TTAAGACCAGTCAGTGGTTGCTCC with a stop codon at the end of the LamR ORF, and SacII CCGCGG AGACCAGTCAGTGGTTGCTCCTAC for LamR::GFP. Fragments were inserted into the pCUP1-GFP plasmid (see above).
To co-express the URA3 pCUP1-SUP35::GFP with wild type or mCherry-tagged LamR, an XhoI/SacII fragment, containing the CUP1 promoter and LAMR, was excised, from either the pRS416-pCUP1-LamR or the pRS416-pCUP1-LamR::GFP construct, and subcloned into the LEU plasmid, pRS415, to confer selective growth in leucineless medium. An mCherry-encoding PCR fragment was synthesized from a pmCherry vector (Clonetech, #632522) using primers: 5′ Primer: SacII CCGCGG ATGGTGAGCAAGGGC; 3′ primer SacII CCGCGG CTACAGCTCGTCCATGC. The SacII/SacII mCherry fragment was cloned into the SacII site of the construct with the CUP1-LAMR insert originating from pRS416-pCUP-LamR::GFP, to generate a LamR::mCherry fusion protein.
Standard yeast media and procedures were used [51]. Yeast transformants were grown in synthetic dextrose media selective for plasmid maintenance: SD-Ura, SD-Leu, or SD-Ura-Leu [51]. To induce the CUP1 promoter, media were supplemented with 25 µM CuSO4.
Yeast Lysates
Yeast cell lysates were prepared from mid-log cultures grown overnight at 30°C in plasmid-selective media supplemented with 25 µM CuSO4 (unless stated otherwise). Cells were harvested by centrifugation at 800×g for 10 min and washed with distilled H2O at RT. Pellets were resuspended in 2× volume lysis buffer [50 mM TrisHCl pH 7.5, 250 mM NaCl, 10 mM MgCl2, 5% w/v glycerol, anti-protease cocktail for yeast (Sigma) and 0.1 M AEBSF (Sigma)] and disrupted by beating with an equal volume of acid washed glass beads (425–600 µm, Sigma) [10 pulses of vortexing for 30 secs each, placing tubes on ice between vortexing to avoid heating]. Cell disruption was monitored microscopically. Lysates were pre-cleared at 800×g for 10 min.
Western Blot Analysis and Antibodies
Protein concentrations were measured using BioRad Dc Protein Reagent. Proteins were separated on 4–15% gradient SDS-polyacrylamide gels (BioRad) under reducing conditions. Proteins were transferred to polyvinylidene fluoride membrane (Millipore) in Tris-glycine buffer pH 7.5 containing 10% methanol. Filters were blocked at RT in 5% non-fat dry milk in TBST [0.1 M TrisHCl pH 7.5, 0.15 M NaCl, and 0.1% Tween-20 (Sigma)]. Incubation with primary antibodies was overnight at 4°C. After TBST wash (4×5 min), appropriate secondary HRP-conjugated antibodies were applied for 90 min at RT. Filters were washed, as above, then developed with ECL (Pierce) and exposed to Highblot CL autoradiography film. Films were scanned using an Epson V600 scanner. Densitometry was performed using NIH ImageJ 1.44f software [http://rsb.info.nih.gov/ij].
Antibodies used were: anti-LamR 1∶1000 (H-2, mouse mAb, 74515 Santa Cruz) in 5% milk; anti-Sup35 1:1000 in PBS (BE4 mouse mAb, [52], a gift from Susan Liebman to ILD); anti-GFP 1∶1000 (Invitrogen rabbit pAb, A11122) in 5% milk; anti-yeast hexokinase-HRP 1∶1000 (Abcam, ab34588) in 5% milk.
Coimmunoprecipitation
Immunoprecipitation was performed using magnetic, Protein G, Dynabeads (Dynal, Invitrogen) according to manufacturer’s protocol. Anti-GFP antibody (10 µg) was adsorbed to 50 µl bead slurry. Control beads were prepared with 10 µg rabbit IgG. Yeast cell lysates (500 µg in 200 µl lysis buffer) were added to prepared antibody-bound beads and rotated overnight at 4°C. Beads were removed magnetically, and supernatant removed as unbound (flow thru, FT) fraction. Beads were washed with 500 µl PBS with 0.02% Tween-20 (PBST), transferred to a new tube with PBST and washed an additional 2×. One ml TBST was added to separated beads. Beads were transferred to a new tube and washed 3× in TBST. Bound protein was removed by incubation of separated beads at 70°C, 10 min in 1× SDS PAGE buffer (50 µl). Eluted protein (25 µl) and 20 µg total cell lysate and FT fraction were separated on SDS PAGE gels and analyzed by western blot.
Fluorescence Microscopy
Cultures of yeast transformants, grown at 30°C in plasmid selective media supplemented with 25 µM CuSO4 from 0.02 OD 600 nm to late-log (48 hours) were viewed with the Plan Fluor 100x/1.3 oil DIC lens of a Nikon TE-2000E fluorescent microscope using a 488 nmex, 507 nmem filter for GFP and a 589 nmex 615 nmem filter for mCherry. Images were captured with a Nikon CoolSnap EZ camera and processed with NIS Elements V2.3 software.
High Speed Centrifugation Analysis
Lysates for centrifugation assays [36] were prepared as described above except that RNAse A (400 µg/ml) was added to yeast lysate buffer to disrupt ribosomes and lysates were precleared at 8000×g, 3 min [53]. Approximately 200 µl lysate was spun, 30 min, at 100,000×g in a Beckman TLA 120.1 rotor (Beckman Optima TLX ultracentrifuge). Supernatants were carefully removed and pellets resuspended in equal volume (200 µl) of lysis buffer. Equal amounts (20 µg) of total, supernatant and pellet protein were analyzed by western blot.
Results
Expression of Human LamR in Yeast Cell
The human LamR was expressed under the control of the copper inducible CUP1 promoter from a low-copy yeast plasmid. Both untagged and C-terminal GFP fusion constructs were made. The size of the expressed proteins was as expected, ∼37 and 64 kDa, respectively, and expression levels were similar for the untagged LamR and the GFP LamR fusion proteins (Fig. 1A, right panel and left panel, respectively). The GFP fusion construct was utilized to enable intracellular visualization of LamR and provide an epitope for immunoprecipitation.
