Highlights
-
•
Mecp2Stop mutant female mice develop Rett-like symptoms late in life (>6 months).
-
•
Symptoms include anomalies in motor, activity and anxiety profiles and were assessed in various behavioural tasks.
-
•
Deficits occurred in ambulatory activity during novelty exploration and habituation to a novel environment.
-
•
Circadian rhythms and anxiety were not affected, but food intake was higher and global activity lower in mutant mice.
Keywords: Mecp2, Rett syndrome, Locomotor activity, Non-associative learning, Mice
Abstract
Numerous experimental models have been developed to reiterate endophenotypes of Rett syndrome, a neurodevelopmental disorder with a multitude of motor, cognitive and vegetative symptoms. Here, female Mecp2Stop mice [1] were characterised at mild symptomatic conditions in tests for anxiety (open field, elevated plus maze) and home cage observation systems for food intake, locomotor activity and circadian rhythms.
Aged 8–9 months, Mecp2Stop mice presented with heightened body weight, lower overall activity in the open field, but no anxiety phenotype. Although home cage activity scans conducted in two different observation systems, PhenoMaster and PhenoTyper, confirmed normal circadian activity, they revealed severely compromised habituation to a novel environment in all parameters registered including those derived from a non-linear decay model such as initial exploration maximum, decay half-life of activity and span, as well as plateau. Furthermore, overall activity was significantly reduced in nocturnal periods due to reductions in both fast ambulatory movements, but also a slow lingering. In contrast, light-period activity profiles during which the amount of sleep was highest remained normal in Mecp2Stop mice.
These data confirm the slow and progressive development of Rett-like symptoms in female Mecp2Stop mice resulting in a prominent reduction of overall locomotor activity, while circadian rhythms are maintained. Alterations in the time-course of habituation may indicate deficiencies in cognitive processing.
1. Introduction
Rett syndrome is a neurological disorder that primarily affects females and is characterised by apparently normal early development up until 6–18 months of age, upon which a period of regression and the development of symptoms including loss of acquired skills, deceleration of head growth, development of ataxia, seizures, scoliosis, breathing anomalies and autistic behaviour is observed [2].
The majority of cases of Rett syndrome result from mutations in the gene encoding methyl-CpG-binding protein 2 (Mecp2) on the X chromosome [3]. Several mouse models that lack or express a truncated MeCP2 protein have been developed, and successfully recapitulate many of the clinical symptoms associated with Rett syndrome [4], [5], [6]. In keeping with the disease, mutant mice have apparently normal early development followed by the emergence of symptoms and progressive dysfunction such as abnormal gait, hypoactivity, irregular breathing and tremors. Male null mutants present with early onset of symptoms rapidly progressing in severity and a short life span of only 8–12 weeks [4], [5]. By contrast, heterozygous females show a late-onset phenotype at 4–12 months and, similar to human patients, these symptoms stabilise so that mice experience a normal life span. Truncation of the Mecp2 allele after codon 308 (Mecp2308/Y) produces a less severe phenotype and mutant males survive until adulthood [6], but Mecp2308 heterozygous females have unbalanced patterns of X-chromosome inactivation predominantly expressing the wild-type allele leading to a high degree of variability in phenotypes [7], [8].
Neuroanatomical studies of brain areas affected in Rett patients reported a reduction in overall brain volume and atrophy in regions associated with motor function including the caudate nucleus [9] and cerebellum [10], [11]. Mecp2 null mice also display abnormalities in the cerebellum and motor cortex [12] and suggest this to be a mechanism for behaviourally recorded reductions in locomotor activity in the open-field [13], [14], [15], [16]. However, it is conceivable that reduced ambulatory activity may not be a direct indicator of locomotion, but instead reflects altered anxiety levels in response to the novel environment, and this would be in agreement with previous studies providing compelling evidence that Mecp2 deficient mice have heightened levels of anxiety [6], [13], [17], [18], [19], [20], [21], [22], [23]. One possible strategy to monitor locomotor activity uncontaminated of novelty-dependent anxiety would be to perform home cage activity scans in these mice. Such long-term continuous recordings may provide additional information on circadian rhythms, amounts of active locomotion relative to lingering, specific patterns of cage exploration as well as feeding and/or drinking orientated behaviours [24]. It would further reveal whether mouse phenotypes include Rett-typical abnormalities in sleep/wake cycle [25], [26] or food intake patterns as a mechanism underlying the observed obesity in patients [27] and Mecp2 mutant mice [4], [5], [16], [18], [19], [28].
Despite Rett occurring predominantly in females, investigations using Mecp2 deficient mouse models have concentrated on males with few exceptions [15], [16], [20], [22]. Mecp2 null males develop post-natal symptoms early (within few weeks) so that recording can take place in young adult subjects and behavioural endpoints are readily met, with the additional benefit of reduced costs. At the same time, this precludes tracing the behavioural progression in longitudinal analyses as the severity of symptoms rapidly progresses. Consequently, extensive data are available for male Mecp2308/Y mice due to their mild phenotypes, but little is known about neurobehavioural anomalies of heterozygous female mice, although females might more realistically mimic the condition of Rett patients at the molecular level [22], [29]. Since behavioural phenotypes of Rett models based on different Mecp2 mutations did not overlap [30], an assessment of each individual model is necessary.
Here, we investigated the anxiety- and motor-related phenotypes of female heterozygous Mecp2Stop mice by attempting to capture symptoms in overall activity through scanning their behaviour in an open field followed by fine-grained investigations of circadian/ultradian activity in different home-cage environments. The benefit of using two home-cage observation systems is that in addition to assessing exploratory activity by way of different tracking parameters (infrared beams or overhead infrared camera) each system has distinctive recording features which complement one another and facilitate in the characterisation of the mice in terms of exploration, circadian and feeding orientated behaviour. The PhenoMaster (TSE Systems, Germany) permits direct measurement of food and water intake and also facilitates the distinction between different types of ambulation. Whereas, the PhenoTyper (Noldus IT, The Netherlands) allows the tracking of behaviours (patrolling and feeding) based on user-defined zones, as an indirect measure of feeding behaviour/food intake. We reveal overall reductions in open field exploration due to lower ambulatory activity in Mecp2Stop mice not related to heightened anxiety levels or endophenotypes affecting circadian rhythms.
