Abstract
The MHC class II-restricted antigen processing pathway generates peptide:MHC complexes in the endocytic pathway for the activation of CD4+ T cells. Gamma-interferon-inducible lysosomal thiol reductase (GILT) reduces protein disulfide bonds in the endocytic compartment, thereby exposing buried epitopes for MHC class II binding and presentation. T cell hybridoma responses and elution of MHC class II bound peptides have identified GILT-dependent epitopes, GILT-independent epitopes, and epitopes that are more efficiently presented in the absence of GILT termed GILT-prevented epitopes. GILT-mediated alteration in the MHC class II-restricted peptidome modulates T cell development in the thymus and peripheral tolerance and influences the pathogenesis of autoimmunity. Recent studies suggest an emerging role for GILT in the response to pathogens and cancer survival.
Keywords: MHC class II, antigen processing and presentation, GILT, autoimmunity, tumor immunity
Introduction
Protein disulfide bonds are common structural elements that maintain the tertiary structure of proteins. Reduction of protein disulfide bonds is an important step in MHC class II-restricted processing and presentation for the activation of CD4+ T cells. Disulfide bond reduction and the acidic pH found in the lysosomal compartment destabilize the tertiary structure of proteins and permit MHC class II binding to full-length proteins and protein fragments (1, 2). Reduction also facilitates lysosomal proteolytic digestion of antigens and generation of MHC class II binding peptides (3). Multiple epitopes require disulfide bond reduction for MHC class II-restricted presentation (3–7). The intracellular reducing activity is primarily associated with lysosomes. Gamma-interferon-inducible lysosomal thiol reductase (GILT) is the only reductase known to be localized in this compartment. GILT is synthesized as a 35 kDa precursor which is tagged with mannose-6-phosphate and targeted to the endocytic pathway; in the early endosomes precursor GILT is cleaved to a 30 kDa mature form which resides in the late endosomes and lysosomes (8). GILT is constitutively expressed by antigen-presenting cells (APCs). Unlike other members of the MHC class II-restricted antigen processing pathway, GILT expression is not regulated by the MHC class II transactivator (CIITA); however, GILT expression is regulated by the transcription factor STAT1 and induced by IFN-γ (9). The diverse biological roles of GILT have recently been reviewed (10, 11). This review will focus on the role of GILT in altering the MHC class II peptidome, subsequent effects on T cell development, tolerance, and autoimmunity, and emerging roles in cancer and infection.
GILT Alters the MHC Class II Peptidome
The reductase function of GILT provides a critical role in MHC class II-restricted processing. Studies investigating the GILT dependency of MHC class II-restricted T cell epitopes have demonstrated epitopes that are dependent and independent of GILT expression for presentation. GILT-dependent class II epitopes have been identified from multiple disulfide bond-containing antigens, including the model antigen hen egg lysozyme (6), melanocyte differentiation antigens tyrosinase and tyrosinase-related protein 1 (TRP1) (4, 12), autoantigen myelin oligodendrocyte glycoprotein (MOG) (13), viral glycoprotein (14), and dust mite allergen Der p 1 (15) (Table 1). The reductase activity of GILT is essential for its function in MHC class II-restricted processing, as mutation of the reductase active site cysteines results in the inability to present a GILT-dependent hen egg lysozyme epitope (16). GILT dependence is not specifically correlated with the presence or proximity of disulfide bonds in the epitope, as two hen egg lysozyme epitopes involving a disulfide bond are GILT-independent (6). Rather, GILT dependence is thought to depend on whether the epitope requires reduction to be exposed for MHC class II binding. Mapping these peptide epitopes to the tertiary structure of the intact, folded protein reveals that GILT-dependent epitopes tend to be buried and, thus, are likely to require reduction to be exposed for MHC class II binding. In contrast, GILT-independent epitopes tend to be surface exposed (6, 17), suggesting that these epitopes are available for MHC class II binding in an acidic pH with proteolytic digestion, but without disulfide bond reduction.
Table 1.
Examples of GILT-dependent, GILT-independent, and GILT-prevented epitopes.