Figure 1. Human LamR is expressed in yeast cells.
A. LamR protein (right panel) or LamR-GFP fusion (left panel) expressed in the [PSI +][PIN+] yeast prion strain grown in synthetic media either supplemented with 25 µM CuSO4 (right panel and lane 2 of left panel) or containing no excess copper (lane1 of left panel). B. Expression of GPF (27 kDa), and LamR-GFP and Sup35-GFP fusion proteins (64 kDa and 104 kDa, respectively) in [psi −][PIN+] (−) and [PSI+][PIN+] (+) yeast strains. Anti-LamR (A) and anti-GFP (B) antibodies were used to detect LamR expression in yeast lysates (25 ug). Numbers in the middle (A) and right (B) refer to protein size markers (kDa). Similar expression levels were observed in [pin −] strains (not shown).
Figure 1A (left panel) shows that the CUP1 promoter induction, in the presence of 25 µM CuSO4, enhanced expression of the 64 kDa LamR-GFP fusion protein compared to basal level expression observed in the cells grown in media with no excess CuSO4. The right panel shows expression of the untagged LamR grown in the presence of 25 µM CuSO4. While the mouse monoclonal LamR antibody H-2, raised against a polypeptide including amino acids 110–150 of the LamR, was able to recognize the human LamR protein in yeast cell lysates, the antibody does not react with any endogenous protein in the yeast extract. Specifically, an expected 30 kDa band for the orthologous RPS0 yeast ribosomal protein is not observed on western blots (Fig. 1A). Moreover, extract from untransformed yeast cells showed no reacting protein bands (not shown). Apparently, the epitope recognized by the LamR H-2 antibody is absent in RPS0 or is not strongly reactive with the H2 antibody.
When plasmids expressing GFP, LamR-GFP or Sup35-GFP, were transformed into yeast strains that either lacked or contained the [PSI +] prion, the GFP, LamR-GFP and Sup35-GFP proteins were expressed at relatively equivalent levels in [psi −][PIN+] and [PSI+][PIN+] yeast strains (Fig. 1B).
LamR Interacts with Yeast Sup35 Protein
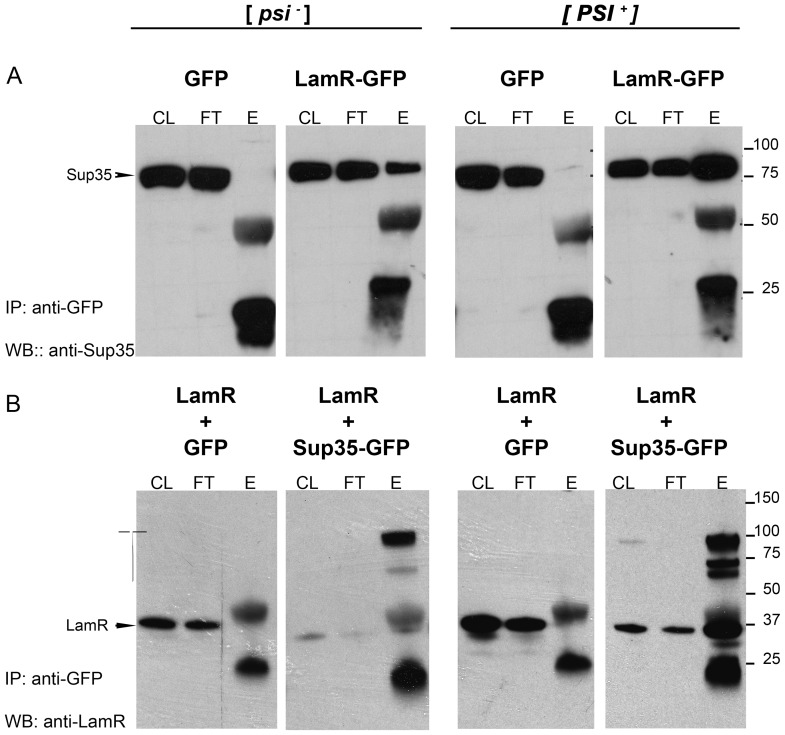
To assess the interaction of the human LamR with yeast Sup35 monomers or Sup35 prion aggregates/oligomers, co-immunoprecipitation experiments were performed. Lysates of CuSO4-induced cells were incubated with protein G-linked magnetic beads bound with GFP antibody. Figure 2 shows western blots of GFP antibody-precipitated lysate samples that were probed with antibody to Sup35 (A) or LamR (B). In lysates expressing LamR-GFP (Fig. 2A, panels 2 and 4), 76 kDa Sup35 protein bands appear in the eluted fractions of both [psi −] and [PSI +] yeast strains, but the relative amount of pulled-down Sup35 is significantly higher in the [PSI +] lysates. Lysate fractions eluted from yeast that expressed only GFP protein did not show a 76 kDa Sup35 protein band indicating a specific interaction between the LamR and Sup35 proteins (Fig. 2A, panels 1 and 3). Also, control beads bound with mouse IgG did not bind Sup35 (not shown).
Figure 2. Human LamR co-immunoprecipitates with yeast Sup35 protein.
Yeast cell lysates (500 ug) from transformants of [psi −][PIN+] and [PSI+][PIN+] strains carrying (A) pCUP1-GFP or pCUP1-LamR::GFP and (B) wild type pCUP1-LamR together with either pCUP-GFP or pCUP-SUP35::GFP were precipitated with anti-GFP antibody. Total eluted proteins (E) were compared with 20 ug cell lysate (CL) and unbound (flow thru, FT) samples; eluted samples represent 12× the amount of CL and FT. Western blots were probed with anti-Sup35 antibody (A) or anti-LamR antibody (B). Numbers on the right (A and B) refer to protein size markers (kDa). High molecular weight LamR bands are observed from 60–100 k Da in the presence of Sup35-GFP (B, panels 2 and 4). The 50 kDa and 25 kDa bands in A and B are the heavy and light chain IgG bands, respectively. Shown are representative experiments; similar results have been obtained for at least three independent experiments.