2. Materials and methods
2.1. Subjects
Female mice in which the endogenous Mecp2 gene was silenced by insertion of a targeted lox stop cassette (Mecp2Stop) were crossed with males hemizygous for the CreESR transgene [1]. For the purpose of this study only heterozygous Mecp2Stop/+ females without the cre-ER transgene and wild-type (WT) littermates were selected. Animals were bred on a C57BL6/J/CBA background. Fifty female mice bred at a commercial vendor (Harlan, UK) and delivered to our facility aged 6 months were used and assigned to two cohorts: Cohort 1: n = 30 (13 Mecp2Stop and 17 WT) were tested in open-field and elevated plus maze; Cohort 2: n = 20 (10 Mecp2stop and 10 WT) were scanned over 7 days in the PhenoTyper (Noldus IT, Wageningen, The Netherlands) and then over a similar time course in the PhenoMaster (TSE Systems, Bad Homburg, Germany) for circadian activity and food/water intake. Mice were group housed with littermates (unless in the recording equipment) and maintained on a 12 h light/dark cycle (lights on 7 am) with free access to food and water. All procedures were performed in accordance with Home Office regulations.
2.2. Assessment of symptoms
Both body weight and symptom score of mice was recorded weekly; scores for individual symptoms associated with Mecp2 deficiency included mobility, gait, hindlimb clasping, tremor, breathing and general condition. Each symptom scored as 0 (absent/as wild-type); 1 (symptom present) or 2 (symptom severe) as previously described [1], [31]. Testing in the open-field, elevated plus maze and home-cages commenced when the average severity symptom score of Mecp2Stop mice was 5/6. At this stage Mecp2Stop mice typically presented with a mild/moderate reduction in mobility, abnormal gait, hindlimb clasping and onset of tremors. However, the general health and condition of the mice was comparable to that of WT.
2.3. Open-field test
Explorative behaviour and emotionality was determined in an open field. A white square arena (50 cm × 50 cm) positioned in a dimly lit room (light intensity: 151 lux) with an overhead video camera connected to a PC based tracking software (Ethovision 3.1, Noldus IT) was set up to continuously record activity of the animals. The software monitored the actual movement based on a body-centred contrast subtracted from background and calculated the following parameters: total distance moved; velocity; time spent in inner/outer zones equidistant from the centre of the arena.
All subjects were acclimatised to the room for 5 min, and then released in the centre of the arena for a total exploration time of 10 min. They were returned to their home cages, the arena cleaned with all purpose maceratable wipes, and the next mouse was introduced. Data were analysed using factorial analysis of variance (ANOVA) with genotype (between-subject) and time (within-subject) as factors followed by appropriate post hoc tests with Bonferroni adjustments. In all analyses, the null hypothesis was accepted for alpha smaller than 0.05.
2.4. Elevated plus maze
The elevated plus maze (EPM) was light grey in colour and comprised of two open arms (35 cm × 5 cm) and two closed arms (35 cm × 5 cm × 15 cm; L × W × H) which extended from a central platform (5 cm × 5 cm). Like arms were arranged opposite to each other in the shape of a plus sign. The apparatus was elevated 42 cm above a table in a dimly illuminated room (light intensity: 142 lux). Prior to testing, mice were transferred to the experimental room and acclimatised for 1 h. At the beginning of a trial mice were individually placed onto the central platform facing an open arm followed by 5 min free exploration. Frequency of entries and time spent in the open/closed arms was recorded online (Ethovision 3.1) and statistically assessed using paired two-tailed t-tests. The maze was thoroughly cleaned using ethanol between subjects.
2.5. Home cage activity scans
Two variants of home-cage observation systems were utilised: (1) the PhenoMaster/LabMaster; (2) the PhenoTyper. In both tests, animals were singly housed and subjected to a 12 h light/dark cycle (lights on 7 am, temperature 23 ± 2 °C, relative humidity of 40–60%). They were placed into the cages containing sawdust bedding at lunchtime and given 2 days of habituation before assessment of circadian/ultradian activity over 3 continuous day–night cycles.
2.5.1. The PhenoMaster/LabMaster
The PhenoMaster system consisted of 4 test cages (42 cm × 26.5 cm × 15 cm) positioned in a metal frame containing regular infrared beams in both X and Z coordinates at a distance of 3 cm and at a rate of 100 Hz. These enable the continuous recording of exploratory activity over days, weeks or even months [32], [33]. Five replications were conducted with Mecp2Stop and WT mice always analysed in parallel and randomly assigned to the test cages. Cage lids were fitted with two weight transducers supporting a feeder and a water bottle, respectively, and transmitting data of food and water intake through an AD converter to a PC. Similarly, beam crossings are stored online and the following parameters calculated: (i) daily food consumption; (ii) daily water consumption; (iii) total distance moved in the cage in hourly bins and separated for day and night time; (iv) fine movements (lingering) are counted as repetitive breaks of the same beam without progression of the body forward; (v) progressive movements (ambulation) are recorded as successive beams are interrupted; and (vi) habituation during the initial 3 h as distance moved in 10-min intervals.
Habituation was modelled and mathematically fitted using a nonlinear exponential decay. This model can be applied for biological processes assuming the rate of decay is always proportional to its remainder [34] and has previously been applied for learning and physiological processes [35]. Here, it provided the best fit for the behaviour of the control group and at the same time highlighted the differences that occurred in Mecp2Stop mice. The one phase exponential decay followed the equation: Y = (Y0 − Plateau)e−kt + Plateau with t representing time of habituation from start to attainment of floor level, and Y0 indexing the total distance moved for time point 0. Over time, the total distance per time-bin (here 10 min) decays to the plateau (baseline activity or floor level reached at 3 h) with the rate constant k and a half life 0.6932/k. Therefore, the rate constant k determines the speed of decay, or in other words the steepness of the habituation curve to reach floor level. In controls, this was achieved after 4 half-life cycles when the ambulatory activity deviated by <5% from the theoretical plateau indicating completion of habituation.
2.5.2. The PhenoTyper
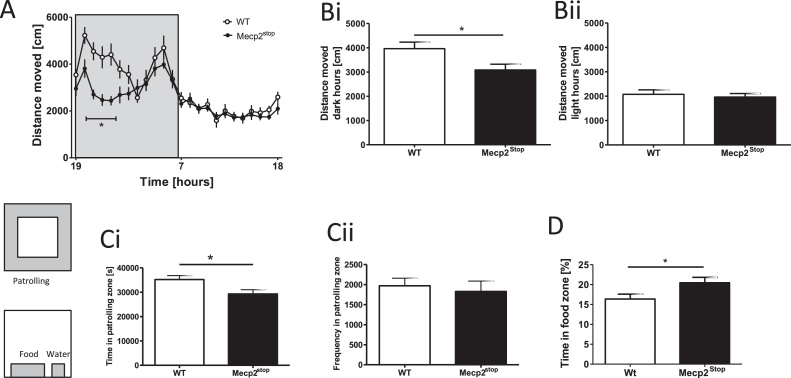
Two sets of 16 PhenoTypers, a video-based observation system for long-term continuous scanning of behavioural activity in mice [24], [36] via a built-in digital infrared sensitive video camera and infrared light sources in a top unit of each cage were used. The body-centred recording of the animal (Ethovision 3.1) was sampled at 12.2 Hz. Data stored online were calculated for the following parameters: (i) total locomotion in the arena summarised in hourly bins during experimental days; (ii) time spent in the food zone adjacent to the feeder; (iii) time spent in the water zone in front of the water bottle (see Fig. 4 for food and water zone locations); (iv) time spent in an outer zone (4.4 cm distance from wall, see Fig. 4) in patrolling the boundaries of the home cage; and (v) frequency of entries into the patrolling zone.
