Ca2+ release through ryanodine receptor (RyR) intracellular Ca2+ release channels plays the leading role in regulation of myocyte contraction. However, Ca2+ signals arising from their less abundant inositol 1,4,5-trisphosphate sensitive counterparts (InsP3R), also localised to intracellular stores, is emerging as an important modulator of Ca2+-sensitive processes in cardiac myocytes. In this month’s issue of Circulation, Rota et al1 report that activation of InsP3R Ca2+ release channels by G-protein coupled receptor (GPCR) signalling regulates the electrical properties of human myocardium. Their results provide new insights into how InsP3 induced Ca2+ release (IICR) modulates cardiomyocyte Ca2+ handling and contractility through influencing sarcolemmal membrane potential and ionic currents.
Ca2+ regulation of myocyte contractility
An elevation in intracellular Ca2+ (known as contractile Ca2+) is a critical step in the sequence of events, termed excitation contraction coupling (ECC) that couples depolarisation of the plasma membrane by the propagating action potential with myocyte contraction.2 Ca2+ is released from sarcoplasmic reticulum (SR) Ca2+ stores via RyRs, which are activated by Ca2+ entering the cell through L-type voltage-gated Ca2+ channels (LTCC) opened in response to membrane depolarisation. The functional coupling between Ca2+ entry through LTCC and RyRs is enabled by the proximity of the sarcolemma and SR, which lie 10-15 nm apart in specialised domains, termed dyads (Fig. 1). Invaginations of the sarcolemma at 1.8 μ intervals across the myocyte distribute these signalling domains throughout the cell volume allowing a rapid and homogenous cell-wide Ca2+ elevation during an action potential.2
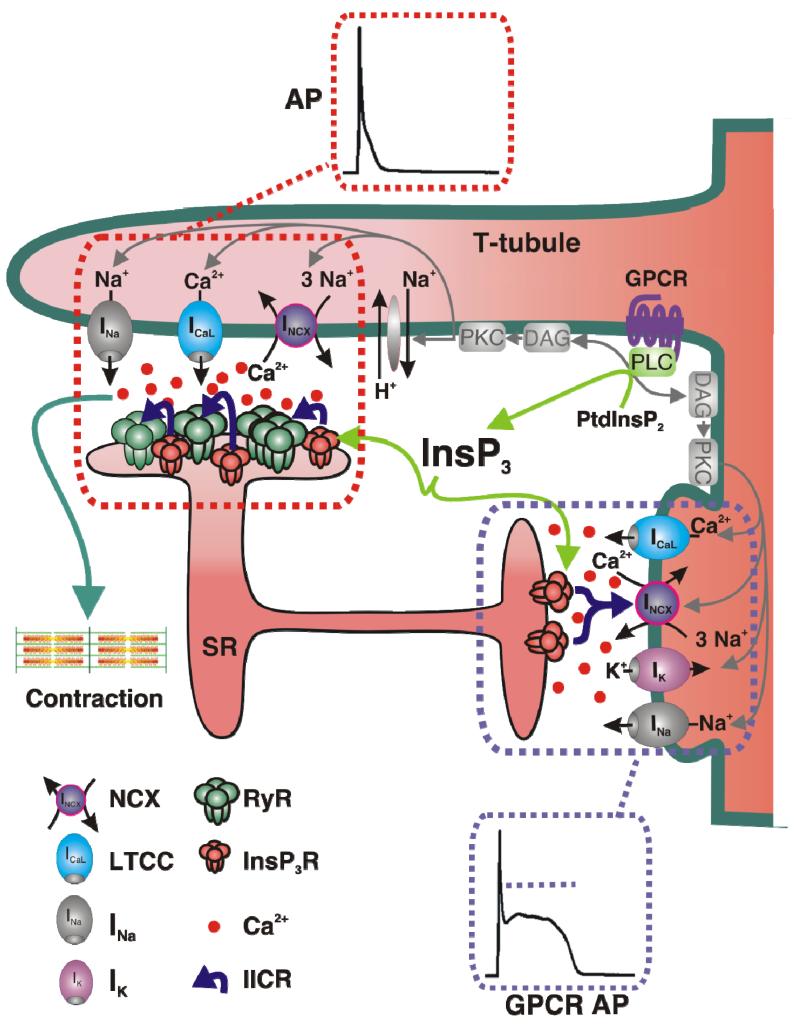
Figure 1. Dual control of excitation contraction coupling (ECC) by GPCR/InsP3 signalling.