Although a significantly lesser amount of Sup35 eluted from LamR beads in the [psi −] strain, the elution of small amounts of Sup35, as opposed to complete absence of Sup35 in eluates, may be due to an interaction of LamR with the monomeric Sup35, or due to the presence of insignificant amounts non-heritable Sup35 oligomers. Indeed, some amounts of aggregated Sup35 are always detected in centrifugation assays in [psi −] strains (see Fig. 3).
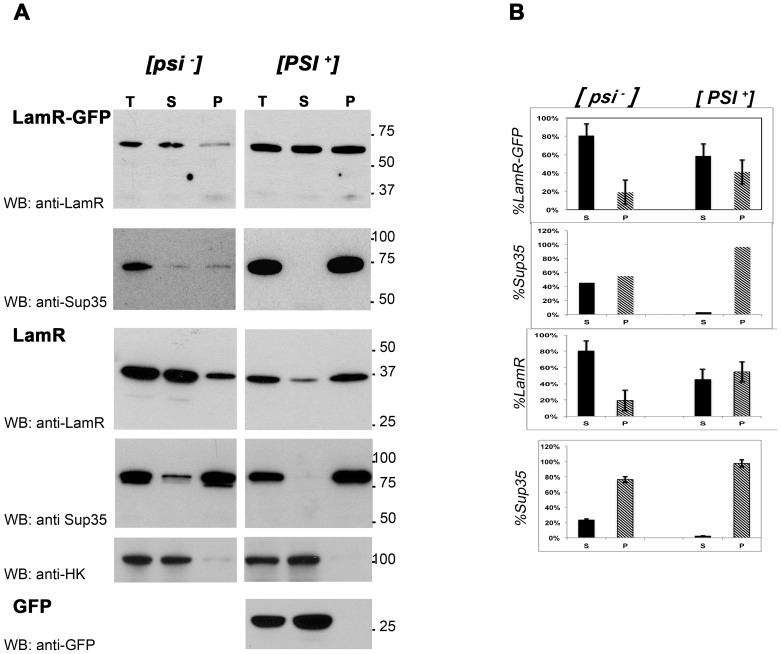
Figure 3. Centrifugation assay demonstrates aggregation of exogenous human LamR in the presence of the [PSI +] prion.
(A) Total lysate (T), supernatant (S), and resuspended pellet (P) (20 µg per sample) of pCUP1-LamR::GFP and pCUP1-LamR transformants of yeast [psi −][pin −] and [PSI+][PIN+] strains were analyzed by western blot using the indicated antibodies. pCUP1-GFP was expressed in the [PSI +] strain as a control (bottom panel), and anti-yeast hexokinase antibody (anti-HK) was used to ensure pellets were free of cytoplasmic proteins (fifth panel from top). Shown are representative experiments out of 3 independent experiments. (B) Corresponding densitometric quantitation of percent distribution between supernatant and pellet fractions was determined from three independent experiments. Bars show standard error of the mean.
Co-expression of wild type LamR with GFP or Sup35-GFP showed similar results (Fig. 2B). Expression of LamR with GFP protein alone did not result in the elution of a 37 kDa LamR band from anti-GFP coated beads in either [psi −] or [PSI +] yeast lysates (panels 1 and 3). In [psi −] yeast co-expressing LamR with Sup35-GFP very small amounts of 37 kDa LamR were observed in anti-GFP eluates, but a very strong band specifically recognized by anti-LamR appeared at approximately 100 kDa. [PSI +] yeast lysates co-expressing LamR with Sup35-GFP contained significantly higher amounts of 37 kDa LamR (Fig. 2B) and even greater amounts of higher molecular weight bands were also observed. Filters, completely stripped of LamR antibody, and reprobed with Sup35 antibody showed coincident higher molecular weight bands (not shown).
The co-immunoprecipitation experiments reveal an interaction between LamR and Sup35, the exact nature of which is not yet clear. Associations may occur between monomeric proteins, oligomers or aggregates. Presence of Sup35 and LamR together in higher molecular weight bands may be caused by anomalous migration of proteins in SDS PAGE gel due to aggregation.
Co-distribution of LamR with Sup35 Prion Protein
Centrifugation analysis provides another method to examine the aggregation of LamR in the presence of the Sup35-based prion. High-speed centrifugation sediments prion aggregates whereas non-aggregated forms of the proteins tend to remain in the supernatant [36]. Total yeast cell lysates, 100,000×g pellets and soluble supernatant fractions were analyzed by western blot. In this experiment, both pCUP1-LamR::GFP and pCUP1-LamR transformants were used, and supernatant and pellet fractions were probed for the exogenous human LamR and the endogenous Sup35 proteins. To ensure that unbroken cells or supernatant lysate did not contaminate the pellet fractions, membranes were probed with antibody to yeast hexokinase 1, which is located only in the cytoplasm (Fig. 3A).
In [PSI +] cell lysates, whether LamR or LamR-GFP was expressed, almost all Sup35 and >40% of LamR were detected in the pellet fraction, indicative of their aggregated state. Conversely, in [psi −] cells, the distribution of both Sup35 and LamR was shifted towards the soluble fraction. The ratio of aggregated LamR to soluble LamR was reversed, with the vast majority of LamR detected in the supernatant (∼80%). This further indicates that LamR becomes insoluble in the presence of the [PSI +] prions.
To further exclude the possibility that GFP contributed to LamR aggregation, analysis of pCUP1-GFP transformants showed that the GFP protein did not produce aggregates regardless of the presence of [PSI +] (Fig. 3A and not shown).
In summary, detection of LamR in the high-speed centrifugal pellets of the [PSI +] lysates supports an association of LamR with aggregated Sup35 protein.
