Abstract
Drosophila has a primitive yet effective blood system with three types of haemocytes which function throughout different developmental stages and environmental stimuli. Haemocytes play essential roles in tissue modeling during embryogenesis and morphogenesis, and also in innate immunity. The open circulatory system of Drosophila makes haemocytes ideal signal mediators to cells and tissues in response to events such as infection and wounding. The application of recently developed and sophisticated genetic tools to the relatively simple genome of Drosophila has made the fly a popular system for modeling human tumorigensis and metastasis. Drosophila is now used for screening and investigation of genes implicated in human leukemia and also in modeling development of solid tumors. This second line of research offers promising opportunities to determine the seemingly conflicting roles of blood cells in tumor progression and invasion. This review provides an overview of the signaling pathways conserved in Drosophila during haematopoiesis, haemostasis, innate immunity, wound healing and inflammation. We also review the most recent progress in the use of Drosophila as a cancer research model with an emphasis on the roles haemocytes can play in various cancer models and in the links between inflammation and cancer.
Keywords: haemocytes, haematopoiesis, plasmatocyte, macrophage, innate immunity, tumor, inflammation
Introduction
Drosophila has undoubtedly been a powerful model organism for the study of nearly all essential and fundamental biological processes. What we have learned from the fruit fly has expanded our knowledge in life science at an unprecedented speed. This is in particular due to the recent availability of the complete annotated genome, a versatile array of genomic modifying techniques and powerful life imaging tools. Cellular and molecular mechanisms underlying many basic biological processes have been discovered to be highly conserved between Drosophila and mammals. For example, the Notch, Hedgehog (Hg) and Wingless (Wnt) pathways first identified in Drosophila embryogenesis and the Runt and Hippo signaling pathways conserved in the Drosophila haematopoiesis and tissue growth are also implicated in the progression of various human cancers (Geissler and Zach, 2012; Harvey et al., 2013). Indeed the past decade has witnessed a rapidly emerging trend for Drosophila to be used in modeling human tumor growth, progression, invasion and metastasis and as a test-bed for therapeutic discovery (reviews in Harris, 2005; Crozatier and Vincent, 2011; Miles et al., 2011; Hsu, 2012; Gonzalez, 2013).
Most forms of human cancers progress step by step from mutations in the oncogene, the tumor suppressor gene and signaling molecules and can eventually kill the host by spreading uncontrollable immortal growth of mutant malignant tissues into different organs. On the route to spread and invade, cancer cells can influence their microenvironment via the interaction with the infiltrated blood cells, gradually disabling the host immunosurvellience and finally breaking the stromal barrier to become invasive and metastatic (Dunn et al., 2004). It is at the metastatic stage that many lives would be claimed. Therefore the outcome from the tug of war in the tumor microenvironment between malignant cancerous cells that undergo constant somatic mutations and surrounding blood cells plays a vital role in the prevention and intervention of tumorigenesis. In addition, chronic inflammation has been well-documented as contributing to and promoting the initiation and progression of various cancers (Coussens and Werb, 2002; Mantovani et al., 2008; Aggarwal et al., 2009). It is now generally accepted that an inflammatory microenvironment is necessary for tumor progression and metastasis (Wu and Zhou, 2009; Grivennikov et al., 2010). Macrophages in particular have been reported to facilitate many aspects of this process in different cancers and also to intervene in the anti-cancer therapies (De Palma and Lewis, 2013; Lee et al., 2013b). Apart from the role of macrophages in cancer development, they have been for many years subjected to extensive research as the key player in inflammatory responses which accompany infection, tissue damage and wound healing (Mantovani et al., 2013; Novak and Koh, 2013). Therefore inflammation, immunity and cancer are inter-linked and any imbalance can result in serious health issues. Blood cells such as macrophages appear to be the link and have a crucial role in influencing and maintaining the equilibrium between protection (immunity and inflammation) and regeneration/tissue homeostasis (where cancer can be considered a malignant proliferative and invasive tissue). Animal models such as mice have revealed invaluable insights into the multi-step interaction of mammalian innate immunity with associated inflammatory responses in defining the cancer microenvironment. These innate immune responses can include the complement pathways (Ricklin and Lambris, 2013), pro-inflammatory cytokine and chemokine production (Sethi et al., 2012; Candido and Hagemann, 2013). However, the multi-layered interaction in the context of a generally slow progression of the human cancer has created fragmentary and controversial results in the mouse model and thus inevitably slows down our progress to understand the disease. Drosophila, on the contrary, as a simply-formed and genetically tractable multi-cellular organism, has been used to dissect processes of development (tissue homeostasis) and innate immunity with such precision that the time is now ripe for us to look into the active dialogs between these fundamental processes in the context of mammalian inflammation and cancer. Drosophila has a primitive open blood circulation system with only three types of blood cells or haemocytes circulating in the haemolymph during a fly's life span. The majority of the circulating haemocytes in the haemolymph are macrophage-like cells that engulf and degrade apoptotic cells and invading pathogens. Haemocytes perform vital roles through their contribution both to cellular and humoral immune responses in the fly. In combination with the currently well-developed Drosophila tumor models, the roles of haemocytes in tumor regression and/or progression can be explored and important clues can be obtained to understand further the inflammatory responses in relation to tumors; the focus can be directed more at the molecular and cellular level by use of sophisticated genetic manipulation and live imaging tools. Tian Xu and colleagues have done pioneering work in this direction by using a Drosophila tumor model to investigate the role of haemocytes in the tumor growth control during a systemic inflammatory response (Pastor-Pareja et al., 2008). In this review, we first give a brief overview of Drosophila haematopoiesis. This is followed by a discussion of the roles of haemocytes in Drosophila at different developmental stages. Next we consider how haemocytes function in tissue injury and wound healing. Drosophila leukemia model and interaction between immunity and tumorigenesis are also discussed. Finally, perspectives for possible future research opportunities in the interplay of inflammation, immunity and cancer revolving around the blood cells are discussed.
Drosophila haematopoiesis
The blood system of Drosophila is rather primitive compared to the great complexity in vertebrates. The fruit fly does not have a vascular network to separate the blood cells from other tissues and organs and its internal organs are bathed in haemolymph. Meanwhile vertebrates have many different types of blood cells. Each type has evolved to perform specialized functions during million years of evolution. On the contrary only three major types of blood cells, collectively termed haemocytes, have been identified in the fruit fly and none of them has acquired the capability to undergo DNA rearrangement and somatic hypermutation to generate a vast repertoire for immunological memory in the B-and T-lymphocytes. Therefore Drosophila relies on a very simple system to fulfil basically all the roles that vertebrate blood cells can play. However there is extensive conservation in the molecular mechanisms of haematopoiesis in both Drosophila and mammals.
As in vertebrates, Drosophila haematopoiesis takes place in two phases: primitive haematopoiesis and definitive haematopoiesis (for more detailed review see Evans et al., 2003; Crozatier and Meister, 2007; Krzemien et al., 2010). Briefly, the site for primitive haematopoiesis resides in the precephalic mesoderm which gives rise to the early wave of haemocyte generation in the embryo (Figure 1A). At the end of embryogenesis, a specialized organ termed Lymph Gland (LG) originating from the lateral mesoderm starts to appear along the dorsal vessel and becomes fully mature during the first half of larval development (Figure 1B). Definite haematopoiesis initiates in the LG (Figures 1B, 2B) and generates terminally differentiated haemocytes at the onset of metamorphosis; the cells are released during the pupal stage with disintegration of the LG. After the disappearance of LG, no haematopoiesis will occur in the pupa or in the adult fly. Haemocytes persisting through the whole life stages of the fly therefore have either embryonic or larval lineage (Holz et al., 2003).
Figure 1.
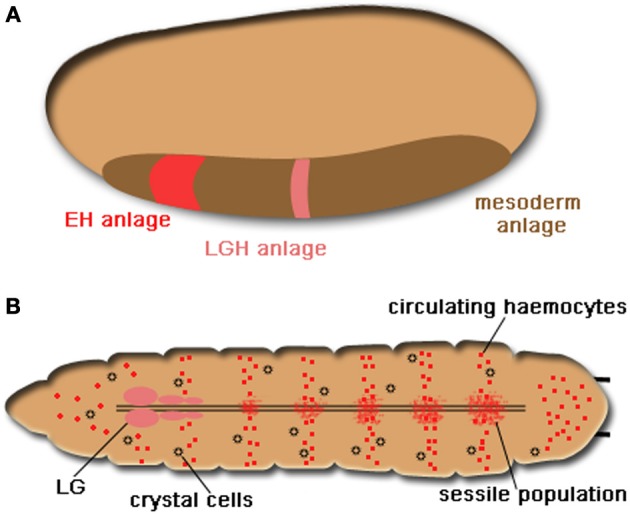
Drosophila haematopoiesis. (A) The embryonic haematopoiesis. Two waves of haemocyte generation take place in Drosophila. During embryogenesis, haemocytes originate from the precephalic mesoderm as indicated in red in the region of mesoderm anlage (shown in brown), The pink region denotes the embryonic origin that will give rise to lymph gland haemocytes (LGH) in the larva. (B) Larval haematopoiesis. The LG (in pink) composes of the primary and secondary lobes and is located in the anterior end of the larva along the dorsal aorta. The sessile haemocyte population distributes diffusely along the segmental borders of the larva and consists of functional differentiated haemocytes and a few prohaemocytes with an embryonic origin (shown in the same red color). Until the end of the third star, circulating haemocytes including plasmatocytes and crystal cells (small black circles) are derived from the embryonic haemocytes.
Figure 2.
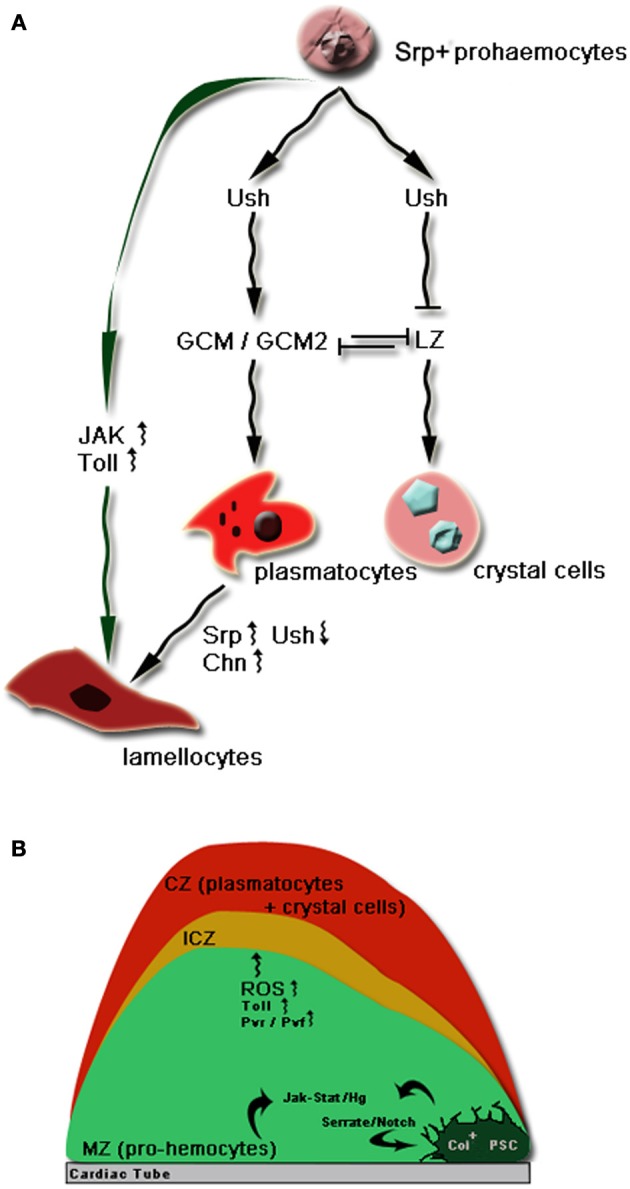
(A) The major transcriptional network in the lineage commitment of Drosophila prohaemocytes (Lebestky et al., 2000; Evans et al., 2003; Waltzer et al., 2010). Prohaemocytes express the early GATA transcription factor, Serpent (Srp) for haematopoietic development (Lebestky et al., 2000). The expression of Srp defines the haemocyte anlage from embryogenesis. Later on the Srp expressing prohaemocytes turn on the transcription of the Drosophila friend of GATA, U-shaped (Ush). Ush together with Srp activates the expression of another transcription factor Gilial cell missing (GCM) and its isoform GCM2. Activation of GCM/GCM2 turns on the expression of plasmocyte cell markers and thus commits prohaemocytes into plasmatocyte specification (Fossett et al., 2001). In a small population of prohaemocytes, the expression of a Drosophila Runx family member of transcription factors, Lozenge (Lz) antagonizes Ush and also inhibits the expression of Gcm/Gcm2. Cells expressing Lz adopt the crystal cell fate (Bataillé et al., 2005). Lamellocytes do not appear in normal circumstances but can be induced rapidly from the LG or sessile haemocytes by parasitic infection in the larva. Most recent lineage tracing studies have pointed out a direct differentiation of lamellocytes from plasmatocytes by the upregulation of Srp and Charlatan (Chn) and downregulation of Ush to suppress the plasmatocyte transcriptional profile (Stofanko et al., 2010). However direct differentiation from the LG prohaemocytes may also contribute to the total population of lamellocytes in particular by the over-activation of JAK/STAT and Toll signaling. (B) Compartments of the primary lobes of the LG and key signaling pathways in larval haemocyte specification (Krzemień et al., 2007). The primary lobes are the major sites for the larval haemocyte differentiation. The primary lobe can be divided into three major compartments: the Cortical Zone (CZ), the Medullary Zone (MZ) and the Posterior Signaling Center (PSC) (Jung et al., 2005). There is also a region that contains a population of intermediate haemocytes from undifferentiated prohaemocytes. This region is sometimes termed Intermediate Cortical Zone (ICZ). The stem cell-like fate of prohaemocytes is regulated by communication between the PSC cells and MZ via filopodia of the PSC cells. Activation of JAK/STAT and Hg signaling by the PSC cells maintains the undifferentiated status of prohaemocytes (Krzemień et al., 2007; Mandal et al., 2007). Meanwhile the transcription factor Collier (Col) expression defines PSC cell identity and is controlled by the Serrate/Notch signaling (Lebestky et al., 2003). Toll signaling is also important in the survival and proliferation of prohaemocytes while increased ROS level and Pvr/Pvf signaling can also contribute to the differentiation of prohaemocytes to plasmatocytes (Qiu et al., 1998; Brückner et al., 2004).