Fig. 4.
Home cage activity as recorded by PhenoTyper. Means ± SEM. (A) Circadian activity over a 24-h period (shaded area indicates dark hours, 19:00–07:00). Mecp2Stop mice were less active relative to WT during the dark hours with a strong locomotor deficit apparent at 2–5 h of the dark phase. Nocturnal activity was significantly higher in WT mice (Bi), but not diurnal locomotion (Bii). Locomotion of WT mice was concentrated towards the edges of their home cage (patrolling zone: grey shaded area) in which they performed more patrolling (Ci), while Mecp2Stop mice spent less time in this zone. This was not related to lower ambulatory activity since both WT and Mecp2Stop mice entered the zone with the same frequency (Cii). A reliable difference was also evident for time spent in food zone (shaded area: in front of food hopper) (D) in keeping with food intake data from the PhenoMaster (Fig. 3D). *p < 0.05.
Data were analysed using Prism 5.0 (GraphPad Software, San Diego, CA, USA) and factorial two-way repeated measures analysis of variance (ANOVAs) with genotype as between and time as within-subject factor; one-way ANOVAs and t-tests using Bonferroni adjustment were conducted for group comparisons of locomotor activity, body weight, food intake and water consumption. Alpha was set to 0.05. Only reliable differences are shown for clarity.
3. Results
3.1. Mecp2Stop mice progressively increased symptom severity and body weight
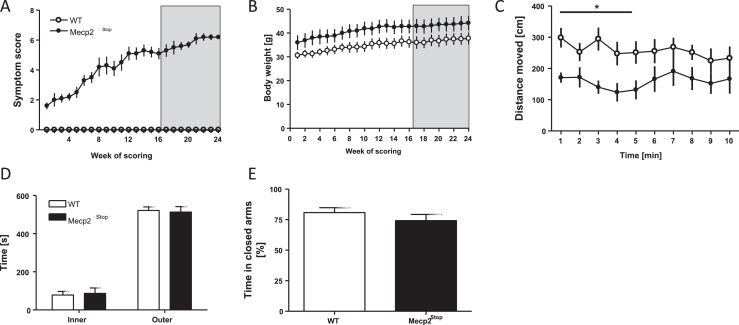
Mecp2Stop female mice already displayed very mild symptoms upon arrival at our animal facility. The symptom score progressively increased from 6 months of age until approximately 10–12 months, when a compound score of 5/6 was met and behavioural testing commenced (grey highlighted area, Fig. 1A). Mecp2Stop mice presented with symptoms of motor impairments including reduced spontaneous locomotor activity, abnormal gait, hindlimb clasping and the presence of mild tremors. The general health and condition of the mice was relatively unaffected. By contrast, WT mice never developed any anomalies in overall mobility, motor coordination or their general health. Throughout this period, Mecp2Stop mice presented with heightened body weight compared to WT (Fig. 1B; F(1,432) = 75.38; p < 0.0001) mimicking a phenotype observed in Rett patients [27].
Fig. 1.
Symptom severity score, body weight and anxiety-related behaviour of Mecp2Stop mice. Means ± SEM. (A) Mecp2Stop mice presented with a progressively increasing symptom score, which attained a mean of 5/6 in weeks 16–24 of testing. (B) Mecp2Stop mice also displayed a sustained increase in body weight compared to WT's. Shaded area indicates period of behavioural assessment. (C) Ambulatory activity in the open field during 10 min of recording plotted in minute bins. Mecp2Stop mice presented with reduced locomotor activity. No reliable habituation occurred in either WT or Mecp2Stop mice. Fear-signalling visits to the centre of the open field (D) or open arms of the elevated plus maze (E) was not affected by transgene knockout. *p < 0.05.
3.2. Reduced locomotor activity in the open-field, but no anxiety phenotype in Mecp2Stop mice
When placed in the open-field, Mecp2Stop mice moved less than WT controls throughout the test (F(1,252) = 6.9; p = 0.01)) (Fig. 1C). Intriguingly, neither genotype showed any habituation during the 10-min recording period. The times spent in the outer/inner zone of the open-field or in closed arms of the elevated plus maze (EPM) were determined as indices of anxiety-like behaviour. No difference between genotypes was obtained for the open field (Fig. 1D) (both groups preferred the outer zone) or the EPM (Fig. 1E).
Based on these data, it seems unlikely that reduced exploratory activity is due to heightened anxiety levels in Mecp2Stop mice however, it may in part be a result of motoric deficits of the mice. We thus sought to confirm this by long-term observations of mice in home-cages. A corollary of motor impairments and an overall reduced exploratory activity in Mecp2Stop mice might be the persistence of this phenotype even when animals are fully habituated to the environment (i.e. home cage). Alternatively, activity differences may only occur during periods when active exploration is the predominant behavioural state of the animal (i.e. during nocturnal food seeking and patrolling). Yet another hypothesis would suggest that anxiety-related lowering of ambulations in Mecp2Stop mice may be observed only during the period of acclimatisation and may wane once this has been completed so that no further phenotype remains. These opposing views were addressed next.
3.3. Contrasting habitation curves between Mecp2Stop mice and WT controls
We placed female mice into novel home cages and recorded their behaviour continuously for 7 days. This period was then further fragmented into phases of particular interest including the first 3 h after placement (habituation) and days 3–5 for assessment of circadian activity post-acclimatisation.