LamR-GFP Forms Fluorescent Foci in [PSI+] Yeast Strains
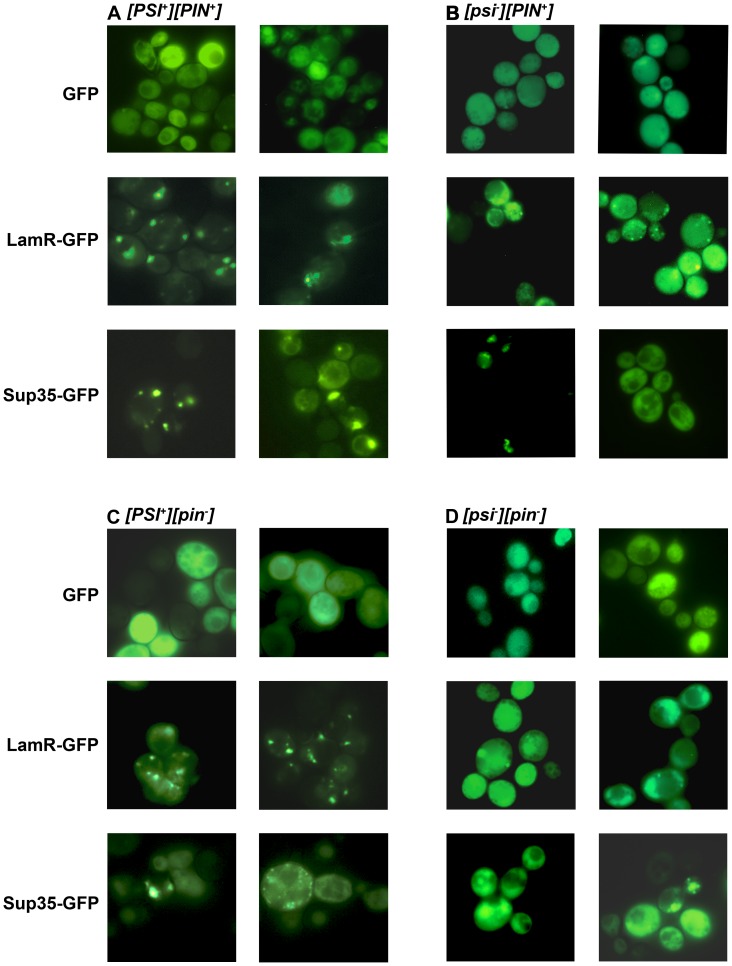
As the co-immunoprecipitation and co-distribution experiments indicated the [PSI +] prion-dependent interaction of LamR and Sup35, we examined whether, like Sup35, LamR forms visible cytoplasmic aggregates in yeast cells. The distribution of GFP, LamR-GFP and Sup35-GFP was examined in [PSI +] and [psi −] cells. Figure 4A displays representative images of yeast cells from two independent transformants of the [PSI +] strain. As expected, yeast cells expressing GFP showed relatively even, diffuse, cytoplasmic distribution of fluorescence. Also, as shown before [36], in [PSI +] cells that expressed Sup35-GFP, Sup35 was detected predominantly in bright fluorescent foci with hardly any protein visible in the cytoplasm. The expression pattern of LamR-GFP strikingly resembled that of Sup35: in most cells LamR was seen in bright foci with very little non-aggregated protein.
Figure 4. Immunofluorescence of LamR-GFP and Sup35-GFP fusion proteins reveals aggregation of LamR in [PSI +] cells.
Late-log cultures of (A) [PSI+][PIN+], (B) [PSI+][pin −], (C) [psi −][PIN+] and (D) [psi − ][pin −] were examined with 100× oil immersion lens of a fluorescent microscope using a 488ex, 507em filter. Representative images from two independent transformants of each yeast strain are displayed.
In [psi −][PIN+] cells (Fig. 4 B) both Sup35 and LamR-GFP were usually distributed evenly in the cytoplasm. However, in some cells both Sup35-GFP and LamR formed [PSI +] like aggregates. The formation of [PSI +] aggregates following overexpression of the Sup35-GFP construct was expected because the strain used in this experiment carries another prion, [PIN+]. The presence of the [PIN+] prion, which is a self-propagating state of the Rnq1 protein [46], [47], [54], [55], allows for the de novo [PSI +] formation upon overexpression of the Sup35 protein [46], [47], [54], apparently due to the direct seeding of the [PSI +] prion by the pre-existing [PIN+] prion aggregates [54], [56], [57], [58]. The LamR aggregates in these [psi −][PIN+] cells could result from either co-aggregating with the newly forming [PSI +] or from interaction of LamR with [PIN+].
To distinguish between these possibilities, we employed [pin −] variants of the same strains, [PSI+][pin–] (Fig. 4C) and [psi −][pin −] (Fig. 4D). In [PSI+][pin–] transformants, aggregation patterns of all proteins, GFP, Sup35-GFP and LamR-GFP were indistinguishable from [PSI+][PIN+] indicating that [PIN+] did not determine the LamR aggregation (Fig. 4C). In [psi −][pin −] cells both Sup35 and LamR-GFP were evenly distributed in the cytoplasm (Fig. 4D). As new [PSI +] formation does not occur when Sup35 is overproduced in the absence of [PIN+] [47], the fact that LamR-GFP was also non-clustered in [pin −][psi −] cells strongly indicates that LamR aggregation in [PSI+][PIN+] (Fig. 4A) and [PSI +][pin −] cells (Fig. 4C) was driven by the [PSI +I] prion, and suggest that LamR binds to the pre-existing and newly forming [PSI +] prion.
