Abstract
[Purpose] The aim of this study was to clarify the effects of the ROM exercise on joint components according to histopathological analysis. [Subjects and Methods] In total, twenty-six 9-week-old adult male Wistar rats were used in this study. The rats were randomly divided into three groups, the immobilization group (n=10), exercise group (n=10), and control group (n=6). The immobilization group and exercise group were anaesthetized and operated on under sterile conditions. The right knee joints in the immobilization group and exercise group were immobilized with external fixation at 120 degrees of flexion. Range of motion exercise was started from the day after immobilization. ROM exercise was performed in the exercise group once a day for 3 minutes, 6 days a week, for 2 weeks. [Result] The joint capsule in the immobilization group and exercise group showed narrowing of the collagen bundles in interstitial spaces but was less dense in the control group. In the immobilized group, a hyperplastic reaction was associated with infiltration into the articular cavity and adhesion to the surface of the articular cartilage. Conversely, in the exercise group, hyperplasia of tissue was localized to the synovial membrane. [Conclusion] This finding may suggest that ROM exercise induces some changes within the joint components and tissue metabolism.
Key words: Joint immobilization, Contracture, ROM exercise
INTRODUCTION
Prolonged immobilization reduces passive range of motion of joints, creating joint contractures1,2,3,4,5,6). This may cause inconveniences with regard to activities of daily living; thus physiotherapy is often used to treat problems related to joint movement limitation. In previous studies, we have reported the histopathological changes that occur in the rat knee joint components during immobilization; however, the changes have not been fully clarified. In addition, controversies still remain regarding the pathosis of joint contracture. Joint immobilization induces muscle atrophy, articular cartilage loss, and proliferation of connective tissue within the joint space1, 7). Immobilization of joints surrounded by edematous soft tissue often produces joint stiffness more quickly and more severely than immobilization of nonedematous extremities8). After two weeks of joint immobilization, the number of synoviocytes in the posterior synovial intima was increased compared with a control group9). Watanabe reported atrophy of fat cells, proliferation of fibroblasts, and narrowing of the spaces between collagen fibers in the rat knee joint after immobilization10). Some studies reported changes in joint components caused by remobilization after immobilization8, 11,12,13,14,15). The range of motion of rat knees immobilized for 8 weeks remained substantially reduced after a 4-week period of unassisted remobilization16). But histopathological findings of the joint components were not described in that study. In the precedent studies, histopathological changes in joint components after remobilization and/or reloading during joint contracture were observed. The purpose of these studies was to observe the healing process. But studies about prevention of joint contracture have not been performed. A few studies have reported the effect of exercise during an immobilization period17, 18). Tatsuta related that exercise during immobilization periods did not improve range of motion but also that granulation tissue did not infiltrate into the articular cavity19). In this study, we used external fixation in order to immobilize the joint and subjected the rats to exercise during the immobilization period. Thereafter, we observed the influence of ROM exercise on the immobilized joint. The purpose of this study was to clarify the effects of the ROM exercise on joint components according to histopathological analysis.
SUBJECTS AND METHODS
The protocol for these experiments were approved by the Animal Care Committee and Institutional Ethics Committee of Kanazawa University, and all procedures for animal care and treatment were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University. In total, twenty-six 9-week-old adult male Wistar rats (weighing 250–275 g) were used in this study. The rats were randomly divided into three groups, the immobilization group (n=10), exercise group (n=10), and control group (n=6). The animals were kept under normal conditions for one week before the start of experiments in order to acclimatize them to the environment. They were housed, 1 per cage, in a room maintained under a 12-hour light-dark cycle. Food and water were given ad libitum.
The immobilization group and exercise group were anaesthetized (pentobarbital sodium, 40 mg/kg bw, ip) and operated on under sterile conditions. The right knee joints of the rats in the immobilization group and exercise group were immobilized with external fixation at 120 degrees of flexion. A 2-mm longitudinal incision was made in the right hind thigh skin; the right femur and tibia were pierced by Kirschner wire with a diameter of 0.8 mm, and the wire was then bent (Fig. 1). Kirschner wires were fixed outside the wounds in a knee flexion posture with screw (4 mm diameter) and nuts. The instruments for external fixation weighed 6 to 7 g. The rat’s knee flexion angle (120 degrees) was determined by the method described in our previous studys13, 21, 22). This immobilization method is low cost, and immobilization can be reversed to perform exercise and then reestablished easily. Although the right hindlimb knee joint was immobilized, the rats were able to move freely without restriction in their respective cages and had free access to food and water.