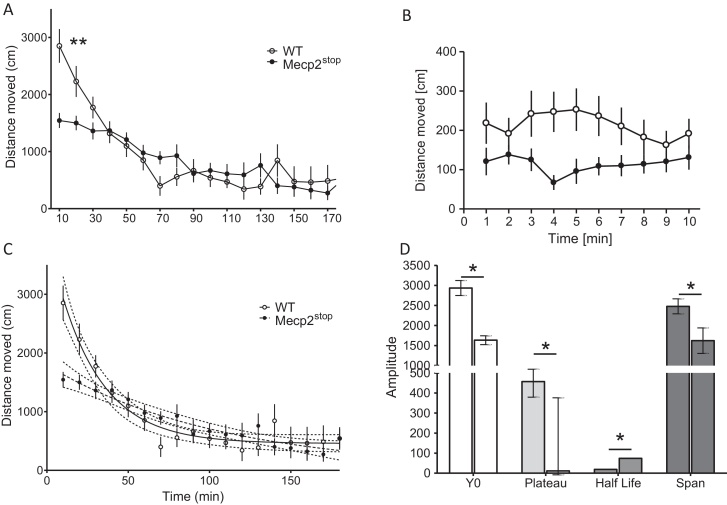
Habituation tested in the PhenoMaster was different between genotypes (interaction between genotype and time point: F(17,238) = 3.9; p < 0.0001), especially during early time points following release into novel home cages (Fig. 2A, asterisks). However, both genotypes presented with a progressive reduction of activity lasting about 1 h, after which floor levels were attained and habituation was completed. In agreement with exploratory activity recorded in the open field, which lasted 10 min, a similar activity profile was observed by the PhenoMaster during the initial exploration period (Fig. 2B). Ambulatory activity (distance moved) was reliably lower in Mecp2Stop mice (F(1,162) = 16; p = 0.001 for genotype, ns for time). Although exploration differed in Mecp2Stop mice from controls, this is unlikely due to differences in anxiety levels (see Fig. 1D and E) and may rather be due to a lack of arousal or motoric dysfunction in the mice.
Fig. 2.
Habituation of Mecp2Stop and WT mice to novel home cages recorded by the PhenoMaster. Animals were recorded upon introduction to the novel cage in 10-min bins for 3 h (habituation complete in WT mice). Mean ± SEM. (A) Activity profiling confirmed higher activity in WT during early phases of habituation (asterisks are for 10–30 min, p < 0.01). (B) High resolution plot of first 10 min in novel cage. Again, Mecp2Stop mice show lower ambulations relative to WT (compare with Fig. 1C). (C) One-phase exponential decay fitted to model typical WT habituation profile following equation y = (Y0 − plateau)e−kt + plateau (for details, see Section 2). Data represent means ± SEM and dotted lines indicate theoretically calculated 95% confidence intervals. As is obvious, Mecp2Stop mice followed a different habituation profile. Consequently maximal locomotor activity (Y0), the asymptotic plateau, the decay half life, and span (range between max and min activity) are significantly different between genotypes (D). Note that left bar for each parameter represents data for WT cohort; data are mean with 95% confidence interval. Asterisks: p < 0.05. Overall, WT mice showed faster habituation. *p < 0.05; **p < 0.01.
A more detailed understanding was achieved through nonlinear modelling of the habituation time course. Extraction of indexes following equation 1 revealed differences in all parameters (Fig. 2D, see asterisks), and the null hypothesis that both habituation curves follow the same nonlinear fit was rejected (Fig. 2C; F(3,282) = 13; p < 0.0001). There were reliable differences in the overall activity level at start of recording (Y0), the half life of the overall decay in activity (t1/2), the plateau level of lowest overall activity achieved at about 4 half life cycles, and the overall span of activities between max and min activation (Fig. 2D). Overall, key values were lower in Mecp2Stop mice underlining the notion of slower habituation and lower general activity. They further contest reliable impairments in non-associative learning in Mecp2Stop mice.
3.4. Mecp2Stop mice present with reduced nocturnal ambulatory activity in the home cage
Long-term scans of activity in home cages were performed to determine abnormalities in motor coordination and circadian activity. Lack of Mecp2 indeed compromises the sleep-wake cycle in 80% of patients [37] for example owing to the failure to activate Period gene expression and clock entrainment in the hypothalamus [38]. A selective decrease in nocturnal home-cage activity but intact circadian rhythms has been observed with Mecp2308 male mice [18], [23], [39]. This however, remains unexplored in our Mecp2 model and we thus placed female mice in home cage observation systems to determine their activity pattern over several days. Two observation systems were chosen for their respective advantages in automatic recording of activity, exploration, food/water intake (PhenoMaster) or fine-grained analysis of ambulatory patterns (PhenoTyper).
3.4.1. Circadian activity recorded by PhenoMaster
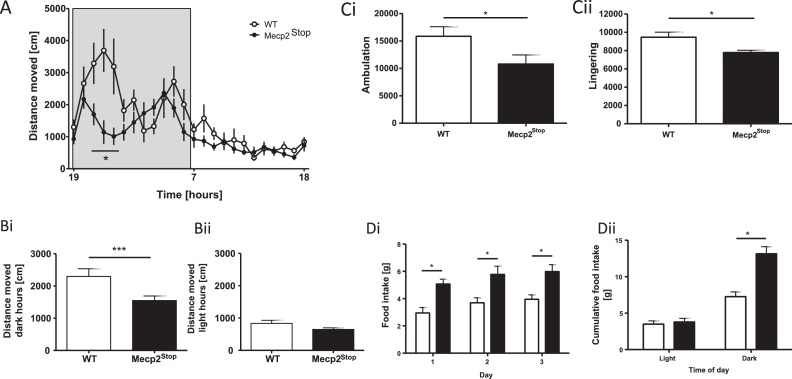
The overall activity pattern of each mouse was recorded continuously for 3 consecutive days and data averaged to give a 24 h profile of activity for each genotype (Fig. 3A). A reduction in activity is particularly obvious in the Mecp2Stop group during the nocturnal activity period (19:00–07:00 indicated by grey bars) while little if any difference occurred during the light period, when mice predominantly sleep. No overall shift of activity onset was recorded suggesting normal circadian activity. Similar to WT controls, Mecp2Stop mice demonstrated peaks in activity during the dark hours and strongly diminished activity during the light phase. Reliable effects of time (F(23,414) = 10.86; p < 0.0001) and genotype (F(1,18) = 4.79; p < 0.05) were in line with the notion that Mecp2Stop mice are less active than WT. Separating the overall activity into nocturnal versus diurnal periods (Fig. 3Bi,ii) further substantiated the ambulatory deficit in Mecp2Stop mice as selective for the nocturnal period (Fig. 3Bi) (df = 18; T = 2.44; p = 0.03). And it is during the dark hours that we observed a reduction in both ambulatory (Fig. 3Ci) (t = 2.1; df = 18; p = 0.03) and fine movements (Fig. 3Cii) (t = 2.7; df = 18; p = 0.01) of Mecp2Stop mice.