LG forms at the end of embryogenesis with a single pair of lobes called the anterior or primary lobes. Larval haematopoiesis takes place primarily in the primary lobes to temporally and spatially regulate the haemocyte differentiation. The primary lobes can be physically divided into three compartments (Figure 2B): the cortical and the medullary zones and the Posterior Signaling Center (PSC) (Jung et al., 2005). PSC is essential in controlling haemostasis in healthy larvae by a direct cell-cell communication via filopodia, thin cytoplasmic extensions (Krzemień et al., 2007; Mandal et al., 2007). This cellular contact provides a platform for the interplay of a network of key signaling pathways required for normal larval haematopoiesis (Figure 2A).
Based on different morphological features three major types of haemocytes can be identified throughout the life cycle of the fruit fly, namely, plasmatocytes, crystal cells and lamellocytes (Figure 2A) (Lanot et al., 2001; Hartenstein, 2006). Plasmatocytes are the dominating haemocyte population during all Drosophila developmental stages. They are macrophage-like cells that are primarily responsible for the removal of apoptotic debris, phagocytosis of invading microbes and repair of damaged tissues. Crystal cells are larger in size than plasmatocytes and are named from the paracystalline inclusions in the cytoplasm. The crystal cell inclusions are believed to contain large quantities of components involved in a process called melanisation involving a cascade of serine proteases leading to melanin synthesis (Jiravanichpaisal et al., 2006). Melanin is important to prevent haemolymph loss in wound sites, immobilize microbial pathogens and facilitate wound healing (see below). In addition, its free radical oxidative by-product can directly kill microorganisms. Although they constitute only a small proportion of 5% of the total population of Drosophila haemocytes in the embryo and larvae, crystal cells are major executioners in Drosophila innate immunity. Lamellocytes are physically distinctive from both plasmatocytes and crystal cells. They are flat and adhesive and are the largest haemocytes observed in Drosophila (Lanot et al., 2001). They do not appear in the embryo or in healthy larvae but can be induced quickly to differentiate from the LG or sessile population along the border of larval segments to engulf foreign particles larger than those that can be phagocytosized by plasmatocytes, such as the parasitoid eggs (Sorrentino et al., 2002; Lee et al., 2009). This process is termed encapsulation. Lamellocytes can also launch a melanisation cascade to kill the parasitic invaders with the aid of crystal cells and thus are essential in the Drosophila immunity against parasite infection (Krzemien et al., 2010).
The key players including primarily transcription factors and the corresponding molecular mechanisms are briefly summarized and illustrated in Figure 2.
Haemocytes in immunity
In the wild, Drosophila feeds on rotting fruits and lives in a microorganism-enriched environment so fruit flies constantly face the danger of physical injury and gastrointestinal infection. The selection pressure from the hostile environment must be one of the driving forces for Drosophila to develop multi-layered defense responses so that it can survive and propagate. Not surprisingly the cellular and molecular mechanisms in the various facets of Drosophila innate immunity have been phylogenetically conserved. Drosophila can mount an array of cellular and humoral responses when challenged by pathogens such as bacteria, fungi, viruses and parasites. The cellular responses include direct engulfment of small objects such as the bacteria, as in phagocytosis, and encapsulation of larger objects such as parasitoid eggs. The humoral responses take place primarily in the haemolymph and three major events can occur during an immune challenge depending on the nature of the invading pathogen: (1) direct killing by Antimicrobial peptide (AMP) released into haemolymph from rapid de novo synthesis in the haemocyte and fat body; (2) direct killing by Hydrogen Peroxide (H2O2) or Nitric Oxide (NO) agents produced during melanisation; and (3) immobilization of opportunistic pathogens by blood coagulation at an open wound (for more in depth reviews see Lemaitre and Hoffmann, 2007; Kounatidis and Ligoxygakis, 2012). In this review, we focus on the roles of haemocytes in the Drosophila host defense and the key signaling pathways in orchestrating the different strategies deployed against a wide range of pathogens.
Molecular basis of haemocyte migration and motility in embryos
Drosophila haemocytes respond to numerous signals during development or following injury and/or infection (Wood and Jacinto, 2007). These signals can include migrating cues during embryogenesis, inflammatory and stress chemoattractants from the injury site and pathogen invasion in the haemolymph or in the tissue. During development, embryonic plasmatocytes are highly motile cells and they migrate from the precephalic mesoderm around stage 10 of the embryogenesis to start to disperse the entire embryo (Tepass et al., 1994). Haemocytes migrate along several invariant main routes throughout the embryo: toward the tail, along the ventral nerve cord, along the dorsal vessel and the gut primordium. The cell migration is guided by Pvr/Pvf signaling. The receptor tyrokinase Pvr is the homolog of the vertebrate Platelet-derived growth factor (PDGF) and Vascular endothelial growth factor (VEGF) receptors. The Pvr has three ligands: PDGF- and VEGF-related factor (Pvf)-1, Pvf-2 and Pvf-3. Haemocytes express Pvr and are attracted by Pvf2 and Pvf3 expressed in different tissues of the embryo. For example, the nerve cord expresses Pvf2 and Pvf3 spatially and temporally in its different compartments to attract haemocytes to move along the Central Nerve System (CNS) (Cho et al., 2002; Wood et al., 2006). The impressively fixed migrating patterns for haemocytes to populate the entire embryo from anterior to posterior and from dosal to ventral rely on sustained motility and cell polarity and highly organized cell shape to enable smooth and rapid movement along the tissue surface. By use of live confocal microscopy, plasmatocytes were discovered to move with large, polarized and actin-rich filopodia and lamellopodia during the embryonic migration (Wood et al., 2006). Toward the end of embryogenesis, these cytoplasmic protrusions become highly dynamic and continually extend and retract to survey the surrounding microenvironment. The Rho family small GTPase Rac, Rac1 and Rac2, function redundantly to control the lamellopodia formation and thus the successful dispersal of haemocytes in the embryo (Paladi and Tepass, 2004). A Drosophila PDZ guanine-nucleotide exchange factor (PDZ-GEF) Dizzy was also identified to be required for the embryonic haemocyte migration (Huelsmann et al., 2006). In the absence of Dizzy, the cytoplasmic protrusions are reduced in size and thus slow down the migration rate of the cells. Overexpression of Dizzy in haemocytes generates cells with abnormally extended protrusions. Dizzy is believed to act upstream of the Ras superfamily member of small GTPase Rap1 to regulate integrin dependent adhesion of haemocytes to the epithelia and to maintain their cellular “microspikes” throughout migration (Huelsmann et al., 2006). Siekhaus et al. discovered that during embryonic haemocyte migration Drosophila haemocytes invade an epithelial barrier as they move into the tail despite an open blood system (Siekhaus et al., 2010). A mutant of RhoL, another Drosophila GTPase homolog specifically expressed in haemocytes blocks this epithelia invasion but not other aspects of guided migration. RhoL interferes with Rap1mediated integrin adhesion by moving Rap1 away from a concentration in the cytoplasm to the leading edge during invasive migration. RhoL therefore functions as a regulator for integrin adhesion and Rap1 localization during the invasion. Inhibition of integrin-based adhesion is necessary to regulate the cadherin interactions that allow plasmatocytes to transmigrate from the head region, through the epithelium, to the posterior of the embryo. These findings revealed a striking similarity of the stepwise migratory process during Drosophila development with vertebrate immune cell transmigration during inflammation.
Apart from moving along fixed developmental migratory patterns, embryonic plasmatocytes respond to the epithelia wound by migrating rapidly to the site of injury. This process also shares many physiological relevancies with the vertebrate inflammation. Similar to the developmental migration, this deployment to the injury site also requires Rac-mediated lamellopodia formation (Stramer et al., 2005). Stramer and co-workers showed that Rho signaling is necessary for haemocytes to retract from sites of cell matrix and disengage from cell-cell contact. During the migration to the wound, CDC42 is required to maintain the plasmatocyte polarity. In contrast to the developmental dispersal of haemocytes in response to Pvr/Pvf cues, the chemotaxic signals from the injury site activate a different mechanism to mobilize plasmatocytes. This Drosophila inflammation induced cell migration depends on a phosphatidyl-inositol 3-kinase (PI3K) signaling which is also used by the mammalian neurophils in response to chemotaxic cues (Stramer et al., 2005). Therefore, it can be concluded that actin protrusion formation controlled by the Rac signaling in the cell motility is essential for plasmatocyte migration in these two different processes. Ena is another player identified recently to regulate the actin protrusions in the embryonic haemocyte (Tucker et al., 2011). Ena is the Drosophila homolog of Mena, member of the evolutionarily conserved Ena/VASP family of actin cytoskeletal regulators. Mena promotes metastasis and invasive motility of breast cancer cells in vivo. Tucker et al. found that Ena stimulates lamellipodial dynamics and positively regulates the number and length of filopodia. Overexpression of Ena in the haemocyte results in dramatic increase in the migration rate. One of the phenotypes can be also observed from overexpression of Mena in the mammalian fibroblast.
Postembryonic haemocyte migration
In the larva, overexpression of Rac in the haemocyte disrupts the sessile haemocytes population and causes a large migration of haemocytes into the circulation. Sessile haemocyte activation and mobilization require the Jun N-terminal kinase (JNK) Basket (Bsk) and Rac1. Bsk is also found to regulate the turnover of focal adhesions in the circulating haemocyte in the larva (Williams et al., 2006). These findings suggest that the Rho and JNK signaling are conserved, underlining their roles in the formation of cytoplasmic protrusions and actin focal adhesions for proper plasmatocyte mobility and migration to support their cellular roles in development and immunity. Two very recent studies on the postembryonic haemocyte migration have shed more light on the cellular dynamics and molecular basis of this process. An ex vivo culturing system using the primary larval or pre-pupal haemocytes has been developed to allow a real time analysis and manipulation to examine the roles of cytoskeleton dynamics in plasmatocyte migration (Sampson and Williams, 2012). From this system, it was found that larval circulating haemocytes are less motile than the pre-pupal haemocytes and thus unable to migrate. The extending and retracting rates of the protrusions appear dormant while the prepupal haemocytes have normal dynamic protrusions potentially required in the morphogenesis. The same study also reinforced the role of Rho family members: Rac1 and Rac2 and CDC42 to sustain the size of the filopodia and lamellopodia in the prepual haemocytes. Absence of these genes caused a static phenotype of pre-pual haemocytes similar to what was observed in haemocytes from third instar larvae. Though an in vivo assay still awaits to confirm these findings, the importance of Rho signaling in the actin cytoskeleton shaping has been strengthened in the ‘walking’ of the haemocyte along the extracellular matrix.
Another in vivo study based on MARCM (Mosaic Analysis with a Repressible Cell Marker System) investigated the integrin adhesion activation and maturation in the migration of the sessile haemocyte population in the late larval stage into pupal stage (Moreira et al., 2013). The Drosophila βPS integrin myosperoid and integrin containing adhesion regulators such as Rhea and Fermitin were found to be required in pupal haemocyte migration.
Cellular immunity mediated by plasmatocytes
Plasmatocytes represent around 90% of the total circulating haemocyte population in all developmental stages of Drosophila. They are professional macrophages which function as sentinels to maintain cell and tissue homeostasis and to recognize pathogen entry for subsequent immune reactions.
In the embryo, mature plasmatocytes function primarily as scavengers to remove apoptotic cell debris during embryogenesis. The clearance of apoptotic cell debris is dependent on scavenger receptors: CD36 homolog Croquemort (Franc et al., 1996), Draper (Manaka et al., 2004) and NimC4/Simu (Kurant et al., 2008). In the larval stage, recognition and rapid engulfment of invading microbes such as bacteria rely on the cell surface receptors Eater (Kocks et al., 2005) and NimC1 (Kurucz et al., 2007) and in the adult fly on Draper also (Cuttell et al., 2008). Loss of function of mutants in those receptors results in functional deficiency of phagocytosis of Gram-positive bacteria such as Staphylococcus. aureus and Gram-negative bacteria such as Escherichia coli. Eater, NimC1, NimC4 and Draper together with CED in C. elegans belong to a large protein family conserved across the metazoan animal kingdom including other insects such as Anopheles and humans (Kurucz et al., 2007). Epidermal Growth Factor (EGF)-like repeats are abundantly found in the extracellular domains of these bacterial phagocytosis receptors and there is evidence to show the direct interaction and binding of the EGF-like repeats with bacteria in Eater (Kocks et al., 2005) and Draper (Hashimoto et al., 2009). These interesting findings suggest that proteins with GF-like repeats may play an evolutionary conserved role in phagocytosis in the entire animal kingdom (Table 1).
