Abstract
Objectives
Our goal was to evaluate the role of three anesthetic techniques in altering the stress response in children undergoing surgery for repair of congenital heart diseases utilizing cardiopulmonary bypass in the setting of fast tracking or early tracheal extubation. Furthermore, we wanted to evaluate the correlation between blunting the stress response and the perioperative clinical outcomes.
Design
Prospective, randomized, double-blinded study.
Setting
Single center from December 2008 to May of 2011.
Patients
Forty-eight subjects (low-dose fentanyl plus placebo, n = 16; high-dose fentanyl plus placebo, n = 17; low-dose fentanyl plus dexmedetomidine, n = 15) were studied between ages 30 days to 3 years old who were scheduled to undergo repair for a ventricular septal defect, atrioventricular septal defect, or Tetralogy of Fallot.
Methods
Children undergoing surgical repair of congenital heart disease were randomized to receive low-dose fentanyl (10 mcg/kg; low-dose fentanyl), high-dose fentanyl (25mcg/kg; high-dose fentanyl), or low-dose fentanyl plus dexmedetomidine (as a 1 mcg/kg loading dose followed by infusion at 0.5mcg/kg/hr until separation from cardiopulmonary bypass. In addition, patients received a volatile anesthetic agent as needed to maintain hemodynamic stability. Blood samples were tested for metabolic, hormonal and cytokine markers at baseline, after sternotomy, after the start of cardiopulmonary bypass, at the end of the procedure and at 24 hours postoperatively.
Measurements and Main Results
Forty-eight subjects (low-dose fentanyl plus placebo, n = 16; high-dose fentanyl plus placebo, n = 17; low-dose fentanyl plus dexmedetomidine, n = 15) were studied. Subjects in the low-dose fentanyl plus placebo group had significantly higher levels of adrenocorticotropic hormone, cortisol, glucose, lactate, and epinephrine during the study period. The lowest levels of stress markers were seen in the high-dose fentanyl plus placebo group both over time (adrenocorticotropic hormone, p = 0.01; glucose, p = 0.007) and at individual time points (cortisol and lactate at the end of surgery, epinephrine poststernotomy; p < 0.05). Subjects in the low-dose fentanyl plus dexmedetomidine group had lower lactate levels at the end of surgery compared with the low-dose fentanyl plus placebo group (p < 0.05). Although there were no statistically significant differences in plasma cytokine levels between the three groups, the low-dose fentanyl plus placebo group had significantly higher interleukin-6:interleukin-10 ratio at 24 hours postoperatively (p < 0.0001). In addition, when compared with the low-dose fentanyl plus placebo group, the low-dose fentanyl plus dexmedetomidine group showed lower norepinephrine level from baseline at poststernotomy, after start of cardiopulmonary bypass, and end of surgery (p ≤ 0.05). Subjects in the low-dose fentanyl plus placebo group had more postoperative narcotic requirement (p = 0.004), higher prothrombin time (p ≤ 0.03), and more postoperative chest tube output (p < 0.05). Success of fast tracking was not significantly different between groups (low-dose fentanyl plus placebo 75%, high-dose fentanyl plus placebo 82%, low-dose fentanyl plus dexmedetomidine 93%; p = 0.39).
Conclusions
The use of low-dose fentanyl was associated with the greatest stress response, most coagulopathy, and highest transfusion requirement among our cohorts. Higher dose fentanyl demonstrated more favorable blunting of the stress response. When compared with low-dose fentanyl alone, the addition of dexmedetomidine improved the blunting of the stress response, while achieving better postoperative pain control.
Keywords: cytokines, early extubation after pediatric cardiac surgery, pediatric cardiac surgery, stress response after cardiopulmonary bypass
Patients undergoing cardiac surgery experience a substantial stress response mediated by the release of stress hormones and cytokines (1–4). Cardiopulmonary bypass (CPB) accentuates this response because of the activation of the immune system by direct contact of the blood to foreign surfaces of the CPB circuit, ischemia-reperfusion injury, and systemic endotoxemia due to translocation of endotoxin from the gut. Although the stress response is well defined in adults, it is variable and less defined in children.
Anand et al (1, 5–8) were among the first to demonstrate that neonates and infants can mount a stress response, with the release of metabolic and hormonal markers. In addition, Anand et al (9) have shown that the use of a high-dose opioid technique effectively blunted or abolished this stress response.
Although blunting the stress response has been the goal of many anesthetic techniques in an effort to improve perioperative outcome after pediatric cardiac surgery (10–14), many studies have failed to show the correlation between blunting the stress response and improved clinical outcome (3, 15–18).