Sup35-GFP and LamR-mCherry Co-localize with [PSI+] or Newly Forming [PSI+]
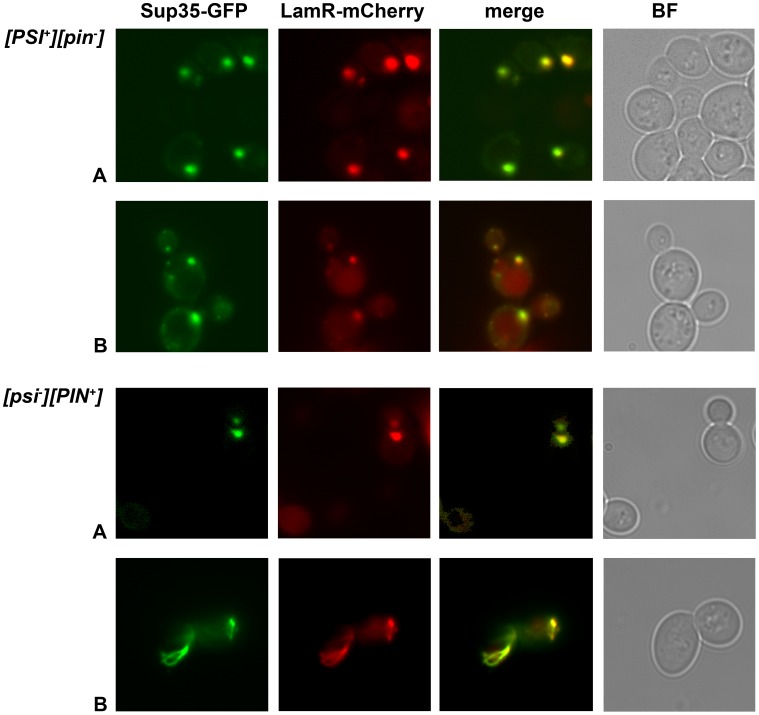
Analysis of fluorescent aggregates in cells co-expressing Sup35-GFP and LamR-mCherry clearly reveals the [PSI+]-dependent interaction between the Sup35 and LamR proteins. Overlapping Sup35 and LamR punctate foci were observed in cells of the weak [PSI+][pin −] strain transformed with pCUP1-SUP35::GFP and pCUP1-LamR::mCherry (Figure 5, two top rows, A and B refer to two independent transformants). Sup35 and LamR foci could also be found in strong prion yeast cells, however, the cytotoxicity associated with over-expression of Sup35 (pCUP1-SUP35::GFP) in the strong [PSI +] strains [59] made it difficult to detect and image aggregate-containing cells (not shown). Also, as expected, if the presence of LamR foci were coupled with the [PSI +] prion, and consistent with observations described in Figure 4D, fluorescently-labeled Sup35 and LamR were evenly dispersed throughout the cytoplasm of [psi −][pin −] yeast transformants (not shown).
Figure 5. LamR co-localizes with visible Sup35 prion aggregates in yeast cells.
pSUP35-GFP and pLamR-mCherry were co-expressed in weak [PSI+][pin −] and [psi −][PIN+] cells. Panels show GFP and mCherry fluorescence and their merged images. Brightfield (BF) images of the cells are shown in the far right panel. Two sets of images (A and B) are shown from independent transformants. Images were taken using a100× oil immersion lens. The images were visualized and merged using Adobe photoshop CS4.
We also followed localization of LamR and Sup35 in [psi–][PIN+] cells where the [PSI +] prion is forming de novo (see Figure 4B above). The newly generated prions can take the form of punctate dots, ring-like or branched ring structures [49]. The two bottom panels of Figure 5 show cell clusters from two independent [psi–][PIN+] transformants. The first cluster (A) contains dot aggregates for Sup35, indicative of heritable [PSI+], and the second cluster – a branched ring (B), which is a non-mature form of [PSI+]. In both cases Sup35 aggregates co-localize with LamR-mCherry visible aggregates. These results suggest that LamR may interact with different prion-like conformers of Sup35. Co-localization is observed in both mother and daughter cells, indicative of a heritable transmission of LamR with [PSI+], although re-association cannot be excluded by these experiments.
LamR-GFP Expression does not Change the [PSI] Status of Yeast Cells
Previous experimental evidence shows that interactions of prion-forming proteins with various cellular proteins may result in either de novo appearance or loss of pre-existing prions; this is true for both interactions between two different prion forming proteins (reviewed in [60]), and between prion-forming proteins and proteins that are not amyloidogenic themselves. Such non-amyloidogenic proteins include various chaperones (reviewed in [61]) or overexpressed interacting partners of prion-forming proteins, like Sup45, which forms a translation termination complex with soluble Sup35 [61], [62]. We tested if LamR expression can cure [PSI +] in [PSI +] strains or promote [PSI +] formation in [psi −] cells. Using standard genetic assays to examine the propagation or loss of Sup35 prions [63], [64], [65], no effect was observed when pCUP1-LamR or pCUP1-LamR::GFP were expressed in [PSI+][PIN+], [psi −][pin −], [PSI +][pin −] or [psi −][PIN+] yeast strains for over 30 replicative generations (not shown).
Discussion
The data presented show an in vivo interaction between LamR and Sup35 protein when Sup35 is present in the prion [PSI +] state. 1) In co-immunoprecipitation assays, Sup35 protein is pulled down with LamR-GFP from yeast cell lysates and, conversely, LamR is pulled down with Sup35-GFP). 2) In centrifugation assays, in [PSI +] cell lysates, LamR was shifted to pellet fractions together with insoluble Sup35 protein. 3) Aggregation patterns of LamR and Sup35 were strikingly similar, with LamR-GFP forming punctate fluorescent foci in [PSI +] cells and in cultures where [PSI +] formation is possible. Furthermore, visible LamR-mCherry co-localized with both pre-existing and newly forming [PSI+]. Although the nature of the interaction between LamR and Sup35 prion protein has not been elucidated, the results indicate the adherence of LamR to [PSI +] prion aggregates, as opposed to, interference or enhancement of the amyloidogenic process.
As part of the protein translation machinery, the yeast and human orthologs of LamR and Sup35, respectively, are highly homologous. The homology, however, is not universally strong throughout the proteins. For both Sup35 and LamR there is an ancient, highly conserved part of the protein and a more recently acquired variable extension. For example, the yeast ortholog of LamR, RPS0, has 252 amino acid residues compared with LamR (RPSA), which contains 295 residues. The additional amino acid residues of mammalian RPSA comprise a C-terminal domain that is thought to have evolved with the gain of the laminin binding function as organisms became multicellular [7]. Likewise, yeast Sup35 (eRF3) is a member of a protein family including ancient EF-Tu/eEF1A elongation factors [35]. The Sup35 region that is homologous with EF-Tu/eEF1A encompasses the GTP- and aminoacyl-tRNA-binding sites, is highly conserved and sufficient for viability and translation termination; respective regions of yeast and human proteins are 57% identical and 75% similar. On the other hand, the ∼200 amino acid long N-terminal extension, present only in Sup35/eRF3 proteins, is more variable. The exact role of this N-terminal extension is not known. In yeast it encompasses the Q/N-rich amyloidogenic region at the extreme N-terminus, which is essential for [PSI +] formation and maintenance [35], [40], [66]. While mammalian eRF factors lack the Q/N-rich region, other segments of their N-terminal extension share similar amino acid composition with Sup35/eRF3, suggesting the possibility of functional conservation.