| GILT-dependent (4, 6, 12–15) | GILT-independent (4, 6, 7) | GILT-prevented (18) | |
|---|---|---|---|
| Model antigens | Hen egg lysozyme 74–88 | Hen egg lysozyme 20–35 | |
| Hen egg lysozyme 46–61 | Hen egg lysozyme 30-53 | ||
| Self antigens | Cysteinylated human IgG κ 188–203 | Human IgG κ 145–159 | Murine iroquois homeodomain protein 408–416 |
| Rat myelin oligodendrocyte glycoprotein 35–55 | Murine lemur tyrosine kinase 3 1080–1103 | ||
| Murine synapse defective 1, Rho | |||
| GTPase, homolog 2 1–16 | |||
| Murine membrane associated guanylate kinase, WW, and PDZ domain containing 1 44–67 | |||
| Murine transmembrane protein 131 130–147 | |||
| Murine short stature homeobox 2 91–99 | |||
| Murine clathrin heavy chain linker domain containing 1 138–152 | |||
| Murine DEAH (Asp-Glu-Ala-His) box polypeptide 9 1253–1264 | |||
| Murine centrosomal protein 68 15–92 | |||
| Murine cyclin B1 68–75 | |||
| Self and tumor antigens | Human tyrosinase 56-70; murine tyrosinase-related protein 1 109–130 | ||
| Viral antigens | HIV-1 gp120 envelope protein 203–212 | Influenza hemagglutinin 107–119 | |
| Allergens | Dust mite Der p 1 110–131 |
Mass spectrometric analysis of the MHC class II bound peptides from resting murine splenocytes shows that at steady state the majority of MHC class II bound self peptides are the same in GILT-expressing and GILT-deficient splenocytes (18). This study also reveals that 5.5% of peptides are at least 10-fold overrepresented from GILT−/− splenocytes with 2% being uniquely presented by GILT−/− splenocytes (Table 1). We term these epitopes, which are presented more efficiently in the absence of GILT, GILT-prevented epitopes. The GILT-prevented epitopes in this study were almost exclusively derived from the N- and C-termini which typically lack structural constraints, suggesting that these regions are readily available for MHC class II binding without reduction. Following disulfide bond reduction by GILT, new regions of the protein become exposed and these GILT-prevented epitopes are presented on MHC class II to a much lesser degree or are not observed. Table 1 summarizes examples of GILT-dependent, GILT-independent, and GILT-prevented epitopes.
Thus, reduction by GILT alters the epitopes available for MHC class II-restricted presentation, such that there are three subsets of epitopes: (1) GILT-independent epitopes, which are presented by both GILT-expressing and GILT-deficient APCs and may be the largest subset, (2) GILT-dependent epitopes which require reduction in order to be presented efficiently, and (3) GILT-prevented epitopes which are more likely to be presented in the absence of GILT. It remains to be determined whether these three epitope subsets are generated in response to a particular immune insult and what percentage of immunodominant epitopes fall into each of these categories.
GILT in CD4+ T Cell Development, Tolerance, and Autoimmunity
We have investigated the role of GILT in modulating CD4+ T cell development, tolerance, and autoimmunity to a GILT-dependent epitope of the autoantigen TRP1 using the TRP1-specific T cell receptor (TCR) transgenic (TRP1Tg) mouse strain (Table 1). TRP1, as well as tyrosinase, TRP2, and gp100, are melanocyte differentiation antigens that are clinically relevant antigens in both autoimmune vitiligo and melanoma. Vitiligo is an autoimmune disease that results in the destruction of melanocytes, the pigment producing cells in the skin. Although the immunopathologic mechanism is not precisely known, lesional CD4+ T cells with a Th17 phenotype, melanocyte-specific CD8+ T cells, and defects in regulatory T (Treg) cells are found in vitiligo patients (19–23). TRP1-specific CD4+ T cells in this mouse strain mediate fur depigmentation (12, 24). An advantage of this model is that T cells recognize a naturally occurring epitope of a clinically relevant, skin-restricted autoantigen expressed in its native genetic context. Thus, the TRP1Tg mouse strain is a useful model for the study of development of autoreactive T cells and initiation of CD4+ T cell-mediated autoimmune responses in the skin. However, since TRP1-specific CD4+ T cells have not been identified as pathologic in human vitiligo, this model may not accurately represent human vitiligo, but rather serves as a model of CD4+ T cell-mediated cutaneous autoimmunity.
Factors that control presentation of the MHC class II-restricted peptidome have the potential to shape the thymic selection. Cortical thymic epithelial cells present self peptide:MHC complexes to CD4+ CD8+ double-positive thymocytes (25–27). Low avidity interaction of the TCR with self peptide:MHC complexes provides a survival signal termed positive selection and leads to downregulation of the unused coreceptor. Due to expression of peripheral tissue-specific self antigens in medullary thymic epithelial cells (mTECs), the thymic medulla is the primary site of negative selection, whereby thymocytes that bind self peptide:MHC complexes with high avidity die by apoptosis (28, 29). Autoimmune regulator (Aire) is a transcriptional regulator which permits the expression of tissue-restricted antigens in mTECs (30). In addition to being a reservoir of peripheral tissue-specific antigens, recent studies have demonstrated that mTECs have the ability to present self antigens and contribute to negative selection (31). Dependent on antigen expression by mTECs, thymic dendritic cells (DCs) serve a major role in antigen presentation and negative selection (32). Autoreactive T cells that escape negative selection may differentiate into regulatory T cells in the thymus or be controlled by peripheral tolerance mechanisms (33–35). Thus, MHC class II-restricted presentation plays a critical role in T cell development.
