Ataxia-telangiectasia (A-T) is a hereditary neurodegenerative disorder that is characterized by progressive cerebellar ataxia, ocular or cutaneous telangiectasia, oculomotor apraxia, choreoathetosis, and dysarthria. In addition, hypersensitivity to radiation, increased malignancy risk, immunodeficiency, and recurrent infection are frequently seen in patients afflicted with this disorder [1]. A-T is caused by mutations in the ataxia-telangiectasia mutated (ATM) gene that is located on chromosome 11q22-23. Mutations to this gene lead to DNA instability and decreased protein kinase activity [2, 3]. The reported prevalence of A-T ranges from 1 in 40,000 to 1 in 300,000 live births, depending on the geographic or ethnic region studied [4, 5]. A-T is the most common cause of progressive ataxia in childhood in most countries, and several cases have been reported in China and Japan [1, 6, 7]. In Korea, 3 cases of A-T have been reported thus far, and only 1 family was genetically confirmed by ATM gene analysis [8-10].
In this report, we present the case of an 18-yr-old female patient with clinical features of A-T, in whom the diagnosis was confirmed by the identification of 2 novel splicing mutations in the ATM gene. The patient visited the Neurology Department for gait disturbance. She had developed gait instability and had experienced frequent falls from the age of 3 yr. She showed slight unsteady gait and slurred speech for her own age group, which progressed slowly. Ocular telangiectasia had been frequently observed for the previous 5 yr. Her perinatal history was unremarkable, and developmental delay was not observed. No family history of A-T was noted.