Fig. 3.
Home cage activity as recorded by PhenoMaster. Means ± SEM. (A) Circadian activity over a 24-h period (shaded area indicates dark hours, 19:00–07:00). Mecp2Stop mice were less active during the dark phase with a strong locomotor deficit apparent at 2–6 h of the dark phase. Indeed, nocturnal activity was significantly higher in WT mice (Bi), but not during light phases (Bii), and was due to reductions in both ambulatory (Ci) and fine movements (Cii). Automatically monitored food intake was higher in Mecp2Stop mice on all recording days (Di) and increased cumulative food intake over 3 days was due to nocturnal eating (Dii). *p < 0.05; ***p < 0.001.
At the same time, food intake was always greater in the Mecp2Stop cohort (F(1,18) = 13.10; p = 0.002) (Fig. 3Di), but no difference in terms of water intake (F < 1, data not shown) was obtained. Analysis of cumulative food intake over 3 days found that food intake did not differ during light hours, but was reliably higher during nocturnal activity periods (Fig. 3Dii; F(1,18) = 45; p < 0.0001 for interaction between genotype and circadian phase – light or dark), during which the majority of food intake occurred. This may readily explain the heightened body weight in Mecp2 knock-out mice (Fig. 1B). This increase in time spent feeding may also account for the reduced exploratory activity (Fig. 3Bi) of the Mecp2Stop mice.
3.4.2. Circadian activity recorded by PhenoTyper
Verification of the data obtained from PhenoMaster was achieved in another home cage observation system. While activity in the PhenoMaster is recorded through the breaking of infrared beams, video camera observed movement of subjects is detected in PhenoTypers and food/water intake is indexed indirectly by either weighing of food hopper/water bottle on a day-by-day basis or by monitoring the time spent in zones adjacent to food and water troughs [24]. Furthermore, identification of the animal is based on centre of gravity so that alteration in a small number of pixels is recognised as movement; this way of activity detection has a higher resolution of ambulatory activity. The average 24 h activity profile from 3 consecutive days of PhenoTyper recordings is presented in Fig. 4A. Mecp2Stop mice displayed normal circadian rhythms with a mouse-typical elevation of locomotor activity during hours of darkness and reduced activity during the light phase. However, as indicated above, Mecp2Stop mice presented with much fewer ambulations during the nocturnal activity period (19:00–07:00, shaded areas) relative to WT mice. Main effects of time (F(23,414) = 65.72; p < 0.0001) and the genotype by time interaction were reliable (F(23,414) = 4.1; p < 0.0001). The main difference appeared for nocturnal locomotor activity (Fig. 4Bi; df = 18; t = 3.08; p = 0.006), but not for diurnal movements (Fig. 4Bii). In an additional step, we explored the activity pattern between the two phenotypes. Typically, animals spend a considerable amount of time patrolling the edges of their environment while less time is spent in exploring the centre of an open home cage. WT mice spent significantly more time patrolling the home cage borders (see diagram in Fig. 4) than Mecp2Stop mice (Fig. 4Ci, t = 2.4, df = 18, p = 0.02). Since Mecp2Stop mice had higher body weights, a 4.4 cm corridor may bias the data in favour of the slimmer WT mice. However, Mecp2Stop animals entered the zone with the same frequency as WT mice (Fig. 4Cii) and we failed to find a correlation between body weight and time in the patrolling zone for either genotype (data not shown). In keeping with the lower body weights of WT controls, the averaged time spent in the food zone for the three recording days confirmed heightened feeding behaviour and food intake in the Mecp2Stop cohort (Fig. 4D; df = 58; t = 2.21; p = 0.03).
4. Discussion
Qualitative assessment of Mecp2Stop heterozygous female mice over a period of 6 months confirmed findings from previous studies [1], [5] in that Mecp2Stop mice recapitulate onset and progression of symptoms associated with Rett syndrome. These include abnormal gait, reduced spontaneous activity, tremors and hindlimb clasping.
4.1. Altered body weight and feeding behaviour in Mecp2Stop mice
In addition to these symptoms, female Mecp2Stop mutants also exhibited increased body weight compared to WT throughout the course of the study. We progressed with examinations at the age of 6 months, at which time a significant weight difference had already been established. Tendency for obesity due to hyperphagia has been widely reported for Mecp2 null [1], [16], [40], Mecp2308 truncated hypomorphic mice with ∼50% reduction in Mecp2 expression [18], [28], [41], and also in conditional Mecp2 knockout mice [13], [19]. Abnormal weight gain was evident with increasing age and most likely due to heightened fat deposition in all Mecp2 mutant mice [19], [28]. Furthermore, region-specific conditional Mecp2 knockout in neurons within either forebrain [13] or hypothalamus [19] caused an obese phenotype, which coincided with a significant reduction in Mecp2 expression in the hypothalamus [28] and is congruent with a possible role of Mecp2 as a transcriptional activator/repressor [42]. However, overweight has not been a consistent phenotype for all mouse models and there are reports of Mecp2 null mice with no difference [15], [23], [43], [44], [45], [46] or even a reduction in body weight [4], [5], [14], [15], [22], [43].
In our investigations, we nevertheless observed compelling evidence not only for heightened body weight, but also for alterations in feeding behaviour since Mecp2Stop mice spent more time in zones associated with the food hopper and they also ingested more chow than WT controls. In contrast to previous work which concentrated on the overall monitoring of food consumption, we here determined for the first time the circumstances during which enhanced feeding orientated behaviour in Mecp2Stop mice occurs. It is precisely during nocturnal hours, when food intake is higher in Mecp2Stop mice and coincident with hypoactivity as recorded in both PhenoMaster and PhenoTyper home cages. Replicating the observation in two different recording environments not only strengthens the quality of the equipment, but also underlines the robustness of the phenotype. It further supports the notion that circadian activity remained normal in our Mecp2Stop mice and the genotype specifically affected food consumption at periods of heightened ambulatory activity and metabolic requirements. Likely mediators of this effect are hypothalamic Sim1-expressing neurones, which when selectively depleted of Mecp2 also caused a hyperphagic phenotype. A possible explanation for this phenotype is either the deregulation of the melanocortin pathway [19] or a rise in leptin levels, which has been observed in Rett patients [47] and is similarly found in obese children and adults [48]. The absence of Mecp2 in the hypothalamus could therefore explain the hyperphagic and obesity-like phenotype of our female Mecp2Stop mice, but requires further confirmatory studies.