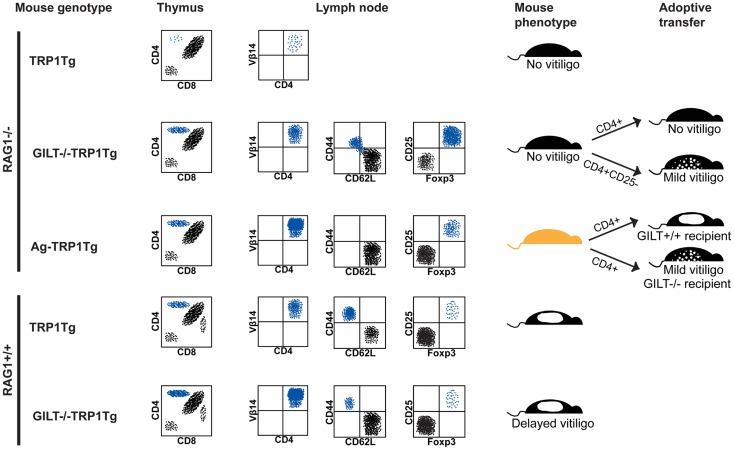
In the thymus of RAG1−/−TRP1Tg mice, CD4+ single-positive TRP1-specific thymocytes do not develop, indicating that thymocytes specific for the self antigen undergo deletion (Figure 1) (24). In GILT−/−RAG1−/−TRP1Tg mice, an increased percentage of CD4+ single-positive TRP1-specific thymocytes develop, demonstrating that GILT is required for thymic deletion of TRP1-specific thymocytes. A similar percentage of CD4+ single-positive TRP1-specific thymocytes develop in TRP1-deficient (Ag−) RAG1−/−TRP1Tg mice as in GILT−/−RAG1−/−TRP1Tg mice, indicating that the absence of TRP1 and GILT have a similar effect on thymic deletion (Figure 1). These studies indicate that GILT plays an important function in the thymic selection of CD4+ T cells specific for GILT-dependent epitopes, suggesting that GILT has an active role in antigen processing by thymic APCs involved in negative selection, including mTECs and thymic DCs.
Figure 1.
GILT in tolerance and autoimmunity. Schematic representation of flow cytometry, phenotype, and adoptive transfer results in the TRP1Tg mouse strains. Staining of thymocytes is shown in a forward scatter and side scatter gate with dead cell exclusion. Staining of lymph node cells is shown gated on Vβ14+CD4+ TRP1-specific T cells. (From top to bottom) in RAG1−/−TRP1Tg mice, TRP1-specific T cells undergo thymic deletion (24). In the absence of GILT or absence of TRP1 antigen (Ag−), there is a similar percentage of CD4+ single-positive thymocytes (24). Although Vβ14+CD4+ TRP1-specific T cells are present in the periphery of GILT−/−RAG1−/−TRP1Tg mice and some have a CD62L−CD44+ effector memory phenotype, these mice do not develop vitiligo (24). There is an increased percentage of Treg cells in GILT−/−RAG1−/−TRP1Tg mice compared to Ag-RAG1−/−TRP1Tg mice (24). Tolerance is partially due to Treg cells, as adoptive transfer of Treg cell-depleted, but not total, CD4+ T cells from GILT−/−RAG1−/−TRP1Tg mice induces mild vitiligo in recipients (24). Ag-RAG1−/−TRP1Tg mice lack TRP1, which is involved in melanin pigment synthesis, and, thus, have a lighter coat color. In Ag-RAG1−/−TRP1Tg mice, all TRP1-specific T cells are naïve and capable of inducing vitiligo following adoptive transfer into GILT+/+ recipients (12). The onset of vitiligo is delayed and the severity is reduced following adoptive transfer into GILT−/− recipients (12). In RAG-expressing TRP1Tg mice, TRP1-specific T cells escape thymic deletion, populate the periphery and induce spontaneous vitiligo (12). In GILT−/−TRP1Tg mice, there are increased TRP1-specific T cells in the thymus and periphery, but fewer TRP1-specific T cells with an effector memory phenotype and a delayed onset of vitiligo (12). There is no difference in the percentage of TRP1-specific Treg cells between TRP1Tg and GILT−/−TRP1Tg mice on the RAG-sufficient background (12).