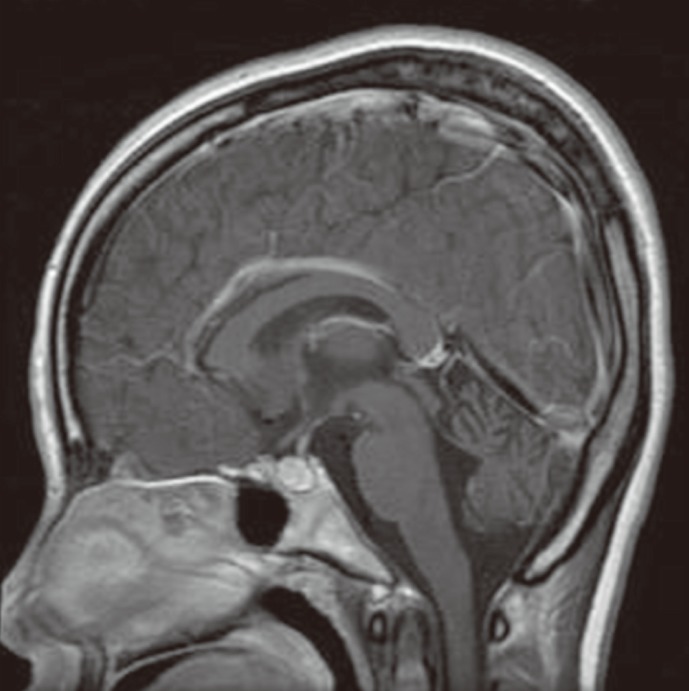
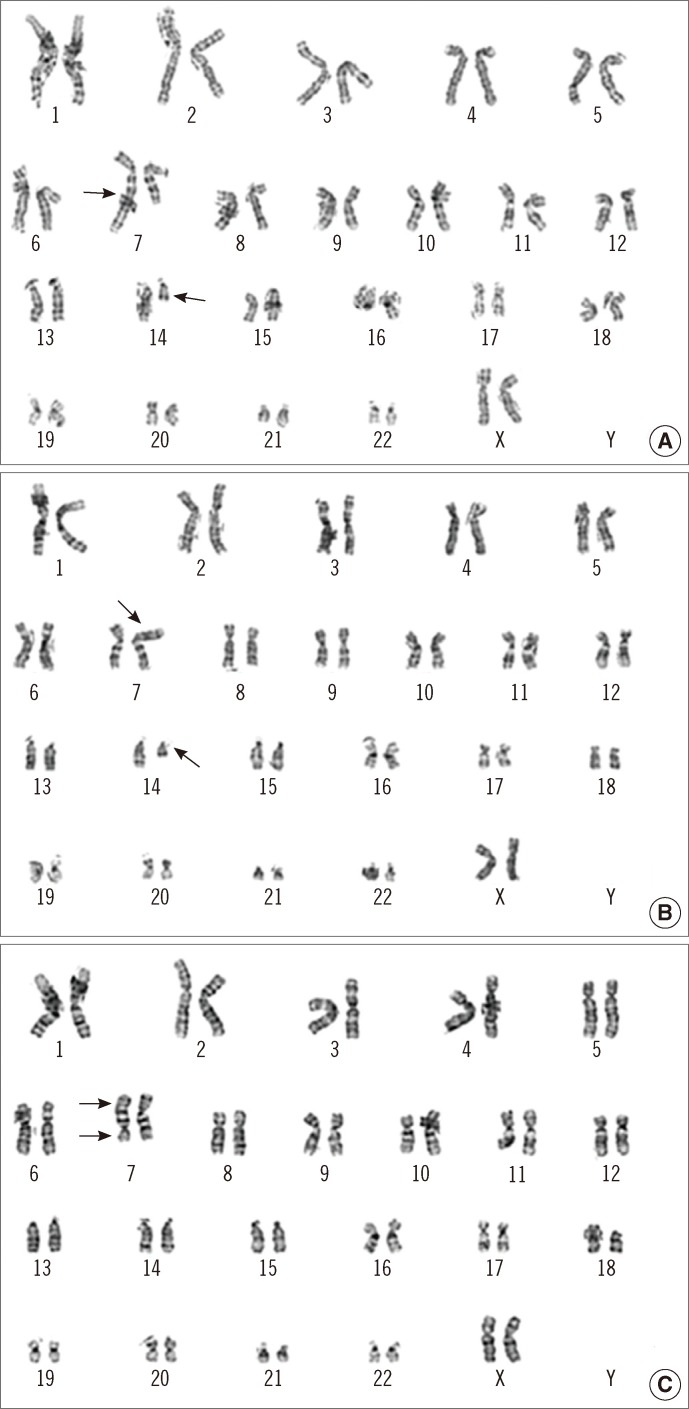
Neurologic examination revealed dysarthria, gaze-evoked nystagmus, and oculomotor apraxia. Deep tendon reflexes were also diminished, and bilateral limb dysmetria and ataxic gait were observed. Sensory dominant polyneuropathy was detected on nerve conduction study. Mild ocular telangiectasia was noted bilaterally, but there was no cutaneous telangiectasia (Fig. 1). The serum α-fetoprotein (AFP) level was elevated (77.1 ng/mL), and brain magnetic resonance imaging (MRI) showed marked bilateral cerebellar atrophy (Fig. 2). There was no history of infection, and serologic testing revealed no immunodeficiency (IgG, 852 mg/dL; IgA, 212 mg/dL; IgE, 1.5 mg/dL; CD4 lymphocyte, 1.19×109/L; CD8 lymphocyte, 0.64×109/L; CD4/CD8 ratio, 1.85). Chromosome analysis, in which the patient's lymphocytes were stimulated with phytohemagglutinin and harvested for 72 hr, revealed balanced translocations or pericentric inversions involving 14q11.2, 7q32, and 7p15.1, besides metaphases with a regular female karyotype (Fig. 3): 46,XX,t(7;14) (q32;q11.2)[6]/46,XX,t(7;14)(p15.1;q11.2)[2]/46,XX,inv(7)(p1?q3?)[2]/46,XX[30].
Fig. 1.
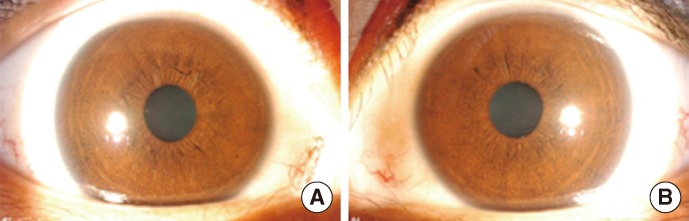
Bilateral ocular telangiectasias. (A) Right eye. (B) Left eye.
Fig. 2.
Sagittal T1-weighted magnetic resonance image of the patient's brain. Pronounced cerebellar atrophy is evident.
Fig. 3.