The sarcolemma of the cardiac myocyte forming a single t-tubule and a caveolus are shown together with the machinery underlying ECC. These membrane regions express GPCR, LTCC, IK, INa, NCX and NHE. Juxtaposed to the t-tubule and caveolar membrane domains are cisternae of the SR, forming dyadic (red box) and peripheral couplings respectively (purple box). The distance between the sarcolemma and underlying SR in both these membrane domains is between 10 and 15 nm allowing rapid Ca2+ diffusion across the dyadic cleft. Based on the model proposed by Rota et al, in which IICR influences NCX and prolongs AP duration (GPCR AP) independently of Ca2+ release via RyRs, InsP3Rs are localised to a junctional coupling devoid of RyRs (purple box). InsP3Rs have also been proposed to influence Ca2+ release via RyRs due a mechanism involving their co-localisation at the dyad (red box). At this location, IICR contributes minimally to regulation of the AP (red box) but could influence NCX activity secondary to RyR activation. InsP3 is produced downstream of GPCR stimulation of PLC and hydrolysis of PtdInsP2. PLC hydrolysis of PtdInsP2 also generates DAG, which can subsequently activate PKC. PKC influences the activity of NCX, NHE, Na+, K+ and Ca2+ channels on the sarcolemma (in grey), which may also affect the electrophysiological properties of the ventricular cardiomyocyte. For clarity, not all proteins involved in ECC are shown in this cartoon.
InsP3R – a different route for modulating contractile Ca2+ in ventricular myocytes
Like RyRs, InsP3Rs are large tetrameric intracellular Ca2+ release channels located in the SR/Endoplasmic Reticulum intracellular Ca2+ store that are activated by Ca2+.3 Unlike RyRs, InsP3Rs also require InsP3 for activation, thereby making them subject to control by extracellular ligands that engage phospholipase C (PLC)-activating plasma membrane receptors, including GPCRs and receptor tyrosine kinase.3 Of the three mammalian InsP3R isoforms (InsP3R1, 2 and 3), InsP3R2 predominates in cardiac muscle.4 Although InsP3Rs are much less abundant than RyRs in the heart (<1:50-1:100 of RyRs),4 substantial evidence exists for InsP3Rs and InsP3-induced Ca2+ signalling in atrial myocytes.5 However, due to their even lower expression in the ventricle, the importance and contribution of InsP3Rs to Ca2+ signals during ECC has been questioned.5
In the current issue of Circulation, Rota et al1 report that human ventricular myocytes express functional InsP3R Ca2+ release channels that are responsible for the modulation of ECC by GPCR ligands such as ATP and endothelin-1 (ET-1). Although a role for InsP3 in stimulating cardiac Ca2+ release was described over two decades ago,6 Rota et al1 are the first to show that the InsP3/InsP3R signalling pathway plays an important role in regulating ECC in human ventricular myocytes. Previous work had suggested that Ca2+release from InsP3R channels located in dyadic cleft augments RyR-mediated ECC and contractility (Fig. 1).7 Using an elegant combination of studies in single ventricular myocytes and intact myocardium from human and mouse, Rota et al1 demonstrate a new mechanism by which InsP3 regulates ECC: InsP3R Ca2+ release signals via an unidentified mechanism to membrane ion channels and the cardiac Na+/Ca2+ exchanger (NCX), resulting in depolarized resting membrane potential and action potential (AP) prolongation (Fig. 1). Hence, Ca2+ release from InsP3R does NOT contribute to contractile Ca2+ directly but rather, the enhanced Ca2+ transients and contractility induced by GPCR ligands and InsP3R signalling are the consequence of the InsP3-induced modulation of membrane ion fluxes. Moreover, since RyR inhibition did not interfere with the InsP3-induced AP regulation, yet intracellular Ca2+ buffering abolished the InsP3 effects on the membrane potential, these results suggest the existence of local Ca2+ signalling microdomains of InsP3R and plasma membrane ion channels or transporters that are distinct from the dyadic domains involved in ECC (Fig. 1), akin to local InsP3-dependent perinuclear Ca2+ signalling involved in excitation-transcription coupling.8, 9 Evidence for perinuclear Ca2+ microdomains containing InsP3Rs has been identified by Ca2+ imaging,8, 9 immunofluorescence9 and electronmicroscopy.10 Whether the same structural domains also couple to sarcolemmal ion channels, or whether such signalling occurs in caveolae-rich microdomains with LTCC that are not involved in ECC11 remains to be seen. Nevertheless, the functional data provided by Rota et al suggest that GPCR/InsP3 signalling is not only a critical player for hypertrophic gene regulation8, 9, 12 but also regulates electrophysiological properties and excitability of cardiac muscle.