In the periphery of RAG1−/−TRP1Tg mice, CD4+Vβ14+ TRP1-specific T cells develop in the absence of GILT or TRP1; however, there is a reduced percentage of TRP1-specific T cells in the lymph nodes of GILT−/−RAG1−/−TRP1Tg mice (Figure 1) (24). Although TRP1-specific T cells in GILT−/−RAG1−/−TRP1Tg mice escape thymic deletion, these T cells are tolerant, as GILT−/−RAG1−/−TRP1Tg mice do not develop vitiligo (Figure 1). This phenotype is not due to inefficient presentation of GILT-dependent antigens in the periphery, because T cells from GILT−/−RAG1−/−TRP1Tg mice do not induce autoimmunity when transferred into GILT-expressing recipients (Figure 1). A portion of the T cells in GILT−/−RAG1−/−TRP1Tg mice are antigen-experienced with a CD62L−CD44+ effector memory phenotype (Figure 1). TRP1-specific T cells from GILT−/−RAG1−/−TRP1Tg mice exhibit diminished IL-2 production after in vitro antigen exposure and diminished production of IL-2 and IFN-γ after in vivo antigen exposure (24). GILT−/−RAG1−/−TRP1Tg mice have a four-fold increased percentage of Treg cells compared to Ag-TRP1Tg mice, with Treg cells composing one-third of the TRP1-specific T cells in the lymph node (Figure 1) (24). T cell tolerance in GILT−/−RAG1−/−TRP1Tg mice is partially mediated by Treg cells, as adoptive transfer of Treg cell-depleted CD4+ T cells, but not total CD4+ T cells, induces mild vitiligo in recipients (Figure 1). In comparison, TRP1Tg mice on the RAG1−/− background do not develop spontaneous autoimmunity, because TRP1-specific T cells are deleted in the thymus (Figure 1). Ag-RAG1−/−TRP1Tg mice do not develop vitiligo because they do not express the autoantigen recognized by the transgenic T cells, although naïve T cells from these mice cause rapid, severe vitiligo following adoptive transfer into GILT and TRP1-expressing mice (Figure 1).
The source of the increased Treg cells, that are found in GILT−/−RAG1−/−TRP1Tg mice compared to Ag-RAG1−/− TRP1Tg mice and contribute to tolerance and prevention of vitiligo, remains to be determined. Increased Treg cells may result from intrathymic development of natural Treg cells, as an intermediate avidity interaction between the TCR on the thymocyte and peptide:MHC complexes on the thymic APCs is thought to promote natural Treg development (31, 36). In the absence of GILT, fewer TRP1 peptide:MHC complexes on thymic APCs may shift the fate of TRP1-specific thymocytes from negative selection to natural Treg cell development. Alternatively, Treg cells can be induced in the periphery following suboptimal antigen stimulation (37, 38). GILT-deficient peripheral APCs are capable of low level presentation of TRP1 (12), and this suboptimal presentation may induce TRP1-specific T cells to undergo peripheral conversion into Treg cells. It remains to be determined whether GILT plays a direct role in the generation of TRP1-specific Treg cells or whether other factors, such as expression of the autoantigen TRP1, are sufficient. In addition, other tolerance mechanisms likely limit the autoreactivity of TRP1-specific T cells that develop in GILT−/−RAG1−/−TRP1Tg mice.
The phenotype of the TRP1Tg mice on the RAG-expressing background differs (12). In RAG-expressing TRP1Tg mice, autoreactive TRP1-specific T cells escape thymic deletion, populate the periphery, and induce vitiligo (12). This difference is likely due to the expression of endogenous TCRs. Although the transgenic TCR Vβ14 chain is the most frequent TCR in RAG1+/+TRP1Tg mice, a diverse array of TCR Vα and Vβ chains are expressed (12). These endogenous TCRs likely rescue autoreactive T cells from thymic deletion as in other TCR transgenic models where autoreactive T cells are deleted on the RAG1−/− background but escape thymic deletion in RAG-expressing mice or with expression of two TCR transgenes on the RAG1−/− background (39, 40). Furthermore, effector function via the autoreactive TCR can be induced through activation of the second TCR (40, 41).
In RAG-expressing GILT−/−TRP1Tg mice, an increased percentage of TRP1-specific T cells is found in the thymus and periphery, suggesting that, as on the RAG1−/− background, deletion of thymocytes recognizing a GILT-dependent epitope is less efficient in the absence of GILT (Figure 1). In GILT−/− compared to GILT-expressing RAG1+/+TRP1Tg mice, the median onset of spontaneous vitiligo is delayed by 7 weeks (12). These mice have a similar small percentage of TRP1-specific Treg cells, so Treg cells do not account for the difference in TRP1-specific T cells or disease onset (Figure 1). This result suggests that on the RAG-expressing background GILT expression does not alter Treg cell development. In the presence of GILT and following the onset of vitiligo there is an increased percentage of TRP1-specific T cells with an effector memory phenotype (12). Adoptive transfer of naïve, TRP1-specific T cells from Ag-RAG1−/−TRP1Tg mice into GILT-deficient and GILT-expressing recipients demonstrates that GILT expression in the peripheral APCs of the recipient increases the severity and accelerates the onset of vitiligo (12). Thus, in the RAG-expressing TRP1Tg model, GILT improves the MHC class II-restricted processing and presentation of TRP1 for enhanced activation of autoreactive T cells and an accelerated onset of vitiligo.