Table 1.
Key genes involved in innate immunity, inflammatory responses and wound healing in Drosophila haemocytes.
| Blood cells | Innate immune response | Key genes |
|---|---|---|
| Plasmatocytes | Phagocytosis / AMP production | Croquemort (Crq) |
| Draper | ||
| NimC4/Sim | ||
| Eater | ||
| NimC1 | ||
| PGPR-LC/PGRP-LE | ||
| Dscam | ||
| Blood coagulation | Hemolectin (hml) | |
| Transglutaminase | ||
| (TF) | ||
| Inflammation / Wound healing | Eiger | |
| Psidin | ||
| Spatzle | ||
| Cytokine-like | Hayan | |
| UPd3 | ||
| Rac1/Rac2 | ||
| Dizzy | ||
| Zir | ||
| Cytoskeleton modulation | RhoL | |
| Rap1 | ||
| Cdc42 | ||
| Ena | ||
| Crystal cells | Wound healing / Melanisation | Srp7 |
| DoxA1 | ||
| CG8193 | ||
| Lamellocytes | Parasite infection | DoxA3 |
| Charlatan |
Another receptor that binds directly microorganisms and participates in phagocytosis is Dscam, which is a member of the Ig superfamily with an essential function in neuron interconnection. By alternative splicing as many as 18,000 isoforms can be theoretically generated in the haemocyte and fat body (Watson et al., 2005). Haemocyte-specific Dscam silencing reduces the phagocytic uptake of bacteria. The existence of a potential extensive repertoire of thousands of Ig-domain-containing proteins in the recognition of a variety of pathogens in Drosophila and other invertebrates has opened up a new avenue in exploring the possibility of an “adaptive” immunity across animal plyla that have been considered as having only innate responses (Watson et al., 2005; Schmucker and Chen, 2009).
Haemocyte-mediated humoral response
In response to pathogens that manage to gain entry into the haemocoel Drosophila can mount a robust systemic immune humoral response. The hallmark of this response is the rapid synthesis of a broad spectrum of AMPs against bacteria and fungi both in the haemocyte and in the fat body. AMPs are secreted into the haemolymph and directly kill the microbes at an optimal concentration. Although the fat body—the Drosophila equivalent of mammalian liver—is the prominent site of AMP synthesis, plasmatocytes play important roles in triggering the AMP production as haemocyte ablation can abolish the AMP expression in larval fat body (Shia et al., 2009). The roles plasmatocytes can play in the systemic immune response can be achieved by signaling between the site of infection and the fat body or by degradation of invading pathogens. To date, a haemocyte-released cytokine, Unpaired-3 (Upd-3) has been proposed to activate the JAK/STAT pathway in response to septic injury by binding to the fat body Domeless (Dom) receptor (Agaisse et al., 2003). Nevertheless the precise role of this pathway and its overall contribution to the host defense remains to be established. Spätzle is another cytokine secreted by haemocytes, processed by a serine protease cascade in the haemolymph and required for the Toll signaling pathway controlled AMP synthesis in the fat body (Shia et al., 2009). The Toll signaling has been well characterized in the Dorsal-Ventral patterning during embryogenesis and AMP production against mainly fungi, Gram positive bacteria, Pseudomonas aeruginosa (a Gram negative bacterium) and also stress/danger signals (reviews in Valanne et al., 2011; Kounatidis and Ligoxygakis, 2012). The core Toll signaling event is the degradation of Drosophila NFκB Inhibitor homolog Cactus followed by the activation and translocation of the Drosophila NFκB transcription factors Dorsal or Dorsal related immunity factor (Dif) into the nucleus. Dorsal and Dif are homologs of mammalian p50 and p65. Apart from these secreted cytokines, a cytoplasmic lysosomal protein called Psidin has been found to be the link between the haemocyte phagocytosis and AMP activation in the fat body in the larval immune response (Brennan et al., 2007). Psidin is required both for the phagocytic degradation of internalized bacteria and for the induction of one of the AMPs, Defensin, in the fat body. This interesting finding suggests a likely “antigen” presentation mechanism of the haemoctye to the fat body for the activation of AMP. Contrary to these findings, plasmatocyte ablation does not affect the antimicrobial responses upon systemic infection in the adult fly (Charroux and Royet, 2009; Defaye et al., 2009). This might suggest that tissue specific humoral responses, such as local expression of AMP and cytokines independent of haemocytes in the gut or in the trachea, play dominant roles in the adult immunity against pathogens.
AMP production in haemocytes
AMP production plays a vital role throughout the life cycle of Drosophila. Many tissues that have direct contact with the microorganisms such as the trachea, the gut and malpighian tubules have the capability to synthesize AMP and kill the microbes locally and efficiently. For microbes that manage to gain entry into the circulation via an open wound or the digestive or reproductive tracts, the fly can mount a systemic humoral response to produce large amounts of AMP from mainly the fat body into the haemolymph. Although haemocytes are not the major organ in the fly for systemic AMP production, the signaling pathways in control of AMP synthesis are activated in haemocytes like in the other tissues during a concerted immune response, in particular in the embryonic haemocytes (reviewed by Lemaitre and Hoffmann, 2007; Kounatidis and Ligoxygakis, 2012).
Melanisation
Melanisation in arthropods is generally believed to play an important and central role in arthropod defense reactions such as wound healing, encapsulation, microbe immobilization and the production of toxic intermediates that are speculated to kill invading microorganisms (Cerenius and Söderhäll, 2004). As described briefly above, crystal cells are the major haemocytes responsible for the melanisation reaction in the larva. Melanisation can be immediately induced at the site of cuticular injury or on the surface of parasites invading the haemocoel. It involves formation of black pigmentations resulting from de novo synthesis and deposition of melanin. Prophenoloxidase (PPO) is the enzyme required in melanisation to catalyze the oxidation of mono- and di-phenols to ortho-quinones, which polymerize into melanin. PPO in normal physical conditions is enzymatically inert. A serine protease known as prophenoloxidase activating enzyme (PPAE) acts upstream to cleave and turn PPO into active phenoloxidase (PO). Like PPO, PPAE also exists as an inactive zymogen and it is processed by a tightly regulated serine protease cascade in a step wise way into enzymatically functional form leading to the final melanin formation. As in other invertebrates, a recent in vitro study on the Drosophila PPOs suggested a direct binding of PPOs to bacteria and fungi which might play a role to initiate their activation (Yang et al., 2013). The Drosophila genome encodes three PPOs: DoxA1, DoxA3 and CG8193 (Irving et al., 2005). Crystal cells express DoxA1 and CG8193 while lamellocytes express exclusively DoxA3, a strong indication that Dox3 participates in the encapsulation that accompanies melanisation. Melanisation is diminished in the domino mutant that lacks haemocytes (Braun et al., 1998) and in the Black cells (Bc) mutant with aberrant crystal cells (Rizki et al., 1980; Corbo and Levine, 1996) and the Lz knockout, which is devoid of crystal cells (Peeples et al., 1969). One serine protease Sp7 has been reported to be involved in PPO activation and expressed also in the crystal cells (Castillejo-López and Häcker, 2005). In the absence of crystal cells in the adult fly, melanisation perhaps relies on the activation of proteolytic cascades in the haemolyph including PPAE, PPO and serine proteases. The cascades are tightly regulated by serine protease inhibitors in the haemolymph such as Serpin27 A to restrict the reaction to the site of injury and to prevent the spread of systemic melanisation (De Gregorio et al., 2002; Ligoxygakis et al., 2002). Two serine proteases MP1 and MP2 are reported to activate the cascade in response to different microbial changes (Tang et al., 2006). This pathogen-specific activation of melanisation can be attributed to PGLP-LC and PGRP-LE expressed both by haemocytes and the fat body (Takehana et al., 2004; Schmidt et al., 2008). However the connection between other types of pathogen receptors (for example, Gram-positive bacteria and fungi etc.) has not been linked to melanisation triggering. To date only one PPAE has been identified in the melanisation cascade of the adult fly (Leclerc et al., 2006).
Encapsulation
Lamellocytes are the major executioner of encapsulation during parasite infection in the Drosophila larva. Encapsulation involves three key steps with coordinated actions from both plasmatocytes and lamellocytes. Firstly circulating plasmatocytes sense and recognize the entry of parasitoid eggs in the haemocoel and attach to the egg chorion. Secondly a massive proliferation and differentiation of sessile compartments and of haemocytes in the LG to lamellocytes is induced via unknown signaling molecules within a few hours to appear in the circulation where the lamellocytes form a multi-layered capsule around the eggs. Eventually the lamellocytes, like the crystal cells, release their cellular content such as PPO to activate the melanisation process and kill the parasites, possibly by the cytotoxic by products from the localized melanisation reaction (Nappi et al., 1995). To date genes that have been reported to play a role in encapsulation process are involved primarily in cell-cell interaction such as αPS4/βPSintegrins (myospheroid) (Irving et al., 2005; Wertheim et al., 2005) and in cytoskeleton remodeling for motility and migration, such as RhoGTPase protein family member Rac1(Williams et al., 2006), Rac2 and CDC42 (Williams et al., 2005). Rac2 and CDC42 are activated by the Drosophila homolog of Rho guanine nucleotide exchange factor (RhoGEF), Zir (Sampson and Williams, 2012; Sampson et al., 2012). Rac1 and Rac2 function in a non-redundant manner. Interaction between Rac1and myospheroid has recently been reported to be required in the directed localization of β-integrin on the cell surface of lamellocytes in response to parasitoid eggs (Xavier and Williams, 2011). Rac1 requires the JNK pathway component Bsk to regulate the formation of actin- and focal adhesion kinase (FAK)-rich placodes in haemocyte migration and both are required for the proper encapsulation of wasp eggs (Williams et al., 2006). A recent screen to target genes involved in the cell adhesion and shape change not only strengthened the previous findings but also discovered more conserved components in these cellular processes that participate in the encapsulation reaction, for example the extracellular matrix proteins (ECM) (Howell et al., 2012). Loss of function of ECM components results in failure to encapsulate. In correlation with the previous discovery on the encapsulation of mechanically damaged self-tissue, it is plausible that exposure of ECM by foreign particle intrusion or deposition of ECM on the eggs can be the initiative signal in encapsulation (Rizki and Rizki, 1980; Howell et al., 2012). Genome-wide analysis of the transcriptional profiles in haemocytes after parasitoid infection has offered many interesting and promising candidate genes that are differentially regulated in the encapsulation reaction (Wertheim et al., 2005). This study also reinforced the importance of the Toll and JAK/STAT signaling pathways in the differentiation and proliferation of lamellocytes in the LG (Sorrentino et al., 2004). In addition, the haemocyte-specific transmembrane protein Hemese has been reported to play a modulatory role to keep lamellocyte proliferation in check from overacting during parasitoid egg infection (Kurucz et al., 2003). Despite these findings, the molecular nature of the signals sent from plasmatocytes for lamellocyte differentiation in the LG remains elusive. It has been proposed that a signal delivered to the PSC initiates lamellocyte differentiation as the PSC-restricted expression of Collier (Col), the Drosophila homolog of human Early B cell factor, is required upon parasite invasion (Crozatier et al., 2004).
Blood coagulation
Haemocytes have essential roles in blood coagulation, not only to maintain haemostasis but also to defend against pathogens. It has been found that Hemolectin (Hml) expressed mainly by plasmatocytes is required in blood coagulation. Blood coagulation led by plasmatocytes is independent of both melanin production and phenoloxidase activity, which is also part of the wound healing process (Goto et al., 2003). By proteomics and pull out analysis, important components of the blood clot have been isolated and subjected to detailed genetic and cellular investigations (Karlsson et al., 2004; Scherfer et al., 2004). Among these the best-characterized clotting factors are Drosophila Transglutaminase (TF) and Fondue. Drosophila TF is the only mammalian blood coagulation factor homolog (Factor XIIIa) found in the fly and uses Fondue as its substrate to form the blood clot. Unlike hemolectin (hml) mutants shown to affect only coagulation (Goto et al., 2003), ubiquitous silencing of fondue also results in cuticle defects in the pupa as well as in the clot forming in larvae (Scherfer et al., 2006). Hml is expressed mainly by plasmatocytes and contains domains found in coagulation factors (Goto et al., 2001, 2003). It is suggested that TF/Fondue acts more actively in cross-linking of fibers formed by Hml, reacting promptly to bleeding and injury (Scherfer et al., 2006; Lindgren et al., 2008). Interestingly TF is most likely expressed in haemocytes (Johansson et al., 2005) while Fondue is expressed in the fat body under control of the Toll signaling pathway (Scherfer et al., 2006). This strongly suggests that cellular and humoral factors are required in blood coagulation with contributions from both haemocytes and the fat body. Therefore, the lack of a signal sequence in TF gene (like PPO, another enzyme expressed in haemocytes) suggests that its release from haemocytes may be a key step in the initiation of coagulation. Most recently Wang et al. proposed a conserved innate immune mechanism based on TF's ability to use a potential microbial surface substrate to sequestrate and immobilize bacteria to the clot formed in blood coagulation (Wang et al., 2010).This interesting piece of work provides direct evidence for the blood coagulation factor to directly bind to microbes in the process of blood clot formation.