Fig. 1.
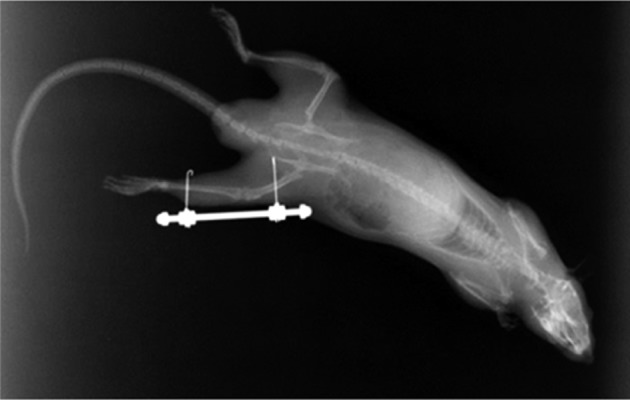
The right femur and tibia were pierced by Kirschner wire with a diameter of 0.8 mm, and the wire was then bent (X-ray).
Range of motion exercise (ROM Ex) was started from the day after immobilization. ROM Ex was performed in the exercise group once a day for 3 minutes, 6 days a week, for 2 weeks. ROM Ex was performed in the left lateral decubitus position under anaesthesia. The mean torque necessary for the rat to passively extend the knee joint was 1 N, and this value was adopted as the strength during the ROM Ex. The exercise consisted in 18 cycles of 10 seconds of exercise (a total of 3 minutes). The exercise time was determinate according to the results of a preliminary experiment. In the first 5 seconds of the exercise cycle, the rat’s right hind limb was maintained in 120 degrees of hip flexion, and in the next 5 seconds, the limb was pulled in the caudal direction with 1 N force applied by a manual force gauge. The ROM exercise intensity was controlled using a force gauge continuously during the 3 minutes. After each exercise, the rat knee was re-immobilized by external fixation at 120 degrees of knee flexion. The control group did not undergo surgery and was not subjected to exercises.
After 2 weeks of intervention, the ROMs of all rat knees (extension limitation) were measured under anaesthesia. In the rats in the immobilization and exercise groups, the external fixation was removed, and shortly after the tight hind limbs of the rats were later pulled with 1 N force in the caudal direction. The knee angles were measured using a goniometer for human fingers. After angle measurement, the knees of the rats were re-immobilized at 120 degrees of flexion by external fixation. Later, the rats were sacrificed by intraperitoneal injection of an overdose of pentobarbital sodium. Immediately after euthanasia, their right hind limbs were disarticulated and dissected out from the hip joint for histopathological study. The right knees of the rats were fixed in 10% buffered formalin and decalcified. After decalcification, they knees were cut in the sagittal plane at the level of the anterior and posterior cruciate ligament. Following neutralization with 5% sodium sulfate solution, the specimens were fixed, decalcified, and neutralized at 4 °C for 72 hours. The specimens were embedded in paraffin, sectioned at 3 μm, and mounted on microscope slides. All sections were stained with hematoxylin and eosin and toluidine blue stains and used for observation in a histopathological examination. Histopathological analysis was performed by examining the synovial membrane, the joint capsule, and the joint cartilage.
Knee limitation data were statistically analyzed using IBM SPSS Statistics for Windows (version 19.0.1, IBM Corp., Armonk, NY, USA). ROM was analyzed using one-factor analysis of variance (ANOVA) with Bonferroni post-hoc multiple comparisons. A value of p<0.05 was accepted as statistically significant. All results are reported as mean±SD values.
RESULTS
All animals survived the experimental period. The histopathological analysis showed no swelling or signs of infection in the knees as a result of the intervention.
The mean values of knee ROM (extension limitation angle) were 19.3±3.0 degrees, 77.3±7.4 degrees, and 51.0±4.9 degrees in the control group, immobilization group, and exercise group, respectively, and each groups showed a statistically significant difference (p<0.05). The mean body weights were 326.0±8.8 g, 310.6±16.6 g, and 304.5±7.7 g in the control group, immobilization group, and exercise group, respectively. There were statistically significant differences (p<0.05) between the control group and other groups.