Multiple sclerosis is an autoimmune disease of the central nervous system that results in multifocal areas of demyelination with loss of oligodendrocytes and astroglial scarring. Bergman et al. investigated the role of GILT in MOG-induced experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (13). Immunization of wild-type mice with rat MOG protein results in EAE that is CD4+ T cell-mediated and is B-cell-independent. MOG contains a disulfide bond between cysteine residues 24 and 98. GILT−/− splenocytes are unable to process rat MOG protein and present MOG35–55, indicating that MOG35–55 is a GILT-dependent epitope (Table 1). Whereas MOG35–55 is the exclusive encephalitogenic epitope produced by rat MOG protein-induced EAE in wild-type mice, lymph node cells from GILT−/− mice immunized with rat MOG protein proliferate in response to several overlapping MOG peptides (MOG67–87, MOG85–105, MOG104–177), but not MOG35–55, indicating that GILT alters the peptides presented and the immunodominant epitopes. GILT−/− mice are resistant to EAE induced by MOG35–55 peptide, which is likely due to the inability of GILT−/− APCs to process endogenous MOG protein to present the MOG35–55 epitope. However, GILT−/− mice develop more severe disease compared to wild-type mice after injection with the extracellular domain of rat MOG protein. This unanticipated finding is due to a switch in the pathogenic mechanism from T cell-mediated autoimmunity in wild-type mice to B-cell-mediated disease in GILT−/− mice. GILT−/− mice immunized with MOG protein generate pathogenic antibodies which bind oligodendrocytes and transfer EAE. These studies indicate that changes in the MHC class II-restricted peptidome by GILT can have a profound impact on the pathogenic mechanism and development of autoimmunity. As many other autoantigens contain disulfide bonds, e.g., TRP2, gp100, types VII and XVII collagen, insulin, glutamic acid decarboxylase, and thyroid-stimulating hormone receptor (5, 42–49), GILT is likely to alter the MHC class II-restricted epitopes that are presented and the pathogenesis of other autoimmune diseases.
GILT and Anti-Tumor Immunity
MHC class II-restricted antigen processing and presentation by cancer cells may influence anti-tumor T cell responses and patient outcome. Diffuse large B-cell lymphoma (DLBCL) is the most common form of aggressive non-Hodgkin lymphoma, and low MHC class II expression is known to be associated with poor survival in DLBCL (50). In this Research Topic, we present the first evidence of the clinical significance of GILT expression (51). In each of four independent gene expression profiling cohorts with a total of 585 DLBCL patients, low GILT mRNA expression is significantly associated with poor overall survival. Low GILT expression is not associated with low expression of the MHC class II gene HLA-DRA, as might be expected since GILT expression is not regulated by CIITA. Low HLA-DRA expression is only associated with poor survival in one of four cohorts, suggesting that GILT is a better predictor of survival than HLA-DRA. Furthermore, the association of low GILT mRNA expression with poor survival is independent of established clinical and molecular prognostic factors. Immunohistochemical analysis of GILT protein expression in 96 DLBCL tumor specimens demonstrates variation in GILT expression within tumor cells with uniformly high GILT expression in tumor-infiltrating APCs, suggesting that GILT expression is lost in tumor cells as opposed to a germline polymorphism affecting GILT expression in all cell types. The loss of GILT expression in DLBCL tumor cells is anticipated to result in diminished processing and presentation of GILT-dependent epitopes and may represent a mechanism of immune evasion. Alternatively, GILT may alter tumorigenesis through modification of the tumor cell redox status, growth rate or function of an oncoprotein. These findings underscore a role for GILT in patient outcome in DBLCL. Further investigation is warranted to determine the mechanism of GILT in lymphoma survival.
MHC class II-expressing melanoma cells are capable of presenting endogenous melanoma antigens and have the potential to stimulate anti-tumor T cell responses (52–55). While defects in MHC class I-restricted processing and presentation have been identified in melanoma and may contribute to immune evasion (56–59), the roles of GILT and the MHC class II pathway are relatively underexplored in melanoma despite their potential to alter immune recognition. Unlike in DLBCL where low MHC class II expression is associated with poor survival, in melanoma ectopic MHC class II expression is associated with increased depth of primary melanoma, poorer survival in primary melanoma, and metastatic disease (60). One potential explanation for this apparent paradox is that melanoma cells may have a defect in the expression of a component of the MHC class II processing pathway, such as GILT. Aberrant expression of MHC class II in melanoma is due to expression of CIITA resulting from activation of the B-cell-specific CIITA promoter III and the IFN-γ-inducible CIITA promoter IV (61, 62). The mechanism of CIITA induction in melanoma is unknown, but MHC class II expression parallels the expression of chemokines CXCL-1 and CXCL-8 and the nuclear expression of NFκB p50 subunit, which are associated with tumor invasiveness and poor prognosis in melanoma (63). Aberrant expression of MHC class II driven by CIITA expression is not anticipated to induce GILT expression (9). In support of this possibility a study of sixteen human melanoma cell lines demonstrated that MHC class II-expressing melanoma cells lack GILT expression and are unable to present an MHC class II-restricted epitope derived from the endogenous melanoma antigen tyrosinase (4) (Table 1). Transfection with GILT enhances presentation of the endogenous tyrosinase epitope. This study suggests that MHC class II expression by melanoma cells in the absence of GILT may be a mechanism of immune evasion, as GILT-deficient, MHC class II-expressing melanoma cells may be unable to generate the GILT-dependent epitopes presented by professional APCs which activate the T cell repertoire. Furthermore, multiple melanoma antigens, including tyrosinase, TRP1, TRP2, and gp100, contain disulfide bonds and likely contain GILT-dependent epitopes (44, 45, 48). In addition to tyrosinase, a naturally occurring epitope from TRP1 is GILT-dependent (Table 1) (12). Further studies are needed to determine the clinical relevance of GILT in melanoma pathogenesis and patient survival.