Any discrepancies between the different mouse models may be explained by genetic modifiers. Towards this end, Guy and colleagues [5] observed changes in body weight in Mecp2 null mice dependent on their genetic background. This was confirmed by Samaco et al. both with hypomorphic Mecp2 mice [41] and more recently Mecp2+/− female [16] cohorts backcrossed on to C57Bl/6 or FVB backgrounds. A factor superimposed here is age and gender, which widely differed between studies. Most reported work on weight reductions concentrated on young male Mecp2 mice ∼4–7 weeks of age [5], [14], [15], [22] for which increased body weight was observed from ∼7 weeks of age onwards [15], [19], [28], [40], [41]. Here, we report hyperphagia/increased body weight in heterozygous female Mecp2 mutant mice which is in agreement with studies of Mecp2+/− females [1], [16] and obesity in some Rett Syndrome patients [27]. By contrast, other studies have reported no effect on body weight of Mecp2+/− female mice [44], [45], [46].
4.2. Reduced locomotion in Mecp2Stop mice may be due to deficits in information/motivational processing
Locomotor activity of Mecp2Stop female mice in the open-field was considerably lower compared to WT, consistent with previous reports of decreased locomotor activity in the same apparatus [5], [6], [13], [15], [16], [49]. This locomotor impairment is in agreement with previous studies from female Mecp2+/− mice that have consistently reported deficits in motor function [5], [15], [16], [44], [50], [51]. However, some experimenters have failed to determine this phenotype for Mecp2308 mice [18], [52], hypomorphic mice [28], conditional deletion of Mecp2 from hypothalamus [19] and Mecp2 null mice [14], [15], [53]. Gemelli et al. [13] used forebrain-specific deletion of Mecp2 and reported an initial reduction in activity in a novel environment lasting for 5 min, before activity levels normalised and remained similar to WT for the habituation period of 2 h. This effect was amplified in our Mecp2Stop mutants lasting for at least 10 min during open-field exposure (Fig. 1) or up to 30 min during habituation to a novel home cage (Fig. 2). What is different in our model is that the resulting habituation curve diverged between genotypes. Although habituation of both WT and Mecp2Stop mice followed a non-linear fit, there were considerable differences pertaining not only to the half-life of activity decay, but also the plateau and peak activity levels, that were higher for WT mice. These data strongly suggest dysfunctional information/motivational processing in Mecp2Stop when exploring novel environments, since putative anomalies in anxiety-related behaviour both in open field and elevated plus maze were excluded (for similar results on other Mecp2 models, see [5], [14], [15], [22], [28], [41], [43], [52], [53]). It contrasts to studies that reported either reduced or elevated anxiety levels in open field, elevated plus maze, zero maze or light/dark box following altered expression of Mecp2 either in specific brain regions [13], [17], [19] or globally [6], [16], [17], [18], [20], [21], [22], [23], [50], [52], previous findings with female Mecp2 +/− mice have observed either a reduction in anxiety [16] or no effect [15]. Studies with mouse models have suggested that the presence of anxiety-like behaviours is dependent on the mutation [54] even though anxiety is a characteristic feature in Rett patients [55], [56].
Overall exploratory activity is a predictor of general cognitive ability ‘g’ in mice [57] and humans [58]. Differences in exploration are most predictive during early periods of exposure to a novel environment (open field, novel cage), but diminish at later stages when asymptotic activity levels are attained. Such a time course follows a one-phase exponential decay function for which we secured a reliable difference between WT and Mecp2Stop mice for Y0, i.e. the maximal locomotor activity at test start. This difference was strikingly similar to the segregation found in the open field underlining the robustness of this observation and implicating Y0 as a critical index for learning capability. This assertion is further strengthened by the observation that non-associative habituation to a novel environment upon first exposure to PhenoMaster or PhenoTyper cages follows different time courses and could be modulated and thus reflect general learning abilities (since in itself it is a form of learning). A mouse with high ‘g’ would more readily acquire information about the environment thereby quickly reducing activity (calculated as half life of activity) and meeting asymptotic plateau faster than mice with lower learning ability. Both habituation rate and plateau were significantly different between genotypes supporting the contention that the rate of information processing which is purported by the working memory system and is likely to be compromised in Mecp2Stop mice.
Such a shunting of information/motivational processing was further confirmed for the female Mecp2Stop cohort during long-term recording of circadian activity in home cages (PhenoMaster and PhenoTyper). While there was no evidence for any circadian anomaly, it became clear that the trigger of activity i.e. change from light to dark phase induced a much lower activation in terms of lingering and ambulation in Mecp2Stop relative to WT. This reduction in nocturnal activity but unaffected circadian activity with Mecp2Stop females corroborates work with male Mecp2308 mice [18], [23], [39]. Aberrant motivation has been used to account for frequently observed variability in intellectual and developmental disabilities, including autism and Rett syndrome. Towards this end, a novel environment, or changes in contexts that are otherwise austere or barren constitutes an environmental challenge and induces motivational operations to seek automatic positive reinforcement [59]. These motivational operations, however, often require attention-maintained behaviour, which seems to be failing in our model possibly due to frontal lobe dysinhibition [60], [61]. While unconfirmed to date for our mice, the global knockdown of Mecp2 may also compromise functions mediated by the prefrontal cortex; indeed, 100% of Rett patients analysed for frontal cortex expression presented with diminished Mecp2 [62]. Thus lowered attention span in Mecp2Stop mice may account for the brief activity peak observed at the beginning of the dark phase, which is then followed by a trough at a period when WT mice maintain a high level of locomotor and exploratory activity. A more fine-grained dissection of the recorded activity, however, suggested a reduction of fine movements, such as head shakes, grooming, or rearings in the knock-out line, with ambulatory progressions also affected (Fig. 3Ci and Cii).
Reduced locomotor activity may also be explained by altered sleep patterns, and these are widely manifest in Rett patients [37], [63]. Especially the emergence of repeated daytime napping and its progression could have affected overall activity observed during nocturnal activity peaks and thereby have caused a lowering of locomotor activity as seen in Mecp2Stop mice. Although both monitoring systems obtained continuous recordings from all subjects, neither can discriminate sleep phases from wake quiescence. Resolution of this issue thus would require detailed vigilance staging of animals based on global electro-encephalograms (EEGs). However, while detailed determination of sleep signatures requires further experimental work, these would offer unlikely explanations for differences in habituation (Y0, decay and asymptotic plateau), which were observed during periods of active wakefulness not interrupted by short naps.