Although interaction of LamR (RPSA) and Sup35 as parts of the ribosomal complex is an obvious hypothesis, our results do not support an interaction that is dependent exclusively upon their ribosomal functions. Indeed, in this case, the interaction is expected to be as efficient when Sup35 is in a soluble non-prion state, whereas, our data suggest that interaction is facilitated by the aggregated protein. In addition, the fact that human eRF3 proteins have not been found to be in complex with LamR in a stringent proteomic study of human LamR binding proteins indicates that interaction does not occur within the framework of a major and constitutive cellular process involving a considerable fraction of each protein [67].
Our data indicate, rather, that interaction between LamR and Sup35 is directed by their newly gained functions, implicating their more recently acquired domains. Acquisition of an extended C-terminus by LamR is thought to be important for the cell surface localization of LamR, its external position allowing for extracellular interactions [7], [20]. The importance of this functional role is reflected by the very high degree of conservation in vertebrates throughout the entire LamR protein sequence [7]. Positioned externally, the LamR C-terminus can bind with laminin and serve as a receptor for various molecules, including PrPC and PrPSc, as cited in the introduction.
The ability of LamR to bind PrPSc [23], [24] has led us to hypothesize that the LamR may have an affinity for structures characteristic of prions and amyloidogenic proteins. Indeed, while there is no evidence that formation of laminin-1, first used to isolate LamR, involves typical amyloid, it contains a fibrous, coiled-coil, α-helical domain that forms a network in the extracellular matrix [68], [69]. Furthermore, amyloidogenic sequences have been identified in laminin-1. Peptides of these sequences form amyloid fibers in vitro and can presumably form in vivo when laminin-1 is fragmented or unstructured while undergoing conformational transformations [70]. Analysis indicates that the extracellular C-terminal domain of LamR is a disordered structure [71]. It is increasingly apparent that disordered domains are common among proteins that form multiple protein-protein interactions [72], [73], and may be both involved with specific binding or engage in protein-protein contacts in a less specific manner. Different regions or conformations of the LamR protein may modulate its interaction with human PrPC vs PrPSc. While specific binding sites have been identified, on each protein, for interaction between LamR and PrPC [22], LamR binding with PrPSc has not been defined.
Significant experimental evidence suggests that both LamR and Sup35-based prions are associated with the actin cytoskeleton, and thus it is plausible that the actin cytoskeleton can mediate LamR and Sup35/[PSI +] interaction. Components of the actin cortical cytoskeleton have been shown to interact with the prion domain of Sup35. This association promotes de novo [PSI +] prion formation and aggregation [74]. LamR has also been shown to interact with the cytoskeletal network within mammalian cells [8], [75], [76], [77], [78]. Specifically, co-localization has been shown with actin filaments in vivo [8], [78] and in vitro [78] and interaction with microfilaments is important for cell adhesion and motility. Ganusova et al. have proposed a model whereby the yeast actin cytoskeleton acts as a scaffold for the amyloid-based aggregation of misfolded proteins, reducing the toxicity of misfolded proteins by sequestration from the cytosol [74]. The cytoskeleton may also serve as a location for protein refolding. Chaperone proteins that contribute to [PSI +] propagation [61], [74] have been found at the cytoskeleton [74]. In this regard, it is noteworthy that LamR amino acid residues 1–120 share some homology with the Hsp70 chaperone protein [79].
In conclusion, our findings reveal the propensity of LamR to interact with different prion-forming proteins and raise the possibility that LamR interaction with mammalian prion protein occurs not only in the capacity of the PrPC receptor, but is implicated in either prion infectivity or prevention of prion infection through a structural affinity for PrPSc. Utilization of the yeast assay system provides a safe and easily manipulated system for further study of LamR binding to prions and amyloids. Structure guided mutagenesis has been used to delineate the laminin-1 binding site of LamR [80]. Similarly, mutagenesis experiments can be designed to probe the interaction of LamR with Sup35 and other amyloid-like proteins. It is hoped that such studies will facilitate an understanding of the multifunctional interactions of the LamR protein.
Acknowledgments
We thank Vincent DiGiacomo, Dr. Efrat Levy and Dr. Lisa Venticinque for their critical reading of the manuscript and helpful discussions.