The Drosophila “inflammatory response”
All organisms have developed various mechanisms to maintain structural and physiological integrity in response both to external injury and to internal disruption. Wound healing, tissue repair and regeneration are essential processes for multi-cellular organisms to survive and proliferate against constant environmental or physical assaults. Therefore it is highly feasible to hypothesize that wound healing is an ancient process that evolved before the divergence of insects and mammals and this view can be supported by evidence from extensive research conducted in various model organisms on the pathways underlying this basic process.
Wound healing depends on complex molecular and cellular networks involving different types of cells and tissues. This complexity increases with the basic mechanisms varying in a tissue-specific, developmental stage dependent, and damage related manner. For example, in fruit fly embryos wound healing occurs rapidly via actin cable assembly and filopodial extension by cells at the wound margin, and proceeds without blood clot formation (Kiehart et al., 2000; Wood et al., 2002). Again, despite the substantial structural differences between Drosophila and mammalian epidermis, embryonic wound healing in mammalian embryos appears to be similar to that in Drosophila. It is also a rapid process involving actin cable formation without apparent haemostatic or inflammatory response (Martin and Lewis, 1992).
Haemocytes in embryonic wound healing
Although blood clots are not formed and required during the embryonic wound healing, microarray analysis comparing the transcriptional profile of wild type and haemocyte-absent embryos still revealed interesting haemocyte signature genes involved specifically in wound healing. These include phospholipase A2 conserved also in the mammalian inflammatory response (Stramer et al., 2008). From this study, a Drosophila ortholog of a novel mouse inflammatory-responsive gene Growth Arrest and DNA Damage-inducible gene 45 (GADD45) (Takekawa and Saito, 1998) was found to be induced in the damaged epidermal cells. This finding reinforces the idea that inflammatory responses are ancient processes for organisms to respond to danger signals. JNK signaling has also been reported to be essential in the epithelial wound healing in the embryo (Rämet et al., 2002; Wood et al., 2002). The earliest signal that triggers the haemocyte attraction to a wound in embryos has been recently identified as the calcium wave from the damaged epithelial cells following immediate laser wounding. Blocking this calcium flash inhibits H2O2 synthesis which relies on the activation of an NAPDH oxidase, DUOX (Razzell et al., 2013). In response to H2O2 that transiently outcompetes developmental migrating cues, haemocytes are quickly recruited to the injury site in the embryo (Moreira et al., 2010). The establishment of calcium flux-induced H2O2 production in the inflammatory response associated with wound healing from Drosophila will certainly give more insights into the signaling events taking place in wound induced inflammation in mammals.
Haemocytes in tissue injury and wound repair in the larva
The mammalian epithelial tissue can summon a set of humoral and cellular reactions lasting from days to months in response to tissue damage until the damage cap is properly closed and the injured cells or tissues are removed and replaced. The reactions typically include the rapid formation of a blood clot at the injury site and recruitment of inflammatory blood cells followed by spreading of the damaged epithelium across the wound gap to restore tissue integrity (Of and Healing, 1999; Singer and Clark, 1999). Likewise, Drosophila larval wound healing shares many similarities to postembryonic wound healing in mammals. By developing an aseptic puncture wounding in the third instar larva in combination with in vivo life imaging, Galko and Krasnow (2004) have established a system to study the process of Drosophila postembryonic wound healing. They have characterized the wound healing process by three key stages: (1) primary clot formation during blood coagulation: larvae bleed following the puncture wounding and the primary clot forms in the wound gap; (2) scab and syncytium formation: the primary clot is further cross-linked and hardened by melanisation to form a scab while epidermal cells surrounding the primary clot migrate toward it and then fuse to form a syncytium; and (3) central syncytium formation: more epidermal cells are attracted to the initial synticium and a larger central syncytium forms. JNK pathway is activated in the epidermal cells of the syncytium in a gradient manner to emanate signals for the epidermal cells to move along or through the wound clot to rebuild a continuous epithelium with its basal lamina and apical cuticle lining. Crystal cells are the haemocyte required in the formation of the scab. The scab stabilizes the wound site, establishes a physical barrier to the external microbes, prevents the over-activation of JNK pathway which can result in chronic wounding and provides a scaffold for re-epithelialisation. Scab forming and wound closure are controlled by independent genetic and signaling pathways as re-epithelialisation can still be activated at the wound gap though it never heals in the absence of a scab (Galko and Krasnow, 2004). Presumably, multiple signals must be produced to spatiotemporally co-ordinate this dynamic flow of cellular events involving crystal cells, epidermal cells and also plasmatocytes to remove cell debris for tissue remodeling in addition to phagocytosis of invading microbial pathogens from the open wound. For example: the signals from damaged sites to initiate blood coagulation as discussed in the previous section are still to be identified. What also remains unknown at present is the signal to attract crystal cells to the primary clot, the signals during the formation of a scab (perhaps from crystal cells or plasmacytes) to negatively modulate the JNK activity and the signal to recruit plasmatocytes to the wound site. These signals may be able to behave like mammalian chemokines or cytokines and possess distinct characteristics in terms of the range and the different signaling pathways they can activate. In addition, there could also be mitogenic signals from apoptotic cells to stimulate cell migration and regeneration (Bergmann and Steller, 2010). Although the identity of these invertebrate inflammatory signaling molecules remains largely unknown, in combination with studies on other arthropods the interconnection between inflammatory responses and wound healing seems to be phylogenetically conserved (Theopold et al., 2004; Eleftherianos and Revenis, 2011). Recent studies have begun to reveal the molecular identities of some of these signals. A blood borne Pvf1 ligand has been found to be expressed by epidermal cells at the wounding edge and to function in an autocrine manner to activate the motility of epidermal cells in wound closure (Wu et al., 2009). In a study by mutant screening using crystal cell rupture and melanisation as the readout, Bidla et al. reported that the rapid rupture of crystal cells and subsequent local melanisation in the clot at injury depended on the JNK pathway and on Eiger, the Drosophila homolog of tumor necrosis factor (Bidla et al., 2007).The most interesting finding is that endogenous signals such as Eiger released from crystal cells and plasmatocytes undergoing apoptosis followed by secondary necrosis can function independently of microbial elicitors in triggering the PPO activation, which can support the idea that endogenously induced ‘death signals’ initiate inflammatory and repair responses. Nevertheless, the molecular nature of the signal to initiate JNK pathway by the mechanical stress for the crystal cells to rupture still remains to be discovered to date.
In a study to reveal the genetic and molecular networks in control of systemic wound response after physical wounding in Drosophila larvae and adult flies, Nam et al. reported that a redox signal released from proPPO activation via the blood borne serine protease Hayan is required for the downstream activation of JNK signaling to protect remote internal tissues from systemic wound response induced by local physical trauma (Nam et al., 2012). Forced expression of Hayan in the haemocyte, but not in other tissues, rescued the wound induced mortality in the hayan loss of function mutant suggesting that a redox dependent mechanism communicates between circulating haemocytes (most probably plasmatocytes) and the remote internal tissues in a process similar to systemic inflammation in mammals.
The cellular and genetic basis of wound healing has recently been studied in detail by using a Drosophila embryo laser wounding model and it is revealed not surprisingly that a coordinated process exists involving myosin, E-cadherin, Echinoid, the plasma membrane, microtubules and the CDC42 small GTPase which respond dynamically during wound repair (Abreu-Blanco et al., 2011, 2012). The wound healing mechanism in Drosophila larvae was also explored by the development of a targeted large scale in vivo RNAi screen in the larval epidermis. Likewise, in the embryo, components in the JNK pathway and genes involved in the remodeling of actin cytoskeleton also actively participate in the larval wound healing (Lesch et al., 2010). Key genes in the haemocyte mediated innate immunity and in the Drosophila inflammatory response are summarized in Table 1. For recent reviews on the topic of wound healing, (see Belacortu and Paricio, 2011; Ríos-Barrera and Riesgo-Escovar, 2013).
Haemocytes in infection induced inflammation
The last decade has witnessed a rapid growth of research in gut immunity in Drosophila (reviews in Royet, 2011; Kounatidis and Ligoxygakis, 2012). In 2009 a genome-wide RNAi screen revealed a large numbers of genes in both haemocytes and the fat body to be regulated following intestinal Serratia macescens infection (Cronin et al., 2009). By ontology enrichment analysis, this study found a strong enrichment of genes in haemocytes implicated in processes including phagocytosis, responses to external stimuli and vesicle trafficking. The critical role of JAK/STAT signaling in the gut immunity was reinforced to function through regulation of intestinal stem cell proliferation which controls the gut epithelial cell haemostasis. Though potential signaling pathways were not the focus of this genome-wide analysis, the large number of genes to be either upregulated or downregulated in haemocytes suggests important modulatory roles that haemocytes can play in organ to organ communication in response to internal infection or inflammation. Recently, Juang and colleagues found that intestinal ROS signal triggers a systemic AMP expression in the fat body following oral feeding of Ecc15 (Erwinia carotovaro subsp.) to larvae. The ROS stress in the gut induces NO expression and transduces the signal to haemocytes by a NO dependent pathway (Wu et al., 2012). NO-dependent signaling mediated by haemocytes has been also observed following gastrointestinal infection by Candida albicans (Glittenberg et al., 2011). In the absence of haemocytes, the AMP production was greatly reduced but not completely abolished in the fat body. Though ROS has long been recognized to be involved in the initiation of inflammatory bowel diseases in humans (Rezaie et al., 2007), this recent finding from Drosophila can offer further insight into the potential role of macrophages in triggering a systemic inflammation during inflammatory bowel diseases. In addition, gut-associated macrophage-like cells were also found in the larval gut and their number was regulated by the PI3K signaling pathway (Zaidman-Rémy et al., 2012). This situation closely resembles the mouse colitis model (for review see Lin and Hackam, 2011). Taken together, research in Drosophila has revealed many similarities in the important signaling roles macrophage-like blood cells can fulfil during either local tissue-specific or systemic inflammation following internal infection. On the other hand, more recently, Panayidou and Apidianakis have given a comprehensive review on using Drosophila as the model to study interlinking mechanisms underlying intestinal cell proliferation, differentiation and maintenance during bacterial infection and intestinal stress. The authors therefore proposed a regenerative inflammation phenomenon independent of haemocytes conserved between Drosophila and mammals in cancer progression (Panayidou and Apidianakis, 2013).
Cancer and immunity in Drosophila
Drosophila as the model to study haematopoiesis and its associated leukemia
In adult mammals such as humans and mice, bone marrow is the organ that houses haematopoietic stem cells (HSC) which give rise to both the myeloid and lymphoid lineage. Mammalian HSC possesses the ability to self-renew and the pluripotency to differentiate into a great variety of blood cells in response to signals from its microenvironment, which has been termed the HSC niche (review in Wang and Wagers, 2011). The HSC niche has been a subject for vigorous research since the concept has been fully accepted (Wang and Wagers, 2011; Lensch, 2012). The dynamic communication between HSC and its niche has been shown to be fundamental in the control and regulation of haematopoietic process in vertebrates. Any dysfunction in the genetic and cellular mechanism underlying the HSC and niche interaction can result in blood borne cancers such as AML (Oh and Humphries, 2012). However, the structural and cellular complexity of the bone marrow niche has hindered the progress of fully understanding the basic genetic and molecular events fundamental to the haematopoietic process and their application in potential human diseases. The Drosophila larval PSC as discussed previously functions as a primitive niche to instruct the different fate that the prohaemocyte would adopt or to help the prohamemocyte to maintain its stem cell status (Crozatier and Meister, 2007). The LG primary lobes represent a very simplified HSC and niche model compared to its mammalian counterpart (Mandal et al., 2004). Although there are obvious restraints such as the limited number of differentiated blood cell types and the complete absence of lymphocytes, this simplicity can further our understanding of the basic signaling and cellular communication mechanisms involved in particular between the HSC and its microenvironment (review in Crozatier and Vincent, 2011).
Signaling pathways in Drosophila haematopoiesis and tumorigenesis
Research in Drosophila haematopoiesis has revealed a number of pathways in control of prohaemocyte proliferation and differentiation. Overexpression of the Drosophila JAK gene hopTum−1 causes proliferation of prohaemocytes and leads to melanotic tumor formation in the LG (Harrison et al., 1995). This discovery preceded the demonstration that mutated constitutive activation of JAK/STAT signaling could result in human leukemia (Lacronique et al., 1997). Compared to the vertebrate system, the Drosophila JAK/STAT pathway is much simplified and shows nearly complete absence of genetic redundancy. The Drosophila genome encodes only three upstream ligands of JAK/STAT pathway Unpaired (Upd1-3) while the mammalian JAK/STAT can be activated by a large group of cytokines and growth factors. Dom is the only transmembrane receptor upstream of one JAK kinase (Hop) and the one STAT transcription factor (STAT92E). Therefore the misexpression of a dominant-active form of STAT92E can also promote tumorigenesis in the eye of the Drosophila adult flies and melanotic tumor formation in the larva (Ekas et al., 2010). A systematic genome-wide RNAi screening for genes required for JAK/STAT pathway activity in cultured Drosophila haemocyte-like cells also identified interacting genes that can function as suppressors of leukemia-like blood cell tumors in humans (Müller et al., 2005). Increasing evidence from clinical research in human AML has pinpointed a role for JAK/STAT signaling pathway to be implicated in AML pathogenesis. In particular an activating mutation on the human JAK2 has been discovered to be responsible for various forms of AML (Lee et al., 2013a; Vainchenker and Constantinescu, 2013).