4.3. Conclusions
Behavioural assessment of Mecp2Stop heterozygous female mice to determine motor and anxiety phenotypes revealed overall reductions in locomotor activity but no effect on anxiety levels or circadian rhythms with reduced activity observed during the dark but not light cycle. In addition to the motor phenotype mice also presented with hyperphagia/increased body weight. As discussed in detail these findings both corroborate and contrast with studies of other mice with altered Mecp2 levels. Following on from the present results further work is needed to determine if the disruption in habituation to the novel home cage environment observed in this study are indeed linked to deficits in cognitive processing of the mice and it would also be beneficial to use EEG to explain potential differences in the sleep/wake composition of these mice during the nocturnal phase of testing when they are consistently less active.
Acknowledgement
We are grateful to DeltaPhenomics, Netherlands, for help in conducting parts of these experiments and advise on an earlier version of this manuscript. Supported by Medical Research Council (G0800401).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg B., Aicardi J., Dias K., Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome. Report of 35 cases. Annals of Neurology. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 3.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X linked MECP2, encoding methyl-Cp G binding protein 2. Nature Genetics. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genetics. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 5.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 6.Shahbazian M., Young J.I., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 7.Watson C.M., Pelka G.J., Radziewic T., Shahbazian M.D., Christodoulou J., Williamson S.L. Reduced proportion of Purkinje cells expressing paternally derived mutant Mecp2308 allele in female mouse cerebellum is not due to a skewed primary pattern of X-chromosome inactivation. Human Molecular Genetics. 2005;14(13):1851–1861. doi: 10.1093/hmg/ddi191. [DOI] [PubMed] [Google Scholar]
- 8.Young J.I., Zoghbi H.Y. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of Rett syndrome. American Journal of Human Genetics. 2004;74:511–520. doi: 10.1086/382228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiss A.L., Faruque F., Naidu S., AbramsM. Beaty T., Bryan R.N. Neuroanatomy of Rett syndrome: a volumetric imaging study. Annals of Neurology. 1993;34:227–234. doi: 10.1002/ana.410340220. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh H., Suzuki I., Maruki K., Miomo M., Hirasawa K., Sasaki N. Magnetic resonance imaging and clinical findings examined in adulthood-studies on three adults with Rett syndrome. Brain and Development. 2001;23(Suppl. 1):S121–S188. doi: 10.1016/s0387-7604(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 11.Murakami J.W., Courchesne E., Haas R.H., Press G.A., Yeung-Courchesne R. Cerebellar, cerebral abnormalities in Rett syndrome: a quantitative MR analysis. American Journal of Roentgenology. 1992;159:177–183. doi: 10.2214/ajr.159.1.1609693. [DOI] [PubMed] [Google Scholar]
- 12.Saywell V., Viola A., Confort-Gouny S., Le Fur Y., Villard L., Cozzone P.J. Brain magnetic resonance study of Mecp2 deletion effects on anatomy and metabolism. Biochemical and Biophysical Research Communications. 2006;340:776–783. doi: 10.1016/j.bbrc.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 13.Gemelli T., Berton O., Nelson E.D., Perrotti L.I., Jaenisch R., Monteggia L.M. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biological Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Pratte M., Panayotis N., Ghata A., Villard L., Roux J.C. Progressive motor and respiratory metabolism deficits in post-weaning Mecp2-null male mice. Behavioural Brain Research. 2011;216:313–320. doi: 10.1016/j.bbr.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Santos M., Silva-Fernandes A., Oliveira P., Sousa N., Maciel P. Evidence for abnormal early development in a mouse model of Rett syndrome. Genes, Brain and Behavior. 2007;6:277–286. doi: 10.1111/j.1601-183X.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 16.Samaco R.C., McGraw C.M., Ward C.S., Sun Y., Neul J.L., Zoghbi H.Y. Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Human Molecular Genetics. 2013;22:96–109. doi: 10.1093/hmg/dds406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi M., Autry A.E., Covington H.E., Monteggia L.M. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. Journal of Neuroscience. 2009;29(13):4218–4227. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Filippis B., Ricceri L., Laviola G. Early postnatal behavioral changes in the Mecp 2-308 truncation mouse model of Rett syndrome. Genes, Brain and Behavior. 2010;9:213–223. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 19.Fyffe S.L., Neul J.L., Samaco R.C., Chao H.T., Ben-Shachar S., Moretti P. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonetti G., Angelucci A., Morando L., Boggio E.M., Giustetto M., Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biological Psychiatry. 2010;67:657–665. doi: 10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 21.McGill B.E., Bundle S.F., Yaylaoglu M.B., Carson J.P., Thaller C., Zoghbi H.Y. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stearns N.A., Schaevitz L.R., Bowling H., Nag N., Berger U.V., Berger-Sweeney J. Behavioral, anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146:907–921. doi: 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 23.De Filippis B., Ricceri L., Fuso A., Laviola G. Neonatal exposure to low dose corticosterone persistently modulates hippocampal mineralocorticoid receptor expression and improves locomotor/exploratory behaviour in a mouse model of Rett syndrome. Neuropharmacology. 2013;68:174–183. doi: 10.1016/j.neuropharm.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Riedel G., Fadda P., McKillop-Smith S., Pertwee R.G., Platt B., Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. British Journal of Pharmacology. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaze D.G., Frost J.D., Jr., Zoghbi H.Y., Percy A.K. Rett's syndrome: characterization of respiratory patterns and sleep. Annals of Neurology. 1987;21:377–382. doi: 10.1002/ana.410210410. [DOI] [PubMed] [Google Scholar]
- 26.Nomura Y. Early behavior characteristics and sleep disturbance in Rett syndrome. Brain and Development. 2005;27:S35–S42. doi: 10.1016/j.braindev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Zappella M., Meloni I., Longo I., Hayek G., Renieri A. Preserved speech variants of the Rett syndrome: molecular and clinical analysis. American Journal of Medical Genetics. 2001;104:14–22. doi: 10.1002/ajmg.10005. [DOI] [PubMed] [Google Scholar]
- 28.Kerr B., Alvarez-Saavedra M., Saez M.A., Saona A., Young J.I. Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Human Molecular Genetics. 2008;17:1707–1717. doi: 10.1093/hmg/ddn061. [DOI] [PubMed] [Google Scholar]
- 29.LaSalle J.M. Paradoxical role of methyl-CpG-binding protein 2 in Rett syndrome. Current Topics in Developmental Biology. 2004;59:61–86. doi: 10.1016/S0070-2153(04)59003-8. [DOI] [PubMed] [Google Scholar]