InsP3 signalling, punching above its weight to control cardiomyocyte function
In ventricular myocardium, InsP3Rs are expressed at exceedingly low levels relative to RyR. The Ca2+ current they conduct is only a quarter of that of RyRs.13 InsP3 is generated at a very low level in cardiac myocytes relative to other tissues,14 thereby limiting the number of channels opened. Together with the exponential decay in Ca2+ concentration with distance from the source channel,15 the low density and Ca2+ flux through InsP3Rs would prevent a significant elevation in intracellular Ca2+. How can this channel then contribute so greatly to cardiomyocyte function – both in regulating ECC and promoting hypertrophic gene transcription? A likely mechanism involves localisation of the InsP3R proximal to its signalling effectors. Analogous to P/Q channels and BKCa channels in smooth muscle,16 the target of InsP3 mediated Ca2+ signals is exposed to the high concentration of Ca2+ at the mouth of the channel. As illustrated in Fig. 1, InsP3Rs localised to the dyad within a few nm of RyRs could “prime” RyRs to activation by Ca2+ influx via LTCC. Moreover, association of Ca2+ arising from InsP3Rs with resident cytosolic Ca2+ buffers would reduce the buffering capacity of the cytosol in the vicinity of the dyad thereby allowing Ca2+ subsequently released via RyRs to have a greater impact upon free Ca2+ levels. In support of this InsP3R-RyR coupling model, block of RyRs with ryanodine, reduces the amplitude of InsP3-stimulated elementary Ca2+ release events in cardiac myocytes.7 This mechanism has also been proposed to explain the increase spark frequency in rabbit and rat ventricular exposed to InsP3,17, 18 and may underlie the positive inotropic action of GPCR/InsP3 signalling in human myocytes. This crosstalk between InsP3Rs and RyRs is likely to make a greater contribution to the augmented GPCR/InsP3 signalling observed in hypertrophic and failing hearts that exhibit significantly elevated InsP3R expression levels.1, 18 Interestingly, Rota et al do not find evidence for InsP3R-RyR coupling in mouse ventricular myocytes. In mice, the positive inotropic effect of GPCR agonists and InsP3 appears to be exclusively the consequence of AP prolongation and suggests an alternative model of coupling of InsP3R that controls cardiac excitation (Fig. 1). The functional results by Rota et al1 suggest that in murine myocardium a pool of functional InsP3R exists outside the dyad that couple directly to membrane ion channels and/or transporters membrane. Rota et al1 demonstrate that GPCR/InsP3 signalling activates NCX and suggest enhanced NCX inward currents as a culprit responsible for the InsP3-induced AP prolongation and membrane depolarization. However, it appears unlikely that the NCX effect reported by Rota et al1 is solely responsible, since enhanced NCX, while prolonging the AP in the short term, would eventually deplete cellular Ca2+ stores and result in reduced contractility. Thus, InsP3 signalling likely modifies other membrane ion channels that either directly prolong the cardiac AP or cause a net increase in Ca2+ influx into the cell. Consistent with this idea, a profound effect on membrane electrophysiology was observed in mouse ventricular myocytes exposed to Fas ligand.19 Fas ligand caused an InsP3-dependent increase in AP duration, depolarized resting membrane potential, decreased Ito and increased arrhythmic events.19 Furthermore, there is extensive crosstalk between signalling messengers downstream of GPCR other than InsP3 that also regulate the activity of LTCC, NCX and the sodium hydrogen exchanger (NHE)20 (Fig. 1), which likely are also involved in the regulation of resting membrane potential and AP duration in response to IICR.
Acknowledgements
Work in the Roderick laboratory is funded by the BBSRC (Epigenetics ISPG) and the British Heart Foundation. The Knollmann laboratory is supported in part by grants from the US National Institutes of Health and the American Heart Association.
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- 1.Rota M, Signore S, Sorrentino A, Ferreira-Martins J, kannappan R, Shafie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, Sanada F, Ogorek B, Zheng H, Liu X, del Monte F, D’Allessandro D, Wunimenghe O, Michler R, Hosoda T, Goichberg P, Leri A, Kajstura J, Anversa P. Inositol 1,4,5-trisphosphate Receptors and Human Left Ventricular Myocytes. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.002764. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Foskett JK, White C, Cheung K-H, Mak D-OD. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 5.Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosek TM, Williams MF, Zeigler ST, Godt RE. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986;250:C807–811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- 7.Harzheim D, Movassagh M, Foo RS-Y, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:11406–11411. doi: 10.1073/pnas.0905485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009;33:472–482. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol. 2010:1–9. doi: 10.1016/j.yjmcc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res. 2010;107:659–666. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J Biol Chem. 2006;281:608–616. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 15.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart J-O, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus H-G, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science (New York, NY) 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 17.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 18.Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F, Sjaastad I, Liu N, Catalucci D, Mikoshiba K, Hisatsune C, Okkenhaug H, Andrews SR, Bootman MD, Roderick HL. Mutual antagonism between IP3RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J Cell Biol. 2012;199:783–798. doi: 10.1083/jcb.201111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shilkrut M, Gealekman O, Rosen D, Berke G, Woodcock E, Binah O. Electrophysiologic perturbations and arrhythmogenic activity caused by activation of the Fas receptor in murine ventricular myocytes: role of the inositol trisphosphate pathway. J Cardiovasc Electrophysiol. 2001;12:185–195. doi: 10.1046/j.1540-8167.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 20.Drawnel FM, archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol. 2013;168:296–317. doi: 10.1111/j.1476-5381.2012.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]