Apart from the JAK/STAT signaling pathway, the Notch, Hg, Wnt, and JNK pathways have all been identified as regulators of prohaemocyte fate (Mandal et al., 2007; Owusu-Ansah and Banerjee, 2009; Sinenko et al., 2009). Hg signaling in the PSC has been identified to maintain the undifferentiated fate of prohaemocytes in the larval medullary zone of the primary lobes in the LG (Mandal et al., 2007). In a mouse B cell lymphoma model, Hg signaling from the stromal cells was also shown to provide an important survival signal for B- and plasma-cell malignancies in vitro and in vivo (Dierks et al., 2007).The Hg signaling pathway was also discovered to be required in the maintenance of cancer stem cells of chronic myeloid leukemia (CML) (Zhao et al., 2009). Loss of Smoothened (Smo) the downstream transmembrane G protein coupled receptor in the JAK/STAT pathway causes depletion of CML stem cells whereas constitutively active Smo augments CML stem cell number and accelerates the disease. These studies are reminiscent of Hg signaling implicated in the communication between PSC niche and the prohaemocyte in Drosophila haematopoiesis.
Notch signaling pathway has been well conserved in vertebrate haematopoiesis, in particular in lymphoid cell commitment (Radtke et al., 2005; Tanigaki and Honjo, 2007). In Drosophila LG, the Serrate-mediated Notch signaling from the PSC is required to maintain normal levels of Col transcription and thus PSC cell identity (Krzemień et al., 2007). In addition, a non-canonical and ligand-independent activation of Notch signaling has also been reported to determine crystal cell fate in the LG. The Drosophila ortholog of mammalian hypoxia-inducible factor–a (HIF-a), Sima, activates full length Notch receptor under conditions of normal oxygen availability and commits the prohemocyte to the crystal cell lineage (Mukherjee et al., 2011). In fact, the first human Notch was originally identified from human Acute lymphoblastic leukemia (ALL) and since then Notch signaling has been discovered to be involved in many forms of human leukemia (review in Pancewicz and Nicot, 2011).
Wnt signaling has been shown to promote proliferation of prohaemocytes and prevent differentiation at the same time by controlling the PSC niche (Sinenko et al., 2009). In addition, Wnt signaling also positively regulates the proliferation and maintenance of PSC cells while inhibition of Wnt signaling results in fewer PSC cells than observed in control flies. Likewise in vertebrates, Wnt signaling has been reported to play important roles in the HSC homeostasis and maintenance of its microenvironment for self renewal (for the most recent review see Seke Etet et al., 2013) and thus has been implicated in many forms of haematologic malignancy such as AML (Gandillet et al., 2011) and CML (Nagao et al., 2011). To elucidate the controversial role of reactive oxygen species (ROS) in the haematopoietic system, work done by Owusu-Ansah and Banerjee set out to make use of the Drosophila haematopoietic model to study levels of ROS in the in vivo proliferation and differentiation of prohaemocytes. It was found that increased levels of ROS promote the differentiation of prohaemocytes while inhibition of the ROS level delays the differentiation of prohaemocytes into mature haemocytes. Interestingly, through a downstream signaling pathway that involves JNK and FoxO activation as well as Polycomb downregulation, increasing the haematopoietic progenitor ROS beyond their basal level triggers premature differentiation of prohaemocytes into all three mature haemocytes found in Drosophila. Therefore a moderately high level of ROS can be the developmental signal for the population of haemocyte progenitor to commit to lineage differentiation (Owusu-Ansah and Banerjee, 2009) (Figure 2B). In mammals, higher ROS level was also observed during the common myeloid progenitor differentiation in response to oxidative stress (Tothova et al., 2007). A recent study by Dragojlovic-Munther and Martinez-Agosto demonstrated that the tumor suppressors TSC and PTEN also have important roles in controlling blood progenitor proliferation through a common TOR- and 4EBP-dependent pathway in the LG. Loss of function of Tsc2 or Pten in prohaemocytes increases TOR signaling and causes overgrowth of the LG by haemocyte hyper-proliferation accompanied by a higher level of ROS. This study illustrates further how TSC and PTEN influence TOR function in response to physical stress such as starvation, hypoxia or increased ROS level during infection (Dragojlovic-Munther and Martinez-Agosto, 2012). Interestingly, the PTEN/mTOR signaling pathway has indeed been the therapeutic target in the treatment of human leukemia (Martelli et al., 2011).
Collectively these studies demonstrate the strength of Drosophila as an excellent model to study the HSC and its microenvironment interaction and shed light on the potential for therapeutic prevention of various hematological malignancies by dissecting its underlining genetic and cellular mechanisms.
Drosophila human leukemia model
Apart from the elucidation of basic molecular signaling pathways in the HSC and its niche interaction, Drosophila can also be used directly to model human leukemia. In addition to overactivation of JAK/STAT signaling, human AMLs can result from the chromosomal translocation of the transcription factor AML1, a RUNX domain protein, to form a protein fusion product with ETO (Hatlen et al., 2012). Targeted expression of human AML1-ETO fusion transcription factor in the haemocyte lineage cells by using the UAS/GAL4 system caused human leukaemic-like phenotypes such as hyper-proliferation of the circulating haemocytes resulting from the expansion of prohaemocytes in the LG (Sinenko et al., 2010). The successful establishment of the AML1-ETO leukemia model in Drosophila allowed a rapid tissue-specific genetic screening to identify suppressors for the hyperproliferation phenotype. The authors thus were able to show that ROS is a signaling factor promoting maintenance of normal as well as aberrant haemocyte precursors which suggested the importance of antioxidant enzymes and their regulators as targets for further study in the context of leukemia (Sinenko et al., 2010). In another independent genetic screening for modifiers in the AML1-ETO Drosophila model, Osman et al. identified calpainB as required for AML1-ETO-induced blood cell disorders in Drosophila by using an in vivo RNAi-based screen for suppressors of AML1-ETO. Remarkably, calpain was also found to interact with AML1-ETO in the human leukemic blood cell line Kasumi-1 (Osman et al., 2009). Therefore these studies have paved new avenues in our understanding of the pathogenesis of haematopoietic associated leukemia by developing and recapitulating the fundamental features of the disease in Drosophila and will contribute significantly to more precise and effective AML therapy.
Drosophila and human solid tumor
During the past two decades, the completion of the Drosophila genome and the development of advanced genome editing tools have given unprecedented stimulus and fast expansion on the use of Drosophila as a model for cancer. In particular, Drosophila has been instrumental for the discovery of three fundamental mechanisms involved in tumor progression and metastasis: (1) the role of Hippo signaling pathway in control of cell growth and survival together with Scrib/Dlg/Lgl signaling pathway in cell polarity to regulate organ sizes; (reviews in Enomoto and Igaki, 2011; Martin-Belmonte and Perez-Moreno, 2012; Harvey et al., 2013); (2) the in situ cell competition mechanism for morphogens during the formation of epithelium where slow growing cells are outcompeted and removed (review in Levayer and Moreno, 2013); and (3) apoptosis induced mitogenic signals in compensatory proliferation to replace surrounding damaged tissues (review in Fan and Bergmann, 2008). All these pathways underline the basic cellular communication, shaping and tissue organization which if disrupted can lead to tumorigenesis and facilitate tumor metastasis.
Despite a very short life span, cancer research to investigate directly the tumor progression and invasion in Drosophila can be dated back to nearly a century ago. It was observed that Drosophila can naturally develop hereditary tumors; carcinogens such as X-rays have been used to discover mutants with abnormal growth (Bridges and Brehme, 1944; Salomon and Jackson, 2008). The first fly strain carrying a mutation in a tumor suppressor gene called lethal giant larvae (lgl) was isolated 70 years ago. However only recently has Lgl been identified as a component of a signaling circuit in the regulation of apico-basal polarity in epithelial cells: Scrib/Dlg/Lgl (Humbert et al., 2008). Loss of function of the genes in this pathway can lead to the formation of neoplasma in the brain and imaginal disks in the developing larva. Based on the neoplastic phenotype observed in the scrib signaling mutants, Gateff was one of the pioneers who carried out more screenings to isolate recessive lethal mutations that could promote neoplastic overgrowth in the brain, imaginal disks or haematopoietic organ (Gateff, 1978). A tissue transplantation technique was developed from Gateff's study to assess the malignancy and invasive capacity of the neoplastic clones arising from various tissues. This technique involves the implantation of cancerous cell clones into the abdomen of wild type adult flies. Flies implanted with malignant cell clones usually die within 2 weeks and histological examination usually observes a massive invasion of cancerous cells into various tissues in the adult fly. This method resembles the tail veil intravenous injection of tumor cell lines into the mouse to model cancer in small rodents. This pioneering work fully established the potential for Drosophila to be used as a whole organism model to recapitulate key stages in cancer pathogenesis. The in vivo lac-Z reporter gene expression system can be utilized to quantify the proliferation rate and metastatic index of donor tumors in a tissue specific context in the normal wild type host (Beaucher et al., 2007). By using mutant clones of labeled cells such as GFP within a specific tissue, the invasive and metastatic behavior of cancer cells can be observed in situ by in vivo live imaging (Brumby and Richardson, 2003; Pagliarini and Xu, 2003). The recent development of MARCM system allows the establishment of large homozygous mutant cells clones within a normal or heterozygous tissue. A similar system is the FLP/FRT site directed recombination that can also generate genetic mosaics in the targeted organ or tissue. The mutant cells usually overexpress a UAS-tagged transgene fused with a reporter gene like GFP to allow imaging. These systems have been used by two independent groups to identify genes in cooperation with known oncogenes to induce tumor growth and invasion in the context of normal tissue (Brumby and Richardson, 2003; Pagliarini and Xu, 2003). By overexpressing an activated Ras (Rasv12) in the Drosophila eye imaginal disk and screening for the entire Drosophila genome, Pagliarini and Xu indentified Scrib as the promoting factor for the metastatic transformation of the otherwise benign tumor caused by overexpression of Ras oncogene alone (Pagliarini and Xu, 2003). Similar work by using the Drosophila eye as an in vivo “test tube” to investigate genetic interactions during tumor progression has proved to be extremely fruitful in dissecting the Scrib tumor suppressor signaling pathway and Hippo pathway and human oncogenes and tumor suppressors such as Ras and PTEN. Critical reviews of the most recent progress in the use of Drosophila in cancer modeling and therapeutic potentials can be found elsewhere (Miles et al., 2011; Stefanatos and Vidal, 2011; Gonzalez, 2013; Tipping and Perrimon, 2013).
Innate immunity in Drosophila tumor progression and tumor invasion model
Like other aspects of tumor progression that can be modeled and studied comparatively in Drosophila, the interplay of innate immunity mediated inflammation and tumor growth and invasion can be investigated accordingly. Of note is the establishment of Drosophila intestinal tumor model to study the molecular and cellular mechanisms linking inflammation and cancer pathogenesis (reviewed in Christofi and Apidianakis, 2013). The Drosophila cytokines Upd1-3 have been reported to activate the JAK/STAT signaling in promoting intestinal stem cell proliferation upon enteric infection or JNK-mediated stress response and thus the innate immunity plays essential roles in gut tissue homeostasis (Jiang et al., 2009). Moreover, overexpression of Drosophila Ras oncogene in association with bacterial infection can result in the formation of intestinal dysplasia (Apidianakis et al., 2009). More recently it is revealed that both the activation of Ras and bacterial induced IMD signaling can activate JNK pathway, which culminates in the up-regulation of matrix metalloproteinase 1 and thus cell invasion and migration (Bangi et al., 2012). Two studies have also emerged to explore the roles of haemocytes in the tumor progression and both relied on induced Rasv12Scrib−/− model in the Drosophila eye disks, which has been used to offer complementary views of systemic innate immunity to tumor growth and invasion. Under the condition of established tumor, the Drosophila haemocyte could be recruited to the tumor site and tumor associated haemocytes are the major source of Drosphila TNFα, Eiger, to promote the growth and invasion of the tumor cells into other tissues (Cordero et al., 2010), while a TNF-dependent mechanism in Drosophila eliminates cells deficient for the polarity tumor suppressors Scrib or Dlg to maintain the tissue homeostasis and keep any malignant growth in check (Brumby and Richardson, 2003). In an earlier study by Tian Xu and co-workers, the same eye imaginal disk derived Rasv12Scrib−/− tumor was found to induce a systemic proliferation of haemocytes via the JNK-JAK/STAT signaling cross-talk conserved also in response to tissue injury. The disrupted tumor basal membrane recruited circulating haemocytes in a manner reminiscence of wound healing (Pastor-Pareja et al., 2008). This study also modeled for the first time in Drosophila the anti-tumor effect of a systemic inflammation response induced by mechanical injury and provided critical insight into the cross-talk between the different signaling pathways in regulating the multiple step progression of tumor.