In the control group, the joint capsule was typically composed of coarse and relatively loose fibrous connective tissues. The joint capsule in the immobilization group and exercise group showed narrowing of the collagen bundles in interstitial spaces but was less dense in the control group (Fig. 2). The surface of the articular cartilage in animals of the control group was smooth, and the hyaline cartilage was directly exposed to the articular cavity (Fig. 3). Chondrocytes were observed in the outer layer of the joint cartilage. The cartilage matrix of the hyaline cartilage was stained with toluidine blue. In the immobilized group, proliferation of granulation-like tissue connecting with synovial tissue was observed. The articular cartilages were covered with the proliferating tissue composed of fibroblast-like spindle-shaped cells (Fig. 4). Negative stain of the proliferating tissue areas were illustrated by toluidine blue stain. Conversely, in the exercise group, hyperplasia of membrane-like tissue was localized to the synovial membrane, and the infiltration of fibroblast-like spindle-shaped cells was localized to the articular cavity (Fig. 5). Notably, the presence of erythrocytes that had leaked into the articular cavity was observed in some exercise group animals (n=6), suggesting the presence of hemorrhage after ROM Ex.
Fig. 2.
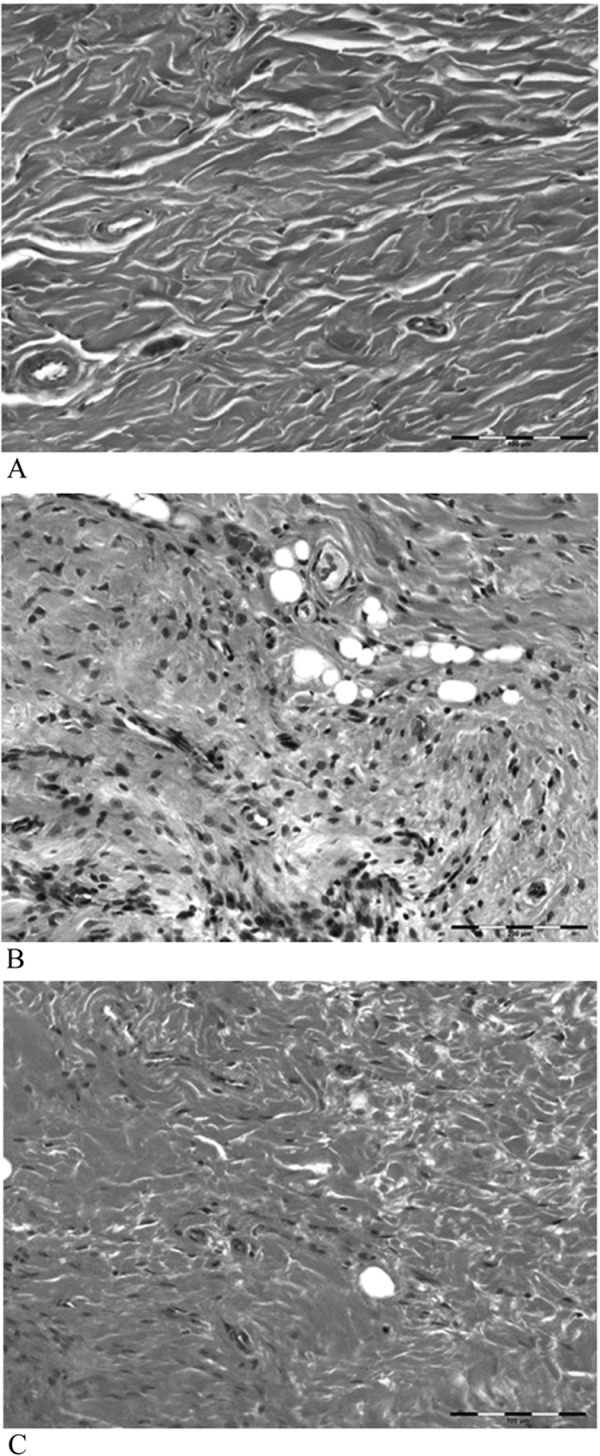
The joint capsule in the control group was typically composed of coarse and relatively loose fibrous connective tissues, but those in the immobilization group and exercise group showed narrowing of the collagen bundles of interstitial spaces; the joint capsule was less dense in the control group. A, control group; B, immobilization group; C, exercise group. HE stain ×200
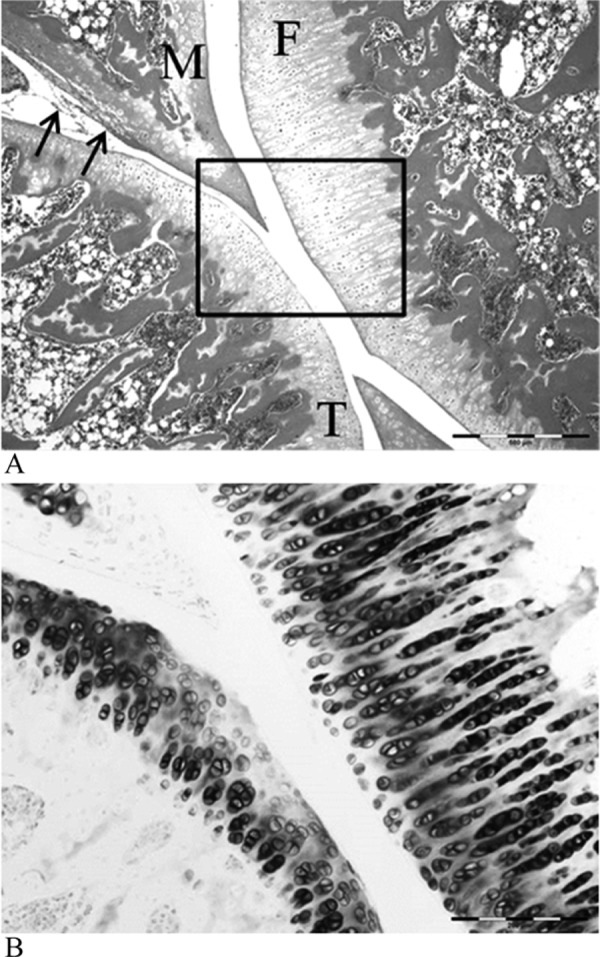
Fig. 3.
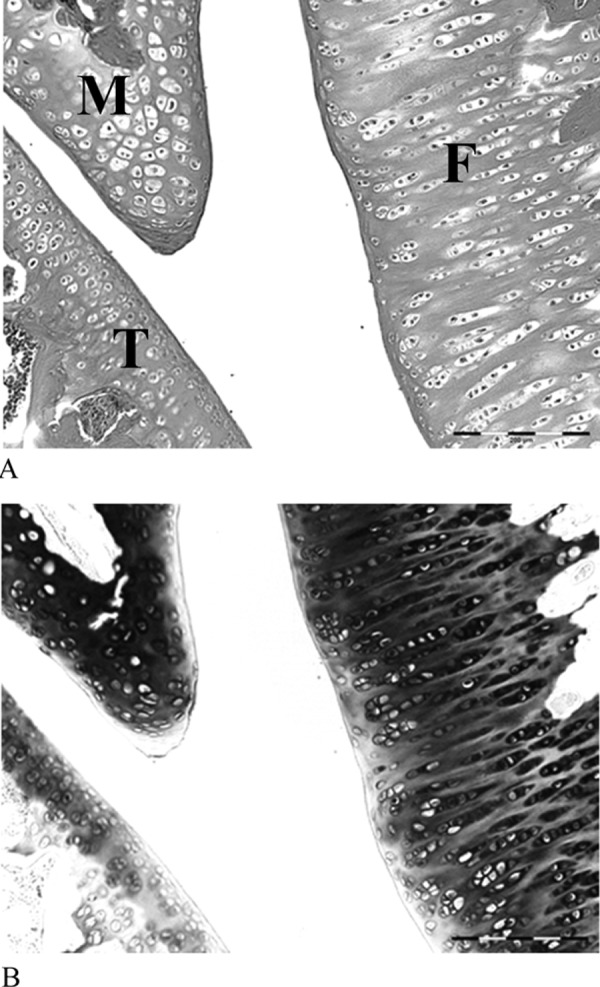
The surface of the articular cartilage in the control group. The surface of the articular cartilage was smooth, and the hyaline cartilage was directly exposed to the articular cavity (A); the cartilage matrix of the hyaline cartilage was stained with toluidine blue (B). F, femur; T, tibia; M, meniscus. HE stain ×200
Fig. 4.