GILT and Anti-Viral Immune Responses
The role of GILT in MHC class II-restricted presentation of viral proteins has been investigated. MHC class II-restricted presentation of an epitope from the human immunodeficiency virus (HIV)-1 envelope protein gp120, which is located at the base of the V1/V2 loops and encompasses a cysteine residue involved in a disulfide bond, is GILT-dependent, and an adjacent epitope in HIV-1 gp120, which includes a downstream disulfide-bonded cysteine residue, is partially dependent on GILT (Table 1) (14). In contrast, MHC class II-restricted presentation of the site 1 epitope of the influenza hemagglutinin major subunit, which requires reduction, is GILT-independent (Table 1) (7). The role of GILT in anti-pathogen CD4+ T cell-mediated responses remains to be studied. In addition to a role in MHC class II-restricted presentation, GILT facilitates cross-presentation of exogenous viral antigens containing disulfide bonds on MHC class I for recognition by CD8+ T cells (17). GILT is required for the cross-presentation of a herpes simplex virus-1 (HSV-1) glycoprotein B (gB) epitope involving residues 498–505 from exogenous recombinant gB or HSV-1-infected cell debris, but not for cross-presentation of an epitope from an HSV-1 cytosolic protein or direct MHC class I-restricted presentation of the gB epitope in infected DCs. GILT is also required for cross-priming of gB-specific CD8+ T cells in vivo, as a smaller percentage of gB-specific CD8+ T cells are generated in HSV-1 infected GILT−/− mice compared to wild-type mice. Similarly, GILT is essential for cross-priming of CD8+ T cells specific for epitopes from the hemagglutinin and neuraminidase envelope proteins of influenza virus. Thus, in addition to a role in MHC class II-restricted presentation for stimulation of CD4+ T cell responses, GILT plays a role in cross-presentation of exogenous antigens on MHC class I and cross-priming of CD8+ T cells, which is important in both viral infection and cancer. It remains to be determined whether alteration of CD4+ and CD8+ T cell epitopes by GILT impacts the overall host response to infection.
Conclusion and Future Directions
Gamma-interferon-inducible lysosomal thiol reductase is a unique reductase in the endocytic compartment which alters the MHC class II peptidome. Certain epitopes require GILT for efficient presentation, others are presented independent of GILT, and some are presented more efficiently in the absence of GILT. GILT-mediated fine changes in the peptide repertoire presented on MHC class II modify thymic selection and shape the T cell repertoire in such a way that influences the pathogenesis of autoimmune disease. Additionally, low GILT expression in tumor cells is associated with poor survival in lymphoma, possibly through loss of immune-mediated destruction. Further investigation is needed to determine the precise mechanisms of GILT’s role in cancer pathogenesis and survival as well as other aspects of CD4+ T cell-mediated immunity, especially in regard to anti-pathogen immune responses. Future studies will elucidate the potential role of GILT as a biomarker for predicting disease outcome and as a therapeutic target to suppress autoimmune disease or improve anti-tumor immunity.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by a National Institutes of Health grant K08-AR054388 (Karen Taraszka Hastings) and a grant from the Arizona Biomedical Research Commission (Karen Taraszka Hastings).