Perspective
Cancer has a complex biology. As a rationalization, Hanahan and Weinberg identified six major successive changes in human tumor development (Hanahan and Weinberg, 2011). The increasing awareness of this daunting complexity has turned more scientists to develop Drosophila as the complementary genetic tool to dissect each of the hallmark processes and to offer fundamental insights into the underlying genetic and cellular basis of the disease. In future, the Drosophila model can continue to be used as the reductionist system to investigate more of the cross-talks between these fundamental cancer biological processes. For example, the contrasting roles of immunity to control tumor growth or to be hijacked by the tumor can be modeled in the fly in a temporal and spatial manner. The unique advantage of Drosophila to be used as a whole organism has started to show promising potential in cancer drug screening and testing recently (Dar et al., 2012). This advantage can be fully explored by modeling simultaneously or sequentially physiological processes such as wound or infection induced innate immune/inflammatory response in the progression of cancer (Figure 3). The study of the role of haemocytes in response to wounding and invasive tumors will shed some light to the fundamentals of macrophages in immunity, inflammation and tumor microenvironment in human cancer.
Figure 3.
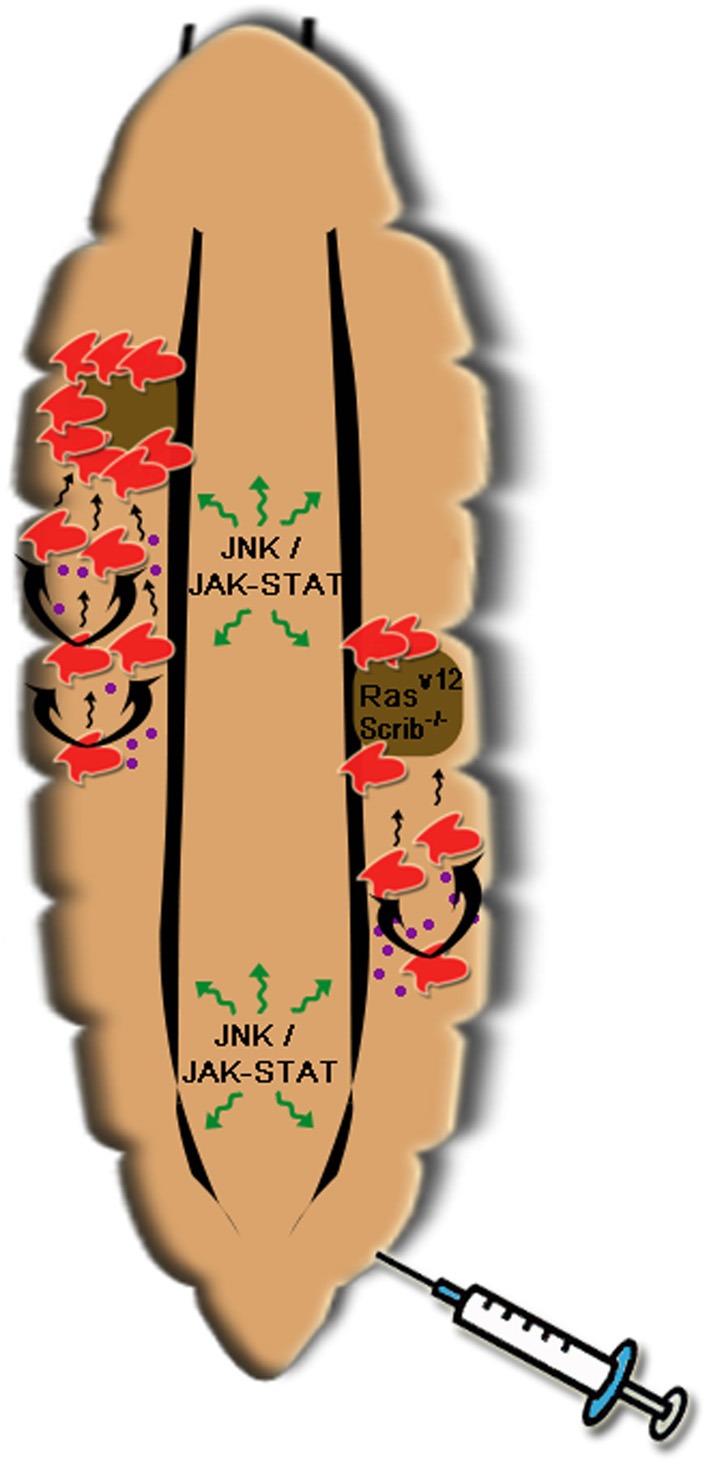
A simple illustration of roles of haemocytes in the Drosophila tumor model. Both tumor (Rasv12Scrib−/−) and wounding (as illustrated by a needle) can trigger proliferation of circulating haemocytes in the larva. This is mediated by cytokines (most probably Upd 1–3 shown in purple dots) induced by JNK signaling in response to wounding or the invasive tumor itself. In responses to the cytokines, JAK/STAT signaling in haemocytes or the fat body (not shown) can be activated and thus can further amplify the cytokine expression to promote haemocyte proliferation. Meanwhile disruption of basal membrane in the tumor attracts the adherent of haemocytes and limits the tumor growth perhaps by the synthesis of Drosophila TNFα, Eiger. This cartoon delineates that a step wise proliferation of haemocytes induced by wounding can be used to counter tumor growth. Modeling of these two processes (tumor growth and inflammation) in the Drosophila larva points out the potential beneficial effect that systemic inflammation can exert on tumorigenesis and will open more avenues for the basic research on innate immunity in particular macrophages' role in human tumor (see text for more detail).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all members of our laboratory for discussions. Our work is funded by the European Research Council and the Biological and Biotechnological Sciences Research Council (UK).
Glossary
Abbreviations
- AMP
antimicrobial peptide
- AML
acute myeloid leukemia
- ALL
acute lymphoblastic leukemia
- Bsk
Basket
- Chn
Charlatan
- CML
chronic myeloid leukemia
- CNS
central nerve system
- Col
Collier
- Dif
Dorsal related immunity
- Dom
Domeless
- ECM
extracellular matrix proteins
- EGF
epidermal growth factor
- FOG
Friend of GATA
- GADD45
growth arrest and DNA damage-inducible gene 45
- Gcm
Glial-cells-missing
- Hg
Hedgehog
- Hml
Hemolectin
- HSC
haematopoietic stem cells
- JNK
Jun N-terminal kinase
- LG
lymph gland
- Lgl
lethal giant larvae
- Lz
Lozenge
- MARCM
mosaic analysis with a repressible cell marker system
- PDGF
platelet-derived growth factor
- PI3K
phosphatidyl-inositol 3-kinase
- PPAE
Prophenoloxidase activating enzyme
- PPO
Prophenoloxidase
- PSC
posterior signaling center
- ROS
reactive oxygen species
- Ser
Serrate
- Smo
Smoothened
- Srp
Serpent
- TF
Transglutaminase
- Upd
Unpaired
- Ush
U-shaped
- VEGF
vascular endothelial growth factor
- Wnt
Wingless.
References
- Abreu-Blanco M. T., Verboon J. M., Liu R., Watts J. J., Parkhurst S. M. (2012). Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J. Cell. Sci. 125(Pt 24), 5984–5997 10.1242/jcs.109066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Blanco M. T., Verboon J. M., Parkhurst S. M. (2011). Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J. Cell. Biol. 193, 455–464 10.1083/jcb.201011018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agaisse H., Petersen U. M., Boutros M., Mathey-Prevot B., Perrimon N. (2003). Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 5, 441–450 10.1016/S1534-5807(03)00244-2 [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Vijayalekshmi R., V, Sung B. (2009). Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 15, 425–430 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y., Pitsouli C., Perrimon N., Rahme L. (2009). Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. U.S.A. 106, 20883–20888 10.1073/pnas.0911797106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangi E., Pitsouli C., Rahme L. G., Cagan R., Apidianakis Y. (2012). Immune response to bacteria induces dissemination of Ras-activated Drosophila hindgut cells. EMBO Rep. 13, 569–576 10.1038/embor.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataillé L., Augé B., Ferjoux G., Haenlin M., Waltzer L. (2005). Resolving embryonic blood cell fate choice in Drosophila: interplay of GCM and RUNX factors. Development 132, 4635–4644 10.1242/dev.02034 [DOI] [PubMed] [Google Scholar]
- Beaucher M., Goodliffe J., Hersperger E., Trunova S., Frydman H., Shearn A. (2007). Drosophila brain tumor metastases express both neuronal and glial cell type markers. Dev. Biol. 301, 287–297 10.1016/j.ydbio.2006.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belacortu Y., Paricio N. (2011). Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev. Dyn. 240, 2379–2404 10.1002/dvdy.22753 [DOI] [PubMed] [Google Scholar]
- Bergmann A., Steller H. (2010). Apoptosis, stem cells, and tissue regeneration. Sci. Signal. 3, re8 10.1126/scisignal.3145re8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidla G., Dushay M. S., Theopold U. (2007). Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell. Sci. 120(Pt 7), 1209–1215 10.1242/jcs.03420 [DOI] [PubMed] [Google Scholar]
- Braun A., Hoffmann J. A., Meister M. (1998). Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. U.S.A. 95, 14337–14342 10.1073/pnas.95.24.14337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Delaney J. R., Schneider D. S., Anderson K. V. (2007). Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr. Biol. 17, 67–72 10.1016/j.cub.2006.11.026 [DOI] [PubMed] [Google Scholar]
- Bridges C. B., Brehme K. S. (1944). The Mutants of Drosophila Melanogaster. Publs Carnegie Instn, 552: vii + 257pp [Google Scholar]
- Brückner K., Kockel L., Duchek P., Luque C. M., Rørth P., Perrimon N. (2004). The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 1, 73–84 10.1016/j.devcel.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E. (2003). scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 10.1093/emboj/cdg548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido J., Hagemann T. (2013). Cancer-related inflammation. J. Clin. Immunol. 33, S79–S84 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- Castillejo-López C., Häcker U. (2005). The serine protease Sp7 is expressed in blood cells and regulates the melanization reaction in Drosophila. Biochem. Biophys. Res. Commun 338, 1075–1082 10.1016/j.bbrc.2005.10.042 [DOI] [PubMed] [Google Scholar]
- Cerenius L., Söderhäll K. (2004). The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 10.1111/j.0105-2896.2004.00116.x [DOI] [PubMed] [Google Scholar]
- Charroux B., Royet J. (2009). Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl. Acad. Sci. U.S.A. 106, 9797–9802 10.1073/pnas.0903971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. K., Keyes L., Johnson E., Heller J., Ryner L., Karim F., et al. (2002). Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108, 865–876 10.1016/S0092-8674(02)00676-1 [DOI] [PubMed] [Google Scholar]
- Christofi T., Apidianakis Y. (2013). Ras-oncogenic Drosophila hindgut but not midgut cells use an inflammation-like program to disseminate to distant sites. Gut. Microbes 4, 54–59 10.4161/gmic.22429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo J. C., Levine M. (1996). Characterization of an immunodeficiency mutant in Drosophila. Mech. Dev. 55, 211–220 10.1016/0925-4773(96)00506-0 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Macagno J. P., Stefanatos R. K., Strathdee K. E., Cagan R. L., Vidal M. (2010). Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev. Cell. 18, 999–1011 10.1016/j.devcel.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L. M., Werb Z. (2002). Inflammation and cancer. Nature 420, 860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin S. J. F., Nehme N. T., Limmer S., Liegeois S., Pospisilik J. A., Schramek D., et al. (2009). Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340–343 10.1126/science.1173164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M., Meister M. (2007). Drosophila haematopoiesis. Cell. Microbiol. 9, 1117–1126 10.1111/j.1462-5822.2007.00930.x [DOI] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J.-M., Vincent A., Meister M. (2004). Cellular immune response to parasitization in Drosophila requires the EBF ortholog collier. PLoS Biol. 2:E196 10.1371/journal.pbio.0020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M., Vincent A. (2011). Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis. Model Mech. 4, 439–445 10.1242/dmm.007351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttell L., Vaughan A., Silva E., Escaron C. J., Lavine M., Van Goethem E., et al. (2008). Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell 135, 524–534 10.1016/j.cell.2008.08.033 [DOI] [PubMed] [Google Scholar]
- Dar A. C., Das T. K., Shokat K. M., Cagan R. L. (2012). Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature 486, 80–84 10.1038/nature11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E., Han S.-J., Lee W.-J., Baek M.-J., Osaki T., Kawabata S., et al. (2002). An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev. Cell. 3, 581–592 10.1016/S1534-5807(02)00267-8 [DOI] [PubMed] [Google Scholar]
- De Palma M., Lewis C. E. (2013). Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23, 277–286 10.1016/j.ccr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Defaye A., Evans I., Crozatier M., Wood W., Lemaitre B., Leulier F. (2009). Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immun. 1, 322–334 10.1159/000210264 [DOI] [PubMed] [Google Scholar]
- Dierks C., Grbic J., Zirlik K., Beigi R., Englund N. P., Guo G. R., et al. (2007). Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat. Med. 13, 944–951 10.1038/nm1614 [DOI] [PubMed] [Google Scholar]
- Dragojlovic-Munther M., Martinez-Agosto J. A. (2012). Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development 139, 3752–3763 10.1242/dev.074203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. P., Old L. J., Schreiber R. D. (2004). The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Ekas L. A, Cardozo T. J., Flaherty M. S., McMillan E. A, Gonsalves F. C., Bach E. A. (2010). Characterization of a dominant-active STAT that promotes tumorigenesis in Drosophila. Dev. Biol. 344, 621–636 10.1016/j.ydbio.2010.05.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I., Revenis C. (2011). Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 3, 28–33 10.1159/000321931 [DOI] [PubMed] [Google Scholar]
- Enomoto M., Igaki T. (2011). Deciphering tumor-suppressor signaling in flies: genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J. Genet. Genomics 38, 461–470 10.1016/j.jgg.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Evans C. J., Hartenstein V., Banerjee U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 5, 673–690 10.1016/S1534-5807(03)00335-6 [DOI] [PubMed] [Google Scholar]
- Fan Y., Bergmann A. (2008). Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell. Biol. 18, 467–473 10.1016/j.tcb.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N., Tevosian S. G., Gajewski K., Zhang Q., Orkin S. H., Schulz R. A. (2001). The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 98, 7342–7347 10.1073/pnas.131215798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc N. C., Dimarcq J. L., Lagueux M., Hoffmann J., Ezekowitz R. A. (1996). Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4, 431–443 10.1016/S1074-7613(00)80410-0 [DOI] [PubMed] [Google Scholar]
- Galko M. J., Krasnow M. A. (2004). Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2:E239 10.1371/journal.pbio.0020239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandillet, A, Park S., Lassailly F., Griessinger E., Vargaftig J., Filby A., et al. (2011). Heterogeneous sensitivity of human acute myeloid leukemia to β-catenin down-modulation. Leukemia 25, 770–780 10.1038/leu.2011.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E. (1978). Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 10.1126/science.96525 [DOI] [PubMed] [Google Scholar]