- 30.Ricceri L., De Filippis B., Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behavioural Pharmacology. 2008;19:501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- 31.Robinson L., Guy J., McKay L., Brockett E., Spike R.C., Selfridge J. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain. 2012;April doi: 10.1093/brain/aws096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edelsbrunner M.E., Painsipp E., Herzog H., Holzer P. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion: exploration, water and food intake. Neuropeptides. 2009;43(6):491–497. doi: 10.1016/j.npep.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theander-Carrillo C., Wiedmer P., Cettour-Rose P., Nogueiras R., Perez-Tilve D., Pfluger P. Ghrelin action in the brain controls adipocyte metabolism. Journal of Clinical Investigation. 2006;116(7):1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motulsky H., Christopoulos A. GraphPad Software Inc; San Diego: 2003. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. [Google Scholar]
- 35.Caeyenberghs K., Balschun D., Roces D.P., Schwake M., Saftig P., D‘Hooge R. Multivariate neurocognitive and emotional profile of a mannosidosis murine model for therapy assessment. Neurobiology of Disease. 2006;23(2):422–432. doi: 10.1016/j.nbd.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 36.de Visser L., van den Bos R., Spruijt B.M. Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behavioural Brain Research. 2005;160(2):382–388. doi: 10.1016/j.bbr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Young D., Nagarajan L., de Klerk N., Jacoby P., Ellaway C., Leonard H. Sleep problems in Rett syndrome. Brain and Development. 2007;29(10):609–616. doi: 10.1016/j.braindev.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Saavedra M., Antoun G., Yanagiya A., Oliva-Hernandez R., Cornejo-Palma D., Perez-Iratxeta C. miRNA-132 orchestrates chromatin remodelling and translational control of the circadian clock. Human Molecular Genetics. 2011;20(4):731–751. doi: 10.1093/hmg/ddq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Filippis B., Fabbri A., Simone D., Canese R., Ricceri L., Malchiodi-Albedi F. Modulation of RhoGTPases improves the behavioural phenotype and reverses astrocytic deficits in a mouse model of Rett syndrome. Neuropsychopharmacology. 2012;37:1152–1163. doi: 10.1038/npp.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacometti E., Luikenhuis S., Beard C., Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samaco R.C., Fryer J.D., Ren J., Fyffe S., Chao H.T., Sun Y. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Human Molecular Genetics. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chahrour M., Jung S.Y., Shaw C., Zhou X., Wong S.T., Qin J. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelka G.J., Watson C.M., Radziewic T., Hayward M., Lahooti H., Christodoulou J. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 44.Bissonette J.M., Knopps S.J. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatric Research. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bissonette J.M., Knopps S.J. Effect of inspired oxygen on periodic breathing in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Journal of Applied Physiology. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- 46.Bissonette J.M., Knopps S.J., Maylie J., Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Autonomic Neuroscience. 2007;136:82–89. doi: 10.1016/j.autneu.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blardi P., De Lalla A., D’Ambrogio T., Zappella M., Cevenini G., Ceccatelli L. Rett syndrome and plasma leptin levels. Journal of Pediatrics. 2007;150(1):37–39. doi: 10.1016/j.jpeds.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 48.Pilcová R., Sulcová J., Hill M., Bláha P., Lisá L. Leptin levels in obese children: effects of gender, weight reduction and androgens. Physiological Research. 2003;52(1):53–60. [PubMed] [Google Scholar]
- 49.Alvarez-Saavedra M., Saez M.A., Kang D., Zoghbi H.Y., Young J.I. Cell-specific expression of wild-type MeCP2 in mouse models of Rett syndrome yields insight about pathogenesis. Human Molecular Genetics. 2007;16(19):2315–2325. doi: 10.1093/hmg/ddm185. [DOI] [PubMed] [Google Scholar]
- 50.Jugloff D.G., Vandamme K., Logan R., Visanji N.P., Brotchie J.M., Eubanks J.H. Targeted delivery of a Mecp2 transgene to forebrain neuronsimproves the behavior of female Mecp2-deficient mice. Human Molecular Genetics. 2008;17:1386–1396. doi: 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- 51.Abdala A.P.L., Dutschmann M., Bissonette J.M., Paton J.F.R. Correction of respiratory disorders in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Human Molecular Genetics. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 53.Kondo M., Gray L.J., Pelka G.J., Christodoulou J., Tam P.P., Hannan A.J. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome—Mecp2 gene dosage effects and BDNF expression. European Journal of Neuroscience. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- 54.Chao H.T., Zoghbi H.Y. MeCP2: only 100% will do. Nature Neuroscience. 2012;15:176–177. doi: 10.1038/nn.3027. [DOI] [PubMed] [Google Scholar]
- 55.Mount R.H., Charman T., Hastings R.P., Reilly S., Cass H. The Rett syndrome behaviour questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2002;43:1099–1110. doi: 10.1111/1469-7610.00236. [DOI] [PubMed] [Google Scholar]
- 56.Hagberg B. Clinical manifestations and stages of Rett Syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- 57.Kazlaukas V., Schuh J., Dall’Igna O.P., Pereira G.S., Bonan C.D., Lara D.R. Behavioral and cognitive profile of mice with high and low exploratory phenotypes. Behavioural Brain Research. 2005;162:272–278. doi: 10.1016/j.bbr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Plomin R. Genetics and general cognitive ability. Nature. 1999;402(Suppl.):C25–C29. doi: 10.1038/35011520. [DOI] [PubMed] [Google Scholar]
- 59.Langthorne P., McGill P., O‘Reilly M. Incorporating “motivation” into the functional analysis of challenging behavior: on the interactive and integrative potential of the motivating operation. Behavior Modification. 2007;31(4):466–487. doi: 10.1177/0145445506298424. [DOI] [PubMed] [Google Scholar]
- 60.Niedermeyer E. Frontal lobe disinhibition: Rett syndrome and attention deficit hyperactivity disorder. Clinical Electroencephalography. 2001;32(1):20–23. doi: 10.1177/155005940103200106. [DOI] [PubMed] [Google Scholar]
- 61.Niedermeyer E., Naidu S.B. Rett syndrome, EEG and the motor cortex as a model for better understanding of attention deficit hyperactivity disorder (ADHD) European Child and Adolescent Psychiatry. 1998;7(2):69–72. doi: 10.1007/s007870050049. [review] [DOI] [PubMed] [Google Scholar]
- 62.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., LaSalle J.M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1(4):1–11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piazza C.C., Fisher W., Kiesewetter K., Bowman L., Moser H. Aberrant sleep patterns in children with Rett syndrome. Brain and Development. 1990;12:488–493. doi: 10.1016/s0387-7604(12)80213-0. [DOI] [PubMed] [Google Scholar]