Abstract
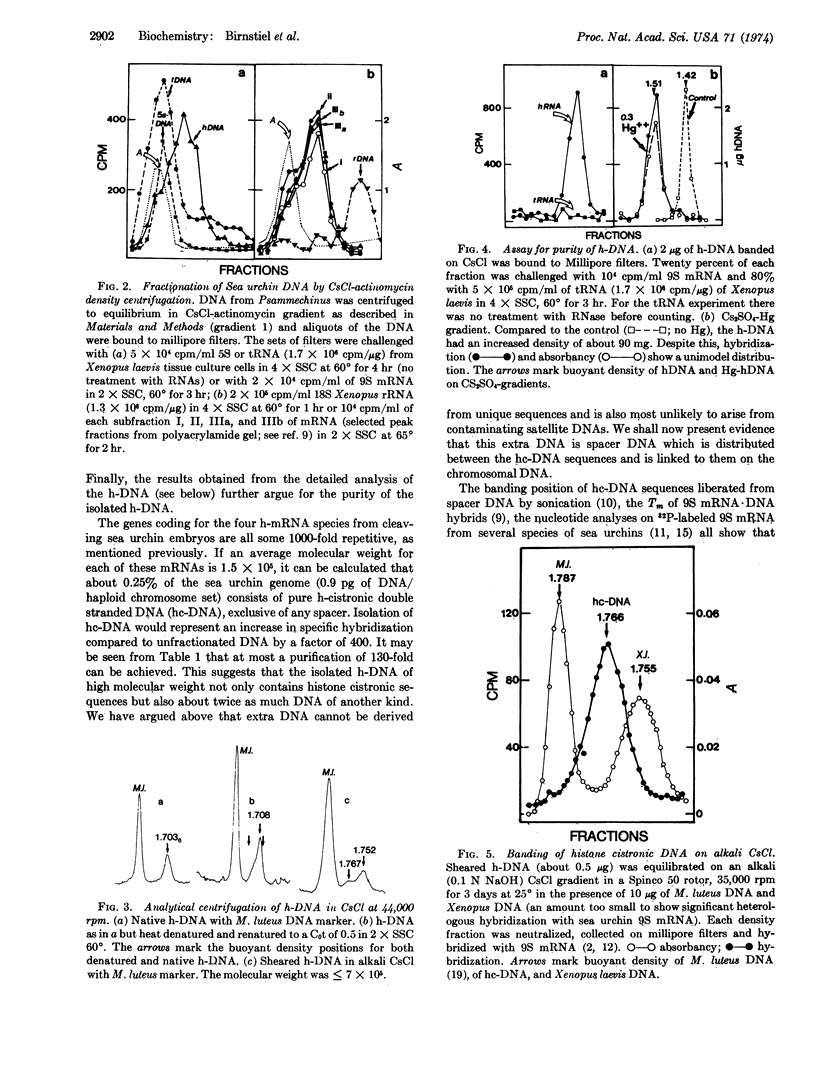
The DNA coding for histone proteins, together with the intervening spacer DNA, has been isolated from the sea urchin, Psammechinus miliaris, by repeated centrifugation on CsCl-asctinomycin gradients. The biophysical data show that a major portion of the spacer is 37% G+C and very divergent in sequence, while the DNA segments coding for histone proteins are high in G+C and have diverged to a lesser extent within the species.
Keywords: histone genes, spacer DNA, synonymous codons, evolution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Wallace H., Sirlin J. L., Fischberg M. Localization of the ribosomal DNA complements in the nucleolar organizer region of Xenopus laevis. Natl Cancer Inst Monogr. 1966 Dec;23:431–447. [PubMed] [Google Scholar]
- Birnstiel M., Speirs J., Purdom I., Jones K., Loening U. E. Properties and composition of the isolated ribosomal DNA satellite of Xenopus laevis. Nature. 1968 Aug 3;219(5153):454–463. doi: 10.1038/219454a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. 5 S DNAs of Xenopus laevis and Xenopus mulleri: evolution of a gene family. J Mol Biol. 1973 Aug 15;78(3):397–415. doi: 10.1016/0022-2836(73)90464-6. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol. 1972 Jan 14;63(1):57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. Purification and some characteristics of 5S DNA from Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3175–3179. doi: 10.1073/pnas.68.12.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. A satellite DNA isolated from human tissues. J Mol Biol. 1967 Feb 14;23(3):619–622. doi: 10.1016/s0022-2836(67)80130-x. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- Kersten W., Kersten H., Szybalski W. Physicochemical properties of complexes between deoxyribonucleic acid and antibiotics which affect ribonucleic acid synthesis (actinomycin, daunomycin, cinerubin, nogalamycin, chormomycin, mithramycin, and olivomycin). Biochemistry. 1966 Jan;5(1):236–244. doi: 10.1021/bi00865a031. [DOI] [PubMed] [Google Scholar]
- Laird C. D., McConaughy B. L., McCarthy B. J. Rate of fixation of nucleotide substitutions in evolution. Nature. 1969 Oct 11;224(5215):149–154. doi: 10.1038/224149a0. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Machattie L., Eron L., Ihler G., Ippen K., Beckwith J. Isolation of pure lac operon DNA. Nature. 1969 Nov 22;224(5221):768–774. doi: 10.1038/224768a0. [DOI] [PubMed] [Google Scholar]
- Stafford D. W., Guild W. R. Satellite DNA from sea urchin sperm. Exp Cell Res. 1969 Jun;55(3):347–350. doi: 10.1016/0014-4827(69)90568-0. [DOI] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. Alkali deamination of cytosine residues in DNA. Biochim Biophys Acta. 1973 Feb 4;294(1):396–404. doi: 10.1016/0005-2787(73)90094-4. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. S., Birnstiel M. L., Purdom I. F., Williamson R. Genes coding for polysomal 9S RNA of sea urchins: conservation and divergence. Nature. 1972 Nov 24;240(5378):225–228. doi: 10.1038/240225a0. [DOI] [PubMed] [Google Scholar]