Giemsa-banded karyograms of the peripheral blood lymphocyte culture. (A) 46,XX,t(7;14)(q32;q11.2). (B) 46,XX,t(7;14)(p15.1;q11.2). (C) 46,XX,inv(7)(p1?q3?). Arrows indicate the breakpoints on each chromosome.
During evaluation for possible malignancy, follicular thyroid cancer was detected, and the patient underwent a right thyroid lobectomy. After 19 yr from disease onset, the overall neurologic deficits, including limb ataxia and gait disturbance, had worsened, but the rate of progression was slower than that described in prior reports of classic A-T [11]. Currently, she can walk independently and complete household chores. To date, no signs of infection have emerged and additional organ involvement has not developed.
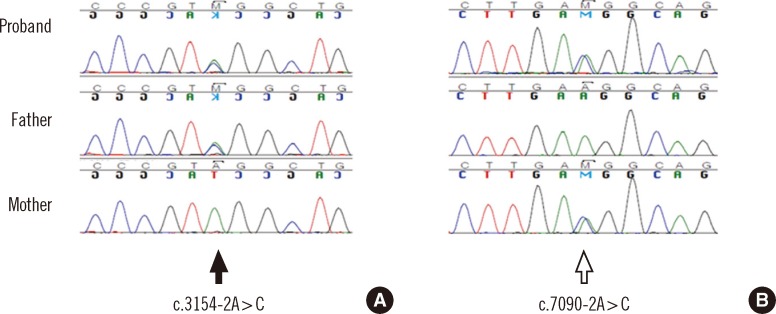
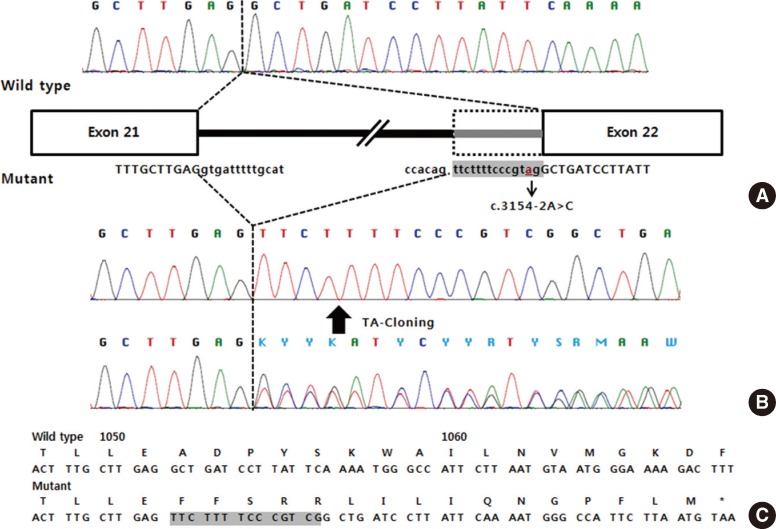
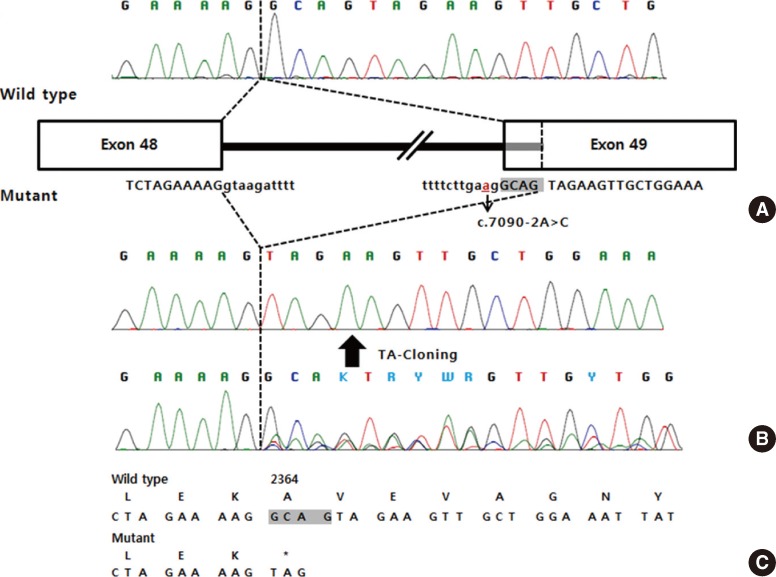
After obtaining informed consent from the patient and her parents, sequence analysis of the ATM gene was performed and 2 novel intronic variants were identified: c.3154-2A>C in intron 21 and c.7090-2A>C in intron 48 on the basis of the reference sequence NM_000051.3 (Fig. 4). Additional genetic analysis of the patient's parents revealed that her father was heterozygous for the c.3154-2A>C variant, whereas her mother was heterozygous for the c.7090-2A>C variant. Since these 2 variants alter the consensus splice acceptor sequences, reverse transcription PCR (RT-PCR) sequencing followed by thymine/adenine (TA) cloning for the PCR product was performed in order to check for possible splicing aberrations. As expected, the c.3154-2A>C variant resulted in the insertion of 14 nucleotides from intron 21 into the transcript, causing a frameshift mutation that disrupted the 3' AG splice site (p.Ala1052Phefs*17; Fig. 5). The c.7090-2A>C variant resulted in the deletion of 4 nucleotides at exon 49, introducing a premature stop codon in exon 49 at amino acid position 2364 (p.Ala2364*; Fig. 6).