Funding Statement
This work was funded by U.S. Public Health Grant CA100687 from the National Cancer Institute to D.M., a generous donation from the Litwin foundation and a Research and License agreement between NYU and Cynvec. This work was also supported by National Institutes of Health (grant 7 R01 GM070934-06) to I.L.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Demianova M, Formosa TG, Ellis SR (1996) Yeast proteins related to the p40/laminin receptor precursor are essential components of the 40 S ribosomal subunit. The Journal of biological chemistry 271: 11383–11391. [DOI] [PubMed] [Google Scholar]
- 2. Scheiman J, Jamieson KV, Ziello J, Tseng JC, Meruelo D (2010) Extraribosomal functions associated with the C terminus of the 37/67 kDa laminin receptor are required for maintaining cell viability. Cell Death Dis 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Susantad T, Smith DR (2008) siRNA-mediated silencing of the 37/67-kDa high affinity laminin receptor in Hep3B cells induces apoptosis. Cellular & molecular biology letters 13: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lesot H, Kuhl U, Mark K (1983) Isolation of a laminin-binding protein from muscle cell membranes. The EMBO journal 2: 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malinoff HL, Wicha MS (1983) Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. The Journal of cell biology 96: 1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao NC, Barsky SH, Terranova VP, Liotta LA (1983) Isolation of a tumor cell laminin receptor. Biochemical and biophysical research communications 111: 804–808. [DOI] [PubMed] [Google Scholar]
- 7. Ardini E, Pesole G, Tagliabue E, Magnifico A, Castronovo V, et al. (1998) The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Molecular biology and evolution 15: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 8. Yannariello-Brown J, Wewer U, Liotta L, Madri JA (1988) Distribution of a 69-kD laminin-binding protein in aortic and microvascular endothelial cells: modulation during cell attachment, spreading, and migration. The Journal of cell biology 106: 1773–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berno V, Porrini D, Castiglioni F, Campiglio M, Casalini P, et al. (2005) The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocrine-related cancer 12: 393–406. [DOI] [PubMed] [Google Scholar]
- 10. Menard S, Tagliabue E, Colnaghi MI (1998) The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast cancer research and treatment 52: 137–145. [DOI] [PubMed] [Google Scholar]
- 11. Sanjuan X, Fernandez PL, Miquel R, Munoz J, Castronovo V, et al. (1996) Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. The Journal of pathology 179: 376–380. [DOI] [PubMed] [Google Scholar]
- 12. Wewer UM, Taraboletti G, Sobel ME, Albrechtsen R, Liotta LA (1987) Role of laminin receptor in tumor cell migration. Cancer research 47: 5691–5698. [PubMed] [Google Scholar]
- 13. Scheiman J, Tseng JC, Zheng Y, Meruelo D (2010) Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol Ther 18: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, et al. (2009) Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. The Journal of clinical investigation 119: 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim KJ, Chung JW, Kim KS (2005) 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. The Journal of biological chemistry 280: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 16. Akache B, Grimm D, Pandey K, Yant SR, Xu H, et al. (2006) The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. Journal of virology 80: 9831–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludwig GV, Kondig JP, Smith JF (1996) A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. Journal of virology 70: 5592–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thepparit C, Smith DR (2004) Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. Journal of virology 78: 12647–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH (1992) High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. Journal of virology 66: 4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, et al. (2008) The 67 kDa laminin receptor: structure, function and role in disease. Bioscience reports 28: 33–48. [DOI] [PubMed] [Google Scholar]
- 21. Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, et al. (2001) The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. The EMBO journal 20: 5863–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rieger R, Edenhofer F, Lasmézas CI, Weiss S (1997) The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med 3: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 23. Kolodziejczak D, Da Costa Dias B, Zuber C, Jovanovic K, Omar A, et al. (2010) Prion interaction with the 37-kDa/67-kDa laminin receptor on enterocytes as a cellular model for intestinal uptake of prions. Journal of molecular biology 402: 293–300. [DOI] [PubMed] [Google Scholar]
- 24. Morel E, Andrieu T, Casagrande F, Gauczynski S, Weiss S, et al. (2005) Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. The American journal of pathology 167: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leucht C, Simoneau S, Rey C, Vana K, Rieger R, et al. (2003) The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO reports 4: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wille H, Bian W, McDonald M, Kendall A, Colby DW, et al. (2009) Natural and synthetic prion structure from X-ray fiber diffraction. Proceedings of the National Academy of Sciences of the United States of America 106: 16990–16995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piro JR, Wang F, Walsh DJ, Rees JR, Ma J, et al. (2011) Seeding specificity and ultrastructural characteristics of infectious recombinant prions. Biochemistry 50: 7111–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crow ET, Li L (2011) Newly identified prions in budding yeast, and their possible functions. Seminars in cell & developmental biology 22: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wickner RB, Edskes HK, Kryndushkin D, McGlinchey R, Bateman D, et al. (2011) Prion diseases of yeast: amyloid structure and biology. Seminars in cell & developmental biology 22: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, et al. (2012) Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ross ED, Minton A, Wickner RB (2005) Prion domains: sequences, structures and interactions. Nat Cell Biol 7: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 32. Shewmaker F, McGlinchey RP, Wickner RB (2011) Structural insights into functional and pathological amyloid. The Journal of biological chemistry 286: 16533–16540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toyama BH, Weissman JS (2011) Amyloid structure: conformational diversity and consequences. Annual review of biochemistry 80: 557–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stansfield I, Jones KM, Kushnirov VV, Dagkesamanskaya AR, Poznyakovski AI, et al. (1995) The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. The EMBO journal 14: 4365–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhouravleva G, Frolova L, Le Goff X, Le Guellec R, Inge-Vechtomov S, et al. (1995) Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. The EMBO journal 14: 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patino MM, Liu JJ, Glover JR, Lindquist S (1996) Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273: 622–626. [DOI] [PubMed] [Google Scholar]
- 37. Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD (1996) Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. The EMBO journal 15: 3127–3134. [PMC free article] [PubMed] [Google Scholar]
- 38. Inge-Vechtomov SG, Tikhodeev ON, Karpova TS (1988) [Selective systems for obtaining recessive ribosomal suppressors in saccharomycete yeasts]. Genetika 24: 1159–1165. [PubMed] [Google Scholar]
- 39. Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268: 880–884. [DOI] [PubMed] [Google Scholar]
- 40. Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW (1996) Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kushnirov VV, Vishnevskaya AB, Alexandrov IM, Ter-Avanesyan MD (2007) Prion and nonprion amyloids: a comparison inspired by the yeast Sup35 protein. Prion 1: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liebman SW, Derkatch IL (1999) The yeast [PSI+] prion: making sense of nonsense. The Journal of biological chemistry 274: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 43. Serio TR, Lindquist SL (2001) The yeast prion [PSI+]: molecular insights and functional consequences. Advances in protein chemistry 59: 391–412. [DOI] [PubMed] [Google Scholar]
- 44. Tessier PM, Lindquist S (2009) Unraveling infectious structures, strain variants and species barriers for the yeast prion [PSI+]. Nature structural & molecular biology 16: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bach S, Tribouillard D, Talarek N, Desban N, Gug F, et al. (2006) A yeast-based assay to isolate drugs active against mammalian prions. Methods 39: 72–77. [DOI] [PubMed] [Google Scholar]
- 46. Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW (1997) Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Derkatch IL, Bradley ME, Masse SV, Zadorsky SP, Polozkov GV, et al. (2000) Dependence and independence of [PSI(+)] and [PIN(+)]: a two-prion system in yeast? The EMBO journal 19: 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou P, Derkatch IL, Liebman SW (2001) The relationship between visible intracellular aggregates that appear after overexpression of Sup35 and the yeast prion-like elements [PSI(+)] and [PIN(+)]. Molecular microbiology 39: 37–46. [DOI] [PubMed] [Google Scholar]
- 50. Jamieson KV, Wu J, Hubbard SR, Meruelo D (2008) Crystal structure of the human laminin receptor precursor. The Journal of biological chemistry 283: 3002–3005. [DOI] [PubMed] [Google Scholar]
- 51.Sherman F, Fink G.R., and Hicks J.B. (1986) Methods in Yeast Genetics. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press.