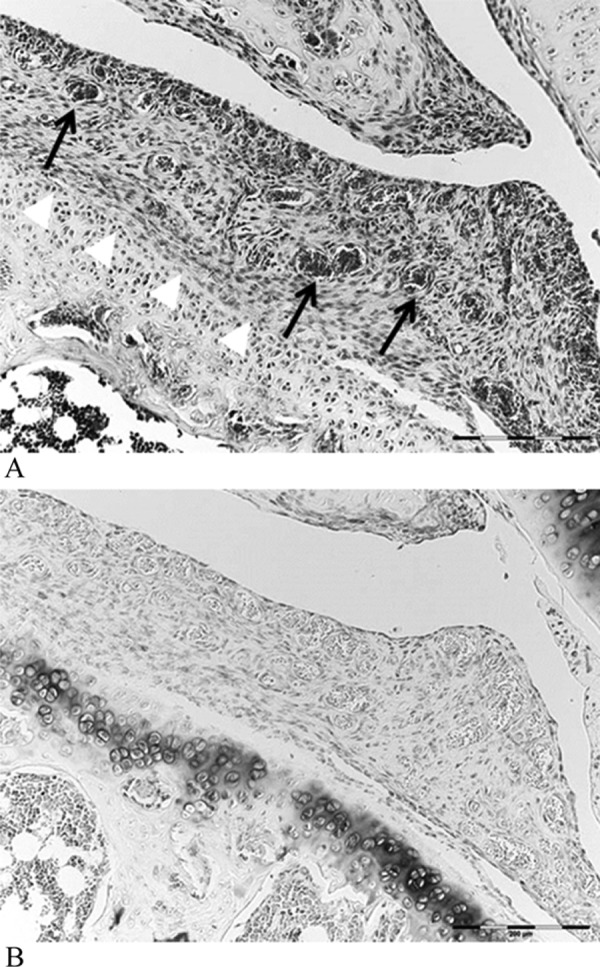
Hyperplastic tissues infiltrated into the articular cavity and adhered to the surface of the articular cartilage (white arrow head), and infiltration of vessels (black arrows) was observed. HE stain (A), toluidine blue stain (B) ×200
Fig. 5.
Hyperplasia of tissue was localized to the meniscus (black arrows), and the surface of the articular cartilage was smooth (A). HE stain ×100. The cartilage matrix of the hyaline cartilage was stained with toluidine blue (B). Toluidine blue stain ×200
DISCUSSION
It is well known that immobilization causes joint contracture and articular cartilage degeneration. There have been many reports concerning joint components changing after immobilization. Proliferation of intracapsular connective tissue and the formation of adhesions are primary responses to limitation of motion5). In this study, we observed the presence of granulation tissue-like organization and infiltration in the joint cavity after two weeks of knee immobilization in rats. The cartilage appeared to be more or less confluent with the overlying connective tissue20). These findings are consistent with those reported in our previous study21, 22). During the first two weeks of immobilization, ROM limitation caused damage in the myogenic component, and after two weeks, the arthrogenic component constituted more than 80% of the total restriction in ROM20). This suggests that in our contracture model, the myogenic factor was stronger than the arthrogenic factor for ROM limitation. ROM limitation was significantly decreased by exercise, presumably due to its property that contributes to the maintenance of muscle extensibility. However, changes were found not only in the muscular component but also in the joint component. Proliferation of intracapsular connective tissue and the formation of adhesions are primary responses to limitation of motion20). In the present study, there were clearly differences between the immobilization group and the exercise group. Extensive hyperplasia of fibroblasts was observed in all animals in the immobilization group, but it was less severe and was focally distributed in the animals in the exercise group. This finding may suggest that ROM exercise induces some change within the joint components and tissue metabolism. Connective-tissue proliferation was present in all three of the knees immobilized for fifteen days, and it was well established at thirty days20). The changes in the joint capsule were observed from the early period, and there were no important differences between the immobilized group and exercised group. In our model, we did not observe a significant influence of exercise on the joint capsule; however we cannot rule out that some changes may be observe in response to other exercise methods. We observed slight intra-articular hemorrhage, which indicates that the exercises used in this study might cause laceration of granulation tissue and adhesion. In this study, it was clarified that ROM Ex maintains range of motion and reduces the changes in the joint components. But the effective frequency and strength were not investigated. Therefore, it is necessary to examine several kinds of frequency and the exercise strength in a future study. A number of studies have performed remobilization following a joint immobilization period, and some have subjected animals to exercise during an immobilization period. These reported studies were still controversial. We necessary to find for effective ROM exercise method.