References
- 1.Jensen PE. Acidification and disulfide reduction can be sufficient to allow intact proteins to bind class II MHC. J Immunol (1993) 150(8 Pt 1):3347–56 [PubMed] [Google Scholar]
- 2.Santoro L, Reboul A, Kerblat I, Drouet C, Colomb MG. Monoclonal IgG as antigens: reduction is an early intracellular event of their processing by antigen-presenting cells. Int Immunol (1996) 8(2):211–9 10.1093/intimm/8.2.211 [DOI] [PubMed] [Google Scholar]
- 3.Collins DS, Unanue ER, Harding CV. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol (1991) 147(12):4054–9 [PubMed] [Google Scholar]
- 4.Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, et al. Absence of G-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med (2002) 195(10):1267–77 10.1084/jem.20011853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen PE. Reduction of disulfide bonds during antigen processing: evidence from a thiol-dependent insulin determinant. J Exp Med (1991) 174(5):1121–30 10.1084/jem.174.5.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, et al. Defective antigen processing in GILT-free mice. Science (2001) 294(5545):1361–5 10.1126/science.1065500 [DOI] [PubMed] [Google Scholar]
- 7.Sinnathamby G, Maric M, Cresswell P, Eisenlohr LC. Differential requirements for endosomal reduction in the presentation of two H2-E(d)-restricted epitopes from influenza hemagglutinin. J Immunol (2004) 172(11):6607–14 [DOI] [PubMed] [Google Scholar]
- 8.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci U S A (2000) 97(2):745–50 10.1073/pnas.97.2.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell PW, Haque A, Klemsz MJ, Kaplan MH, Blum JS. Induction of the antigen-processing enzyme IFN-gamma-inducible lysosomal thiol reductase in melanoma cells is STAT1-dependent but CIITA-independent. J Immunol (2004) 173:731–5 [DOI] [PubMed] [Google Scholar]
- 10.Hastings KT, Cresswell P. Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase. Antioxid Redox Signal (2011) 15(3):657–68 10.1089/ars.2010.3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West LC, Cresswell P. Expanding roles for GILT in immunity. Curr Opin Immunol (2013) 25(1):103–8 10.1016/j.coi.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausch MP, Irvine KR, Antony PA, Restifo NP, Cresswell P, Hastings KT. GILT accelerates autoimmunity to the melanoma antigen tyrosinase-related protein 1. J Immunol (2010) 185(5):2828–35 10.4049/jimmunol.1000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman CM, Marta CB, Maric M, Pfeiffer SE, Cresswell P, Ruddle NH. A switch in pathogenic mechanism in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in IFN-gamma-inducible lysosomal thiol reductase-free mice. J Immunol (2012) 188(12):6001–9 10.4049/jimmunol.1101898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sealy R, Chaka W, Surman S, Brown SA, Cresswell P, Hurwitz JL. Target peptide sequence within infectious human immunodeficiency virus type 1 does not ensure envelope-specific T-helper cell reactivation: influences of cysteine protease and gamma interferon-induced thiol reductase activities. Clin Vaccine Immunol (2008) 15(4):713–9 10.1128/CVI.00412-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West LC, Grotzke JE, Cresswell P. MHC class II-restricted presentation of the major house dust mite allergen Der p 1 Is GILT-dependent: implications for allergic asthma. PLoS One (2013) 8(1):e51343. 10.1371/journal.pone.0051343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings KT, Lackman RL, Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol (2006) 177(12):8569–77 [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science (2010) 328(5984):1394–8 10.1126/science.1189176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogunovic B, Srinivasan P, Ueda Y, Tomita Y, Maric M. Comparative quantitative mass spectrometry analysis of MHC class II-associated peptides reveals a role of GILT in formation of self-peptide repertoire. PLoS One (2010) 5(5):e10599. 10.1371/journal.pone.0010599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res (2010) 23(2):276–86 10.1111/j.1755-148X.2010.00688.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One (2012) 7(5):e37513. 10.1371/journal.pone.0037513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, Schittek B, et al. HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol (2001) 116(6):891–7 10.1046/j.1523-1747.2001.01363.x [DOI] [PubMed] [Google Scholar]
- 22.Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol (2003) 121(3):550–6 10.1046/j.1523-1747.2003.12413.x [DOI] [PubMed] [Google Scholar]
- 23.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med (1998) 188(6):1203–8 10.1084/jem.188.6.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rausch MP, Hastings KT. GILT modulates CD4(+) T-cell tolerance to the melanocyte differentiation antigen tyrosinase-related protein 1. J Invest Dermatol (2012) 132(1):154–62 10.1038/jid.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell (1994) 76(1):17–27 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- 26.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol (2009) 10(11):1155–61 10.1038/ni.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolic-Zugic J, Bevan MJ. Role of self-peptides in positively selecting the T-cell repertoire. Nature (1990) 344(6261):65–7 10.1038/344065a0 [DOI] [PubMed] [Google Scholar]
- 28.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med (2005) 202(1):33–45 10.1084/jem.20050471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol (2001) 2(11):1032–9 10.1038/ni723 [DOI] [PubMed] [Google Scholar]
- 30.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science (2002) 298(5597):1395–401 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- 31.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol (2010) 11(6):512–9 10.1038/ni.1874 [DOI] [PubMed] [Google Scholar]