- 52. Bagriantsev S, Liebman S (2006) Modulation of Abeta42 low-n oligomerization using a novel yeast reporter system. BMC biology 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DePace AH, Santoso A, Hillner P, Weissman JS (1998) A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell 93: 1241–1252. [DOI] [PubMed] [Google Scholar]
- 54. Derkatch IL, Bradley ME, Hong JY, Liebman SW (2001) Prions affect the appearance of other prions: the story of [PIN(+)]. Cell 106: 171–182. [DOI] [PubMed] [Google Scholar]
- 55. Sondheimer N, Lindquist S (2000) Rnq1: an epigenetic modifier of protein function in yeast. Molecular cell 5: 163–172. [DOI] [PubMed] [Google Scholar]
- 56. Derkatch IL, Uptain SM, Outeiro TF, Krishnan R, Lindquist SL, et al. (2004) Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proceedings of the National Academy of Sciences of the United States of America 101: 12934–12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osherovich LZ, Weissman JS (2001) Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI(+)] prion. Cell 106: 183–194. [DOI] [PubMed] [Google Scholar]
- 58. Vitrenko YA, Gracheva EO, Richmond JE, Liebman SW (2007) Visualization of aggregation of the Rnq1 prion domain and cross-seeding interactions with Sup35NM. The Journal of biological chemistry 282: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 59. Chernoff YO, Derkach IL, Inge-Vechtomov SG (1993) Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Current genetics 24: 268–270. [DOI] [PubMed] [Google Scholar]
- 60. Derkatch IL, Liebman SW (2007) Prion-prion interactions. Prion 1: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perrett S, Jones GW (2008) Insights into the mechanism of prion propagation. Current opinion in structural biology 18: 52–59. [DOI] [PubMed] [Google Scholar]
- 62. Derkatch IL, Bradley ME, Liebman SW (1998) Overexpression of the SUP45 gene encoding a Sup35p-binding protein inhibits the induction of the de novo appearance of the [PSI+] prion. Proceedings of the National Academy of Sciences of the United States of America 95: 2400–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chernoff YO, Uptain SM, Lindquist SL (2002) Analysis of prion factors in yeast. Methods Enzymol 351: 499–538. [DOI] [PubMed] [Google Scholar]
- 64. Liebman SW, Bagriantsev SN, Derkatch IL (2006) Biochemical and genetic methods for characterization of [PIN+] prions in yeast. Methods 39: 23–34. [DOI] [PubMed] [Google Scholar]
- 65. Tuite MF, Cox BS (2007) The genetic control of the formation and propagation of the [PSI+] prion of yeast. Prion 1: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ter-Avanesyan MD, Kushnirov VV, Dagkesamanskaya AR, Didichenko SA, Chernoff YO, et al. (1993) Deletion analysis of the SUP35 gene of the yeast Saccharomyces cerevisiae reveals two non-overlapping functional regions in the encoded protein. Molecular microbiology 7: 683–692. [DOI] [PubMed] [Google Scholar]
- 67. Venticinque L, Meruelo D (2012) Comprehensive proteomic analysis of nonintegrin laminin receptor interacting proteins. Journal of proteome research 11: 4863–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hunter I, Schulthess T, Engel J (1992) Laminin chain assembly by triple and double stranded coiled-coil structures. The Journal of biological chemistry 267: 6006–6011. [PubMed] [Google Scholar]
- 69. Paulsson M, Deutzmann R, Timpl R, Dalzoppo D, Odermatt E, et al. (1985) Evidence for coiled-coil alpha-helical regions in the long arm of laminin. The EMBO journal 4: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kasai S, Urushibata S, Hozumi K, Yokoyama F, Ichikawa N, et al. (2007) Identification of multiple amyloidogenic sequences in laminin-1. Biochemistry 46: 3966–3974. [DOI] [PubMed] [Google Scholar]
- 71. Ould-Abeih MB, Petit-Topin I, Zidane N, Baron B, Bedouelle H (2012) Multiple folding states and disorder of ribosomal protein SA, a membrane receptor for laminin, anticarcinogens, and pathogens. Biochemistry 51: 4807–4821. [DOI] [PubMed] [Google Scholar]
- 72. Dunker AK, Oldfield CJ, Meng J, Romero P, Yang JY, et al. (2008) The unfoldomics decade: an update on intrinsically disordered proteins. BMC genomics 9 Suppl 2 S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tompa P (2011) Unstructural biology coming of age. Current opinion in structural biology 21: 419–425. [DOI] [PubMed] [Google Scholar]
- 74. Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, et al. (2006) Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Molecular and cellular biology 26: 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cody RL, Wicha MS (1986) Clustering of cell surface laminin enhances its association with the cytoskeleton. Experimental cell research 165: 107–116. [DOI] [PubMed] [Google Scholar]
- 76. Fujimura Y, Umeda D, Kiyohara Y, Sunada Y, Yamada K, et al. (2006) The involvement of the 67 kDa laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochemical and biophysical research communications 348: 524–531. [DOI] [PubMed] [Google Scholar]
- 77. Keppel E, Schaller HC (1991) A 33 kDa protein with sequence homology to the 'laminin binding protein' is associated with the cytoskeleton in hydra and in mammalian cells. Journal of cell science 100 (Pt 4): 789–797. [DOI] [PubMed] [Google Scholar]
- 78. Venticinque L, Jamieson KV, Meruelo D (2011) Interactions between laminin receptor and the cytoskeleton during translation and cell motility. PloS one 6: e15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen MS, Fornace A Jr, Laszlo A (1996) Characterization of an hsp70 related clone encoding a 33 kDa protein with homology to a protein which associates with polysomes. Biochimica et biophysica acta 1297: 124–126. [DOI] [PubMed] [Google Scholar]
- 80. Jamieson KV, Hubbard SR, Meruelo D (2011) Structure-guided identification of a laminin binding site on the laminin receptor precursor. J Mol Biol 405: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]