Acknowledgments
We are grateful to the preparation staff in the Morpho-Functional Pathology Department, Graduate School of Medical Science, Kanazawa University. We also thank Pleiades T Inaoka of the Department of Physical Therapy, Kanazawa University. This research was supported by a Grant-in-Aid for Scientific Research (22500454) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Field PL, Hueston JT: Articular cartilage loss in long-standing flexion deformity of the proximal interphalangeal joints. Aust N Z J Surg, 1970, 40: 70–74 [DOI] [PubMed] [Google Scholar]
- 2.Enneking WF, Horowitz M: The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am, 1972, 54: 973–985 [PubMed] [Google Scholar]
- 3.Videman T: Experimental osteoarthritis in the rabbit: comparison of different periods of repeated immobilization. Acta Orthop Scand, 1982, 53: 339–347 [DOI] [PubMed] [Google Scholar]
- 4.Schollmeier G, Uhthoff HK, Sarkar K, et al. : Effects of immobilization on the capsule of the canine glenohumeral joint. Clin Orthop Relat Res, 1994, 304: 37–42 [PubMed] [Google Scholar]
- 5.Trudel G, Jabi M, Uhthoff HK: Intraarticular tissue proliferation after immobility: Methods of assessment and preliminary results in rat knee joint. J Rheumatol, 1998, 25: 945–950 [PubMed] [Google Scholar]
- 6.Vanwanseele B, Lucchinetti E, Stussi E: The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage, 2002, 10: 408–419 [DOI] [PubMed] [Google Scholar]
- 7.Evans B, Eggers G, Butler J: Experimental immobilization and remobilization of rat knee joints. J Bone Joint Surg Am, 1960, 42: 737–758 [Google Scholar]
- 8.Peacock EE: Some biochemical and biophysical aspects of joint stiffness: Role of collagen synthesis as opposed to altered molecular bonding. Ann Surg, 1966, 164: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudel G, Jabi M, Uhthoff HK: Localized and adaptive synoviocyte proliferation characteristics in rat knee joint contractures secondary to immobility. Arch Phys Med Rehabil, 2003, 84: 1350–1356 [DOI] [PubMed] [Google Scholar]
- 10.Watanabe M, Hoso M, Hibino I, et al. : Histopathological changes of joint capsule after Joint immobility compared with aging in rats. J Phys Ther Sci, 2010, 22: 369–374 [Google Scholar]
- 11.Takemura K, Hoso M, Yoshikubo H, et al. : Histopathological effects of the stretching on joint components after two-week knee joint immobilization in rats. Rigaku Ryohogaku, 2004, 31: 76–85(in Japanese) [Google Scholar]
- 12.Usuba M, Miyanaga M, Miyakawa S, et al. : Effect of heat in increasing the range of knee motion after the development of a joint contracture: an experiment with an animal model. Arch Phys Med Rehabil, 2006, 87: 247–253 [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Hoso M, Yoshikubo H, et al. : Histopathological effects of the stretching on joint capsule after contracture; a study of knee joint immobility model in the rat. Rigakuryoho Kagaku, 2008, 24: 403–409(in Japanese) [Google Scholar]
- 14.Peltz CD, Sarver JJ, Dourte LM, et al. : Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res, 2010, 28: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando A, Suda H, Hagiwara Y, et al. : Experimental joint contracture correction with low torque long duration repeated stretching. Tohoku J Exp Med, 2011, 224: 77–85 21558763 [Google Scholar]
- 16.Trudel G, Zhou J, Uhthoff HK: Four weeks of mobility after 8 weeks of immobility fails to restore normal motion. Clin Orthop Relat Res, 2008, 466: 1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono T, Tsuboi M, Oki S, et al. : Preliminary report: another perspective on the effect of prolonged stretching for joint contractures. J Phys Ther Sci, 2007, 19: 97–101 [Google Scholar]
- 18.Tatsuta N, Nakajima M, Akiyama J, et al. : A device for external fixation for immobilization of the knee joint in rats. Rigakuryoho Kagaku, 2008, 23: 73–77(in Japanese) [Google Scholar]
- 19.Tatsuta N, Nakajima M, Akiyama J, et al. : Effect of range of motion exercise on different trequency of joint movable range with fixed joint. Rigakuryoho Kagaku, 2009, 24: 427–433(in Japanese) [Google Scholar]
- 20.Trudel G, Uhthoff H: Contractures Secondary to Immobility: Is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil, 2000, 81: 6–13 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Hoso M, Takemura K, et al. : Histopathological changes in joint components during contracture –A studyof the long term knee joint immobility model in the rat. Rigakuryoho Kagaku, 2007, 22: 67–75(in Japanese) [Google Scholar]
- 22.Matsuzaki T, Hoso M, Tachino K: Establishment of new method for making rat knee joint contracture and changes of the joint components. J Turuma Health Sci Soc, 2008, 32: 43–47(in Japanese) [Google Scholar]