- Geissler K., Zach O. (2012). Pathways involved in Drosophila and human cancer development: the Notch, Hedgehog, Wingless, Runt, and Trithorax pathway. Ann. Hematol. 91, 645–669 10.1007/s00277-012-1435-0 [DOI] [PubMed] [Google Scholar]
- Glittenberg M. T., Kounatidis I., Christensen D., Kostov M., Kimber S., Roberts I., et al. (2011). Pathogen and host factors are needed to provoke a systemic host response to gastrointestinal infection of Drosophila larvae by Candida albicans. Dis. Model Mech. 4, 515–525 10.1242/dmm.006627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. (2013). Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 13, 172–183 10.1038/nrc3461 [DOI] [PubMed] [Google Scholar]
- Goto A., Kadowaki T., Kitagawa Y. (2003). Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev. Biol. 264, 582–591 10.1016/j.ydbio.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Goto A., Kumagai T., Kumagai C., Hirose J., Narita H., Mori H., et al. (2001). A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. Biochem. J. 359, 99–108 10.1042/0264-6021:3590099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R., Karin M. (2010). Immunity, inflammation, and cancer. Cell 140, 883–899 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Harris H. (2005). A long view of fashions in cancer research. Bioessays 27, 833–838 10.1002/bies.20263 [DOI] [PubMed] [Google Scholar]
- Harrison D. A., Binari R., Nahreini T. S., Gilman M., Perrimon N. (1995). Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14, 2857–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V. (2006). Blood cells and blood cell development in the animal kingdom. Annu. Rev. Cell. Dev. Biol. 22, 677–712 10.1146/annurev.cellbio.22.010605.093317 [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Zhang X., Thomas D. M. (2013). The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Tabuchi Y., Sakurai K., Kutsuna M., Kurokawa K., Awasaki T., et al. (2009). Identification of lipoteichoic acid as a ligand for draper in the phagocytosis of Staphylococcus aureus by Drosophila hemocytes. J. Immunol. 183, 7451–7460 10.4049/jimmunol.0901032 [DOI] [PubMed] [Google Scholar]
- Hatlen M. A., Wang L., Nimer S. D. (2012). AML1-ETO driven acute leukemia: insights into pathogenesis and potential therapeutic approaches. Front. Med. 6:3 10.1007/s11684-012-0206-6 [DOI] [PubMed] [Google Scholar]
- Holz A., Bossinger B., Strasser T., Janning W., Klapper R. (2003). The two origins of hemocytes in Drosophila. Development 130, 4955–4962 10.1242/dev.00702 [DOI] [PubMed] [Google Scholar]
- Howell L., Sampson C. J., Xavier M. J., Bolukbasi E., Heck M. M. S., Williams M. J. (2012). A directed miniscreen for genes involved in the Drosophila anti-parasitoid immune response. Immunogenetics 64, 155–161 10.1007/s00251-011-0571-3 [DOI] [PubMed] [Google Scholar]
- Hsu T. (2012). Complex cellular functions of the von Hippel-Lindau tumor suppressor gene: insights from model organisms. Oncogene 31, 2247–2257 10.1038/onc.2011.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsmann S., Hepper C., Marchese D., Knöll C., Reuter R. (2006). The PDZ-GEF dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development 133, 2915–2924 10.1242/dev.02449 [DOI] [PubMed] [Google Scholar]
- Humbert P. O., Grzeschik N. A., Brumby A. M., Galea R., Elsum I., Richardson H. E. (2008). Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27, 6888–6907 10.1038/onc.2008.341 [DOI] [PubMed] [Google Scholar]
- Irving P., Ubeda J. M., Doucet D., Troxler L., Lagueux M., Zachary D., et al. (2005). New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell. Microbiol. 7, 335–350 10.1111/j.1462-5822.2004.00462.x [DOI] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 10.1016/j.cell.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiravanichpaisal P., Lee B. L., Söderhäll K. (2006). Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology 211, 213–236 10.1016/j.imbio.2005.10.015 [DOI] [PubMed] [Google Scholar]
- Johansson K. C., Metzendorf C., Söderhäll K. (2005). Microarray analysis of immune challenged Drosophila hemocytes. Exp. Cell. Res. 305, 145–155 10.1016/j.yexcr.2004.12.018 [DOI] [PubMed] [Google Scholar]
- Jung S. H., Evans C. J., Uemura C., Banerjee U. (2005). The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521–2533 10.1242/dev.01837 [DOI] [PubMed] [Google Scholar]
- Karlsson C., Korayem A. M., Scherfer C., Loseva O., Dushay M. S., Theopold U. (2004). Proteomic analysis of the Drosophila larval hemolymph clot. J. Biol. Chem. 279, 52033–52041 10.1074/jbc.M408220200 [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Galbraith C. G., Edwards K. A., Rickoll W. L., Montague R. A. (2000). Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell. Biol. 149, 471–490 10.1083/jcb.149.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Cho J. H., Nehme N., Ulvila J., Pearson A. M., Meister M., et al. (2005). Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123, 335–346 10.1016/j.cell.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Kounatidis I., Ligoxygakis P. (2012). Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biol. 2, 120075 10.1098/rsob.120075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien J., Crozatier M., Vincent A. (2010). Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int. J. Dev. Biol. 54, 1117–1125 10.1387/ijdb.093053jk [DOI] [PubMed] [Google Scholar]
- Krzemień J., Dubois L., Makki R., Meister M., Vincent A., Crozatier M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325–328 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- Kurant E., Axelrod S., Leaman D., Gaul U. (2008). Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell 133, 498–509 10.1016/j.cell.2008.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz E., Márkus R., Zsámboki J., Folkl-Medzihradszky K., Darula Z., Vilmos P., et al. (2007). Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654 10.1016/j.cub.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Zettervall C., Sinka R., Vilmos P., Pivarcsi A., Ekengren S., et al. (2003). Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 100, 2622–2627 10.1073/pnas.0436940100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacronique V., Boureux A., Valle V. D., Poirel H., Quang C. T., Mauchauffé M., et al. (1997). A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278, 1309–1312 10.1126/science.278.5341.1309 [DOI] [PubMed] [Google Scholar]
- Lanot R., Zachary D., Holder F., Meister M. (2001). Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230, 243–257 10.1006/dbio.2000.0123 [DOI] [PubMed] [Google Scholar]
- Lebestky T., Chang T., Hartenstein V., Banerjee U. (2000). Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288, 146–149 10.1126/science.288.5463.146 [DOI] [PubMed] [Google Scholar]
- Lebestky T., Jung S. H., Banerjee U. (2003). A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 17, 348–353 10.1101/gad.1052803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V., Pelte N., El Chamy L., Martinelli C., Ligoxygakis P., Hoffmann J. A., et al. (2006). Prophenoloxidase activation is not required for survival to microbial infections in Drosophila. EMBO Rep. 7, 231–235 10.1038/sj.embor.7400592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Daver N., Kantarjian H. M., Verstovsek S., Ravandi F. (2013a). The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin. Cancer Res. 19, 327–335 10.1158/1078-0432.CCR-12-2087 [DOI] [PubMed] [Google Scholar]
- Lee H. W., Choi H. J., Ha S. J., Lee K. T., Kwon Y. G. (2013b). Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta 1835, 170–179 10.1016/j.bbcan.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Lee M. J., Kalamarz M. E., Paddibhatla I., Small C., Rajwani R., Govind S. (2009). Virulence factors and strategies of Leptopilina spp.: selective responses in Drosophila hosts. Adv. Parasitol. 70, 123–145 10.1016/S0065-308X(09)70005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J. (2007). The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 10.1146/annurev.immunol.25.022106.141615 [DOI] [PubMed] [Google Scholar]
- Lensch M. W. (2012). An evolving model of hematopoietic stem cell functional identity. Stem Cell Rev. 8, 551–560 10.1007/s12015-012-9347-x [DOI] [PubMed] [Google Scholar]
- Lesch C., Jo J., Wu Y., Fish G. S., Galko M. J. (2010). A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics 186, 943–957 10.1534/genetics.110.121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levayer R., Moreno E. (2013). Mechanisms of cell competition: themes and variations. J. Cell. Biol. 200, 689–698 10.1083/jcb.201301051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P., Pelte N., Ji C., Leclerc V., Duvic B., Belvin M., et al. (2002). A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 21, 6330–6337 10.1093/emboj/cdf661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Hackam D. J. (2011). Worms, flies and four-legged friends: the applicability of biological models to the understanding of intestinal inflammatory diseases. Dis. Model. Mech. 4, 447–456 10.1242/dmm.007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M., Riazi R., Lesch C., Wilhelmsson C., Theopold U., Dushay M. S. (2008). Fondue and transglutaminase in the Drosophila larval clot. J. Insect Physiol. 54, 586–592 10.1016/j.jinsphys.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Manaka J., Kuraishi T., Shiratsuchi A., Nakai Y., Higashida H., Henson P., et al. (2004). Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J. Biol. Chem. 279, 48466–48476 10.1074/jbc.M408597200 [DOI] [PubMed] [Google Scholar]
- Mandal L., Banerjee U., Hartenstein V. (2004). Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 36, 1019–1023 10.1038/ng1404 [DOI] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V., Banerjee U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320–324 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. (2008). Cancer-related inflammation. Nature 454, 436–444 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013). Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 10.1002/path.4133 [DOI] [PubMed] [Google Scholar]
- Martelli A. M., Evangelisti C., Chappell W., Abrams S. L., Bäsecke J., Stivala F., et al. (2011). Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia 25, 1064–1079 10.1038/leu.2011.46 [DOI] [PubMed] [Google Scholar]
- Martin P., Lewis J. (1992). Actin cables and epidermal movement in embryonic wound healing. Nature 360, 179–183 10.1038/360179a0 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Perez-Moreno M. (2012). Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 12, 23–38 10.1038/nrc3169 [DOI] [PubMed] [Google Scholar]
- Miles W. O., Dyson N. J., Walker J. A. (2011). Modeling tumor invasion and metastasis in Drosophila. Dis. Model Mech. 4, 753–761 10.1242/dmm.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira C. G. A, Jacinto A., Prag S. (2013). Drosophila integrin adhesion complexes are essential for hemocyte migration in vivo. Biol. Open 2, 795–801 10.1242/bio.20134564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S., Stramer B., Evans I., Wood W., Martin P. (2010). Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 20, 464–470 10.1016/j.cub.2010.01.047 [DOI] [PubMed] [Google Scholar]
- Mukherjee T., Kim W. S., Mandal L., Banerjee U. (2011). Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 332, 1210–1213 10.1126/science.1199643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Kuttenkeuler D., Gesellchen V., Zeidler M. P., Boutros M. (2005). Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436, 871–875 10.1038/nature03869 [DOI] [PubMed] [Google Scholar]
- Nagao R., Ashihara E., Kimura S., Strovel J. W., Yao H., Takeuchi M., et al. (2011). Growth inhibition of imatinib-resistant CML cells with the T315I mutation and hypoxia-adaptation by AV65–a novel Wnt/β-catenin signaling inhibitor. Cancer Lett. 312, 91–100 10.1016/j.canlet.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Nam H. J., Jang I. H., You H., Lee K. A., Lee W. J. (2012). Genetic evidence of a redox-dependent systemic wound response via Hayan protease-phenoloxidase system in Drosophila. EMBO J. 31, 1253–1265 10.1038/emboj.2011.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A. J., Vass E., Frey F., Carton Y. (1995). Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell. Biol. 68, 450–456 [PubMed] [Google Scholar]
- Novak M. L., Koh T. J. (2013). Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 93, 875–881 10.1189/jlb.1012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Of B., Healing W. (1999). Cutaneous wound healing. N. Engl. J. Med. 738–746 [DOI] [PubMed] [Google Scholar]
- Oh I. H., Humphries R. K. (2012). Concise review: multidimensional regulation of the hematopoietic stem cell state. Stem Cells 30, 82–88 10.1002/stem.776 [DOI] [PubMed] [Google Scholar]