Fig. 4.
Genomic DNA sequence analysis of the ATM gene in the patient and in her parents. (A) The solid arrow indicates a heterozygous A>C transition (c.3154-2A>C). (B) The open arrow indicates a heterozygous A>C transition (c.7090-2A>C).
Fig. 5.
Molecular effects of the c.3154-2A>C mutation. (A) The cDNA sequence from a normal control, and schematic representation of normal/aberrant mRNA splicing of the ATM gene. (B) The cDNA sequence from the patient. (C) The predicted amino acid sequence (inserted nucleotides are shaded).
Abbreviation: TA, thymine/adenine.
Fig. 6.
Molecular effects of the c.7090-2A>C mutation. (A) The cDNA sequence from a normal control, and schematic representation of normal/aberrant mRNA splicing of the ATM gene. (B) The cDNA sequence from the patient. (C) The predicted amino acid sequence (deleted nucleotides are shaded).
Abbreviation: TA, thymine/adenine.
In Korea, A-T is extremely rare that the diagnosis in our patient might have been delayed. Although curative treatments for A-T are not available, early diagnosis is of particular importance, given the associated increased risk of malignancies and the high risk of side effects due to subsequent cancer treatment [11, 12]. Furthermore, T lymphocytes from patients with A-T demonstrate specific aberrations involving chromosomes 7 and 14 in 5-10%, with breakpoints occurring at the sites of immunoglobulin and T cell receptor genes [13]. Translocations may induce tumorigenic processes, resulting in the preferential proliferation of an aberrant cell clone with neoplastic potential. This increased level of nonspecific translocations may contribute to the elevated frequency of malignancies in homozygote and heterozygote carriers of ATM gene mutations [13]. Therefore, this case emphasizes the importance of clinical suspicion of A-T, even though it is a rare diagnosis in Korea. Genetic study for the ATM gene is useful in the diagnosis, especially in the early stages, of classic A-T or in variant A-T [1].
Mutations of the ATM gene cause A-T, resulting in an abnormal cellular response to DNA damage, which leads to multi-organ impairment. Recently, Verhagen et al. [4] postulated that phenotypic manifestations in A-T have a continuous spectrum that depends on the presence of the ATM protein and kinase activity. Patients with truncating mutations of the ATM gene and an absence of ATM kinase activity presented with the severe classic form of A-T. Patients with classic, childhood-onset A-T developed neurologic deficits at around 1 yr of age, and were wheelchair-bound before the age of 12 yr. Conversely, missense or splice-site mutations of the ATM gene tend to present clinically milder forms of A-T. Compared to classic A-T, the milder form of A-T had an older age of onset, and independent walking was better preserved [4]. In our case, the patient presented with an ataxic gait in early childhood, but the rate of ataxia progression was relatively slow. At the age of 22 yr, she can still walk independently and complete household chores.
Variable immunodeficiency is a common clinical manifestation of A-T. In a large cohort study of 100 patients with A-T, immunoglobulin deficiencies were frequently observed, including that of IgG4 (65%), IgA (63%), IgG2 (48%), and IgE (23%) [14]. However, immunodeficiency was not found in patients with variant A-T, which is defined as the absence or presence, later in life, of some of the hallmark findings of classic A-T [11]. Our patient exhibited no evidence of immunodeficiency or history of infection, suggesting a possibility of variant A-T.