- 32.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med (2009) 206(7):1505–13 10.1084/jem.20082449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol (2002) 3(8):756–63 [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol (2003) 4(4):330–6 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 35.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol (2001) 2(4):301–6 10.1038/86302 [DOI] [PubMed] [Google Scholar]
- 36.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol (2007) 19(2):176–85 10.1016/j.coi.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 37.Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes (2005) 54(12):3395–401 10.2337/diabetes.54.12.3395 [DOI] [PubMed] [Google Scholar]
- 38.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol (2005) 6(12):1219–27 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- 39.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med (1994) 180(6):2089–99 10.1084/jem.180.6.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zal T, Weiss S, Mellor A, Stockinger B. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc Natl Acad Sci U S A (1996) 93(17):9102–7 10.1073/pnas.93.17.9102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity (2008) 28(5):662–74 10.1016/j.immuni.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, et al. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci (1988) 319(1195):369–456 10.1098/rstb.1988.0058 [DOI] [PubMed] [Google Scholar]
- 43.Battaglioli G, Liu H, Hauer CR, Martin DL. Glutamate decarboxylase: loss of N-terminal segment does not affect homodimerization and determination of the oxidation state of cysteine residues. Neurochem Res (2005) 30(8):989–1001 10.1007/s11064-005-6772-0 [DOI] [PubMed] [Google Scholar]
- 44.Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell (2001) 12(11):3451–64 10.1091/mbc.12.11.3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Borron JC, Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res (2002) 15(3):162–73 10.1034/j.1600-0749.2002.02012.x [DOI] [PubMed] [Google Scholar]
- 46.Graves PN, Vlase H, Davies TF. Folding of the recombinant human thyrotropin (TSH) receptor extracellular domain: identification of folded monomeric and tetrameric complexes that bind TSH receptor autoantibodies. Endocrinology (1995) 136(2):521–7 10.1210/en.136.2.521 [DOI] [PubMed] [Google Scholar]
- 47.Morris NP, Keene DR, Glanville RW, Bentz H, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem (1986) 261(12):5638–44 [PubMed] [Google Scholar]
- 48.Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J Biol Chem (2000) 275(41):32200–7 10.1074/jbc.M005186200 [DOI] [PubMed] [Google Scholar]
- 49.Schacke H, Schumann H, Hammami-Hauasli N, Raghunath M, Bruckner-Tuderman L. Two forms of collagen XVII in keratinocytes. A full-length transmembrane protein and a soluble ectodomain. J Biol Chem (1998) 273(40):25937–43 10.1074/jbc.273.40.25937 [DOI] [PubMed] [Google Scholar]
- 50.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med (2002) 346(25):1937–47 10.1056/NEJMoa012914 [DOI] [PubMed] [Google Scholar]
- 51.Phipps-Yonas H, Cui H, Sebastio N, Brunhoeber PS, Haddock E, Deymier MJ, et al. Low GILT expression is associated with poor patient survival in diffuse large B-cell lymphoma. Front Immunol (2013) 4:425. 10.3389/fimmu.2013.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins PF, El-Gamil M, Li YF, Zeng G, Dudley M, Rosenberg SA. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J Immunol (2002) 169(10):6036–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robila V, Ostankovitch M, Altrich-Vanlith ML, Theos AC, Drover S, Marks MS, et al. MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J Immunol (2008) 181(11):7843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Touloukian CE, Leitner WW, Robbins PF, Li YF, Kang X, Lapointe R, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res (2002) 62(18):5144–7 [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Bartido S, Yang G, Qin J, Moroi Y, Panageas KS, et al. A role for a melanosome transport signal in accessing the MHC class II presentation pathway and in eliciting CD4+ T cell responses. J Immunol (1999) 163(11):5820–6 [PubMed] [Google Scholar]
- 56.Belicha-Villanueva A, Golding M, McEvoy S, Sarvaiya N, Cresswell P, Gollnick SO, et al. Identification of an alternate splice form of tapasin in human melanoma. Hum Immunol (2010) 71(10):1018–26 10.1016/j.humimm.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst (1996) 88(2):100–8 10.1093/jnci/88.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, et al. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res (2001) 61(24):8647–50 [PubMed] [Google Scholar]
- 59.Wang RF, Parkhurst MR, Kawakami Y, Robbins PF, Rosenberg SA. Utilization of an alternative open reading frame of a normal gene in generating a novel human cancer antigen. J Exp Med (1996) 183(3):1131–40 10.1084/jem.183.3.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaloudik J, Moore M, Ghosh AK, Mechl Z, Rejthar A. DNA content and MHC class II antigen expression in malignant melanoma: clinical course. J Clin Pathol (1988) 41(10):1078–84 10.1136/jcp.41.10.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deffrennes V, Vedrenne J, Stolzenberg MC, Piskurich J, Barbieri G, Ting JP, et al. Constitutive expression of MHC class II genes in melanoma cell lines results from the transcription of class II transactivator abnormally initiated from its B cell-specific promoter. J Immunol (2001) 167(1):98–106 [DOI] [PubMed] [Google Scholar]
- 62.Goodwin BL, Xi H, Tejiram R, Eason DD, Ghosh N, Wright KL, et al. Varying functions of specific major histocompatibility class II transactivator promoter III and IV elements in melanoma cell lines. Cell Growth Differ (2001) 12(6):327–35 [PubMed] [Google Scholar]
- 63.Martins I, Sylla K, Deshayes F, Lauriol J, Ghislin S, Dieu-Nosjean MC, et al. Coexpression of major histocompatibility complex class II with chemokines and nuclear NFkappaB p50 in melanoma: a rational for their association with poor prognosis. Melanoma Res (2009) 19(4):226–37 10.1097/CMR.0b013e32832e0bc3 [DOI] [PubMed] [Google Scholar]