- Osman D., Gobert V., Ponthan F., Heidenreich O., Haenlin M., Waltzer L. (2009). A Drosophila model identifies calpains as modulators of the human leukemogenic fusion protein AML1-ETO. Proc. Natl. Acad. Sci. U.S.A. 106, 12043–12048 10.1073/pnas.0902449106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E., Banerjee U. (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 10.1038/nature08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini R. A., Xu T. (2003). A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 10.1126/science.1088474 [DOI] [PubMed] [Google Scholar]
- Paladi M., Tepass U. (2004). Function of Rho GTPases in embryonic blood cell migration in Drosophila. J. Cell. Sci. 117(Pt 26), 6313–6326 10.1242/jcs.01552 [DOI] [PubMed] [Google Scholar]
- Panayidou S., Apidianakis Y. (2013). Regenerative inflammation: lessons from Drosophila intestinal epithelium in health and disease Pathogens 2, 209–231 10.3390/pathogen2020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancewicz J., Nicot C. (2011). Current views on the role of Notch signaling and the pathogenesis of human leukemia. BMC Cancer 11:502 10.1186/1471-2407-11-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Wu M., Xu T. (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Model Mech. 1, 144–154 10.1242/dmm.000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples E. E., Geisler A., Whitcraft C. J., Oliver C. (1969). Comparative studies of phenol oxidase activity during pupal development of three lozenge mutants (lzs, lz, lzk) of Drosophila melanogaster. Genetics 62, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Pan P. C., Govind S. (1998). A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125, 1909–1920 [DOI] [PubMed] [Google Scholar]
- Radtke F., Wilson A., MacDonald H. R. (2005). Notch signaling in hematopoiesis and lymphopoiesis: lessons from Drosophila. BioEssays 27, 1117–1128 10.1002/bies.20315 [DOI] [PubMed] [Google Scholar]
- Rämet M., Lanot R., Zachary D., Manfruelli P. (2002). JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 241, 145–156 10.1006/dbio.2001.0502 [DOI] [PubMed] [Google Scholar]
- Razzell W., Evans I. R., Martin P., Wood W. (2013). Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 23, 424–429 10.1016/j.cub.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie A., Parker R. D., Abdollahi M. (2007). Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 52, 2015–2021 10.1007/s10620-006-9622-2 [DOI] [PubMed] [Google Scholar]
- Ricklin D., Lambris J. D. (2013). Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190, 3831–3838 10.4049/jimmunol.1203487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Barrera L. D., Riesgo-Escovar J. R. (2013). Regulating cell morphogenesis: the Drosophila Jun N-terminal kinase pathway. Genesis 51, 147–162 10.1002/dvg.22354 [DOI] [PubMed] [Google Scholar]
- Rizki R. M., Rizki T. M. (1980). Hemocyte responses to implanted tissues in Drosophila melanogaster larvae. Rouxs Arch. Dev. Biol. 213, 207–213 [DOI] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M., Grell E. H. (1980). A mutant affecting the crystal cells in Drosophila melanogaster. Rouxs Arch. Dev. Biol. 181, 91–99 [DOI] [PubMed] [Google Scholar]
- Royet J. (2011). Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster. Cell. Mol. Life Sci. 68, 3651–3660 10.1007/s00018-011-0828-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R. N., Jackson F. R. (2008). Tumors of testis and midgut in aging flies. Fly 2, 265–268 [DOI] [PubMed] [Google Scholar]
- Sampson C. J., Valanne S., Fauvarque M.-O., Hultmark D., Rämet M., Williams M. J. (2012). The RhoGEF Zizimin-related acts in the Drosophila cellular immune response via the Rho GTPases Rac2 and Cdc42. Dev. Comp. Immunol. 38, 160–168 10.1016/j.dci.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Sampson C. J., Williams M. J. (2012). Real-time analysis of Drosophila post-embryonic haemocyte behaviour. PLoS ONE 7:e28783 10.1371/journal.pone.0028783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfer C., Karlsson C., Loseva O., Bidla G., Goto A., Havemann J., et al. (2004). Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Curr. Biol. 14, 625–629 10.1016/j.cub.2004.03.030 [DOI] [PubMed] [Google Scholar]
- Scherfer C., Qazi M. R., Takahashi K., Ueda R., Dushay M. S., Theopold U., et al. (2006). The Toll immune-regulated Drosophila protein Fondue is involved in hemolymph clotting and puparium formation. Dev. Biol. 295, 156–163 10.1016/j.ydbio.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Schmidt R. L., Trejo T. R., Plummer T. B., Platt J. L., Tang A. H. (2008). Infection-induced proteolysis of PGRP-LC controls the IMD activation and melanization cascades in Drosophila. FASEB J. 22, 918–929 10.1096/fj.06-7907com [DOI] [PubMed] [Google Scholar]
- Schmucker D., Chen B. (2009). Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev. 23, 147–156 10.1101/gad.1752909 [DOI] [PubMed] [Google Scholar]
- Seke Etet P. F., Vecchio L., Bogne Kamga P., Nchiwan Nukenine E., Krampera M., Nwabo Kamdje A. H. (2013). Normal hematopoiesis and hematologic malignancies: role of canonical Wnt signaling pathway and stromal microenvironment. Biochim. Biophys. Acta. 1835, 1–10 10.1016/j.bbcan.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Sethi G., Shanmugam M. K., Ramachandran L., Kumar A. P., Tergaonkar V. (2012). Multifaceted link between cancer and inflammation. Biosci. Rep. 32, 1–15 10.1042/BSR20100136 [DOI] [PubMed] [Google Scholar]
- Shia A. K. H., Glittenberg M., Thompson G., Weber A. N., Reichhart J. M., Ligoxygakis P. (2009). Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes. J. Cell. Sci. 122(Pt 24), 4505–4515 10.1242/jcs.049155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekhaus D., Haesemeyer M., Moffitt O., Lehmann R. (2010). RhoL controls invasion and Rap1 localization during immune cell transmigration in Drosophila. Nat. Cell. Biol. 12, 605–610 10.1038/ncb2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A, Hung T., Moroz T., Tran Q. M., Sidhu S., Cheney M. D., et al. (2010). Genetic manipulation of AML1-ETO-induced expansion of hematopoietic precursors in a Drosophila model. Blood 116, 4612–4620 10.1182/blood-2010-03-276998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A., Mandal L., Martinez-Agosto J. A., Banerjee U. (2009). Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell. 16, 756–763 10.1016/j.devcel.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A. J., Clark R. A. (1999). Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 10.1197/j.aem.2006.03.189 [DOI] [PubMed] [Google Scholar]
- Sorrentino R. P., Carton Y., Govind S. (2002). Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243, 65–80 10.1006/dbio.2001.0542 [DOI] [PubMed] [Google Scholar]
- Sorrentino R. P., Melk J. P., Govind S. (2004). Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics 166, 1343–1356 10.1534/genetics.166.3.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos R. K., A, Vidal M. (2011). Tumor invasion and metastasis in Drosophila: a bold past, a bright future. J. Genet. Genomics 38, 431–438 10.1016/j.jgg.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Stofanko M., Kwon S. Y., Badenhorst P. (2010). Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PLoS ONE 5:e14051 10.1371/journal.pone.0014051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Winfield M., Shaw T., Millard T. H., Woolner S., Martin P. (2008). Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 9, 465–471 10.1038/embor.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Wood W., Galko M. J., Redd M. J., Jacinto A., Parkhurst S. M., et al. (2005). Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell. Biol. 168, 567–573 10.1083/jcb.200405120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana A., Yano T., Mita S., Kotani A., Oshima Y., Kurata S. (2004). Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 23, 4690–4700 10.1038/sj.emboj.7600466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M., Saito H. (1998). A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–30 10.1016/S0092-8674(00)81619-0 [DOI] [PubMed] [Google Scholar]
- Tang H., Kambris Z., Lemaitre B., Hashimoto C. (2006). Two proteases defining a melanization cascade in the immune system of Drosophila. J. Biol. Chem. 281, 28097–28104 10.1074/jbc.M601642200 [DOI] [PubMed] [Google Scholar]
- Tanigaki K., Honjo T. (2007). Regulation of lymphocyte development by Notch signaling. Nat. Immunol. 8, 451–456 10.1038/ni1453 [DOI] [PubMed] [Google Scholar]
- Tepass U., Fessler L. I., Aziz a, Hartenstein V. (1994). Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120, 1829–1837 [DOI] [PubMed] [Google Scholar]
- Theopold U., Schmidt O., Söderhäll K., Dushay M. S. (2004). Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25, 289–294 10.1016/j.it.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Tipping M., Perrimon N. (2013). Drosophila as a model for context-dependent tumorigenesis. J. Cell. Physiol. 1–20 10.1002/jcp.24427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., Cullen D. E., et al. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 10.1016/j.cell.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Tucker P. K., Evans I. R., Wood W. (2011). Ena drives invasive macrophage migration in Drosophila embryos. Dis. Model Mech. 4, 126–134 10.1242/dmm.005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W., Constantinescu S. N. (2013). JAK/STAT signaling in hematological malignancies. Oncogene 32, 2601–2613 10.1038/onc.2012.347 [DOI] [PubMed] [Google Scholar]
- Valanne S., Wang J. H., Rämet M. (2011). The Drosophila Toll signaling pathway. J. Immunol. 186, 649–656 10.4049/jimmunol.1002302 [DOI] [PubMed] [Google Scholar]
- Waltzer L., Gobert V., Osman D., Haenlin M. (2010). Transcription factor interplay during Drosophila haematopoiesis. Int. J. Dev. Biol. 54, 1107–1115 10.1387/ijdb.093054lw [DOI] [PubMed] [Google Scholar]
- Wang L. D., Wagers A. J. (2011). Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell. Biol. 12, 643–655 10.1038/nrm3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wilhelmsson C., Hyrsl P., Loof T. G., Dobes P., Klupp M., et al. (2010). Pathogen entrapment by transglutaminase–a conserved early innate immune mechanism. PLoS Pathog. 6:e1000763 10.1371/journal.ppat.1000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F. L., Püttmann-Holgado R., Thomas F., Lamar D. L., Hughes M., Kondo M., et al. (2005). Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309, 1874–1878 10.1126/science.1116887 [DOI] [PubMed] [Google Scholar]
- Wertheim B., Kraaijeveld A. R., Schuster E., Blanc E., Hopkins M., Pletcher S. D., et al. (2005). Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 6, R94 10.1186/gb-2005-6-11-r94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. J., Ando I., Hultmark D. (2005). Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes Cells 10, 813–823 10.1111/j.1365-2443.2005.00883.x [DOI] [PubMed] [Google Scholar]
- Williams M. J., Wiklund M.-L., Wikman S., Hultmark D. (2006). Rac1 signalling in the Drosophila larval cellular immune response. J. Cell. Sci. 119(Pt 10), 2015–2024 10.1242/jcs.02920 [DOI] [PubMed] [Google Scholar]
- Wood W., Faria C., Jacinto A. (2006). Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell. Biol. 173, 405–416 10.1083/jcb.200508161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., Jacinto A. (2007). Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat. Rev. Mol. Cell. Biol. 8, 542–551 10.1038/nrm2202 [DOI] [PubMed] [Google Scholar]
- Wood W., Jacinto A., Grose R., Woolner S., Gale J., Wilson C., et al. (2002). Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell. Biol. 4, 907–912 10.1038/ncb875 [DOI] [PubMed] [Google Scholar]
- Wu S. C., Liao C. W., Pan R. L., Juang J. L. (2012). Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 11, 410–417 10.1016/j.chom.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Wu Y., Brock A. R., Wang Y., Fujitani K., Ueda R., Galko M. J. (2009). A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr. Biol. 19, 1473–1477 10.1016/j.cub.2009.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou B. P. (2009). Inflammation: a driving force speeds cancer metastasis. Cell Cycle 8, 3267–3273 10.4161/cc.8.20.9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier M. J., Williams M. J. (2011). The Rho-family GTPase Rac1 regulates integrin localization in Drosophila immunosurveillance cells. PLoS ONE 6:e19504 10.1371/journal.pone.0019504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Lu A., Peng Q., Ling Q. Z., Ling E. (2013). Activity of fusion prophenoloxidase-GFP and its potential applications for innate immunity study. PLoS ONE 8:e64106 10.1371/journal.pone.0064106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman-Rémy A., Regan J. C., Brandão A. S., Jacinto A. (2012). The Drosophila larva as a tool to study gut-associated macrophages: PI3K regulates a discrete hemocyte population at the proventriculus. Dev. Comp. Immunol. 36, 638–647 10.1016/j.dci.2011.10.013 [DOI] [PubMed] [Google Scholar]
- Zhao C., Chen A., Jamieson C. H., Fereshteh M., Abrahamsson A., Blum J. (2009). Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779 10.1038/nature07737 [DOI] [PMC free article] [PubMed] [Google Scholar]