In our mutation analysis, the patient was identified as a compound heterozygote with 2 novel genetic mutations. The c.3154-2A>C mutation, located in intron 21, represented a novel mutation. However, an A>G mutation at the same site was previously described by Laake et al. [15] and was reported to create a new cryptic 5' splice site that caused a frameshift mutation. This scenario was identical to the putative protein product detected in our patient. In addition, the c.7090-2A>C mutation, located in intron 48, was also a novel variant that resulted in a premature stop codon. This finding was confirmed by RT-PCR.
In summary, we report a Korean female with clinical features of A-T, carrying 2 novel splicing mutations in the ATM gene. Although A-T is extremely rare in Korea, patients exhibiting clinical features of the disease should be checked for this disease in order to prevent its delayed diagnosis.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Gatti R. Ataxia-Telangiectasia. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. Gene Reviews. Seattle: University of Washington; 1993. [Google Scholar]
- 2.McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinnon PJ. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol. 2012;7:303–321. doi: 10.1146/annurev-pathol-011811-132509. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen MM, Last JI, Hogervorst FB, Smeets DF, Roeleveld N, Verheijen F, et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia-telangiectasia: a genotype-phenotype study. Hum Mutat. 2012;33:561–571. doi: 10.1002/humu.22016. [DOI] [PubMed] [Google Scholar]
- 5.Woods CG, Bundey SE, Taylor AM. Unusual features in the inheritance of ataxia telangiectasia. Hum Genet. 1990;84:555–562. doi: 10.1007/BF00210809. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Tang B, Xia K, Hu Z, Shen L, Tang J, et al. Mutation analysis of the ATM gene in two Chinese patients with ataxia telangiectasia. J Neurol Sci. 2006;241:1–6. doi: 10.1016/j.jns.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Morio T, Takahashi N, Watanabe F, Honda F, Sato M, Takagi M, et al. Phenotypic variations between affected siblings with ataxia-telangiectasia: ataxia-telangiectasia in Japan. Int J Hematol. 2009;90:455–462. doi: 10.1007/s12185-009-0408-0. [DOI] [PubMed] [Google Scholar]
- 8.Kang DW, Ahn SS, Jeon BS. A case of ataxia telangiectasia. J Korean Neurol Assoc. 1997;15:895–899. [Google Scholar]
- 9.Song MH, Kim EJ, Sung TJ, Shin SH, Lee KH, Kim HD. A case of progressive elevation of serum gamma-GTP level in ataxia-telangiectasia. J Korean Child Neurol Soc. 2006;14:363–368. [Google Scholar]
- 10.Huh HJ, Cho KH, Lee JE, Kwon MJ, Ki CS, Lee PH. Identification of ATM mutations in Korean siblings with ataxia-telangiectasia. Ann Lab Med. 2013;33:217–220. doi: 10.3343/alm.2013.33.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhagen MM, Abdo WF, Willemsen MA, Hogervorst FB, Smeets DF, Hiel JA, et al. Clinical spectrum of ataxia-telangiectasia in adulthood. Neurology. 2009;73:430–437. doi: 10.1212/WNL.0b013e3181af33bd. [DOI] [PubMed] [Google Scholar]
- 12.Gilad S, Chessa L, Khosravi R, Russell P, Galanty Y, Piane M, et al. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am J Hum Genet. 1998;62:551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stumm M, Neubauer S, Keindorff S, Wegner RD, Wieacker P, Sauer R. High frequency of spontaneous translocations revealed by FISH in cells from patients with the cancer-prone syndromes ataxia telangiectasia and Nijmegen breakage syndrome. Cytogenet Cell Genet. 2001;92:186–191. doi: 10.1159/000056900. [DOI] [PubMed] [Google Scholar]
- 14.Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr. 2004;144:505–511. doi: 10.1016/j.jpeds.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 15.Laake K, Jansen L, Hahnemann JM, Brondum-Nielsen K, Lönnqvist T, Kääriäinen H, et al. Characterization of ATM mutations in 41 Nordic families with ataxia telangiectasia. Hum Mutat. 2000;16:232–246. doi: 10.1002/1098-1004(200009)16:3<232::AID-HUMU6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]