Over the last decade, many retrospective studies have demonstrated the safety of fast tracking, with early tracheal extubation after cardiac surgery (19–26). The key component of the anesthetic management of patients who are in the fast-track pathway is the use of lower total dose of opioid in addition to inhalational anesthetic agents, either alone (20) or in addition to other adjunct medications, such as propofol (27), or very short-acting opioids, such as remifentanil (28). Also, some advocate the use of regional techniques, such as thoracic epidurals or single-shot caudal morphine, to facilitate early extubation (19, 29, 30).
Recently, dexmedetomidine has been increasingly used as an adjunct during cardiac surgery in both children and adults (31–35). Mukhtar et al (33) reported that the use of dexmedetomidine in addition to low-dose fentanyl has resulted in lower levels of hormonal and metabolic stress markers, such as glucose, epinephrine, norepinephrine, and cortisol, when compared with low-dose fentanyl alone.
We therefore designed a prospective, randomized, double-blinded, clinical trial to test the hypothesis that varying anesthetic techniques will result in differential modulation of the stress response in children undergoing surgery for the repair of congenital heart disease, as evidenced by lower metabolic, hormonal, and cytokine stress markers. Our secondary objective was to evaluate the possible role of dexmedetomidine in blunting some of these stress markers while achieving early tracheal extubation. Our third objective was to evaluate the possible relationship between this blunting of the stress response and different clinical outcomes, including postoperative inotropic score, pain control, coagulation profile, blood transfusion requirements, prevalence of infection, and length of stay in the cardiothoracic intensive care unit (CTICU) in addition to the length of stay in the hospital.
Methods
This study was approved by the Institutional Review Board at Nationwide Children's Hospital, and written informed consent was obtained from a parent. The study was registered with clinical trials.gov (ID NCT00848393). An investigational new drug (IND) application was obtained (IND# 101911). Inclusion criteria included patients > 30 days old to 3 years old who were undergoing repair for a ventricular septal defect, atrioventricular septal defect, or Tetralogy of Fallot. The study was conducted in the period between December 2008 and May 2011.
Patients were randomly assigned by the operating room pharmacy to one of three groups according to a set randomization table: group 1 (low-dose fentanyl plus placebo, LDF) received 10 mcg/kg of fentanyl prior to CPB. Group 2 (high-dose fentanyl plus placebo, HDF) received 25 mcg/kg of fentanyl prior to CPB. For placebo for both group 1 and group 2, normal saline was administered at a volume equivalent to dexmedetomidine of 1 mcg/kg over 10 minutes and then given as an infusion equivalent to 0.5 mcg/kg/hr, continued until separation from CPB. Group 3 low-dose fentanyl plus dexmedetomidine, LDF + DEX received 10 mcg/kg of fentanyl prior to CPB and dexmedetomidine at 1 mcg/kg loading dose over 10 minutes followed by an infusion at 0.5 mcg/kg/hr, continued until separation from CPB. All patients were maintained on isoflurane in addition to the study drugs. Isoflurane was titrated under the discretion of the attending anesthesiologist.
After placement of standard ASA monitors, including electrocardiogram (ECG), pulse oximetry (Spo2), and noninvasive blood pressure cuff, all patients underwent inhalational induction using sevoflurane in a mixture of oxygen and nitrous oxide followed by placement of a peripheral intravenous cannula. Endotracheal intubation was facilitated using 0.2 mg/kg of pancuronium. After endotracheal intubation, a second peripheral intravenous cannula and an arterial cannula were placed. In addition, a bispectral index (BIS) probe (Aspect 119 Medical Systems, Troy, MI) was placed on the left forehead.
At the start of the case, the attending anesthesiologist received two syringes for the study drugs. The first syringe was labeled “study drug: fentanyl.” Half of the drug volume was administered at induction of anesthesia, and the second half of the volume was administered just prior to skin incision. The second syringe was labeled “study drug: dexmedetomidine/placebo at a concentration of 4 μg/mL.” The dexmedetomidine/placebo was administered after obtaining the intravenous access as a loading dose of 1 mcg/kg over 10 minutes and then as an infusion at a rate of 0.5 μg/kg/hr. The dexmedetomidine/placebo infusion was discontinued after separation from CPB.
Acute Normovolemic Hemodilution
During acute normovolemic hemodilution (ANH), it is our protocol to minimize volume replacement and to guide our fluid replacement by hemodynamic variables, such as changes in heart rate, ECG, and mean arterial pressure. In addition, we monitor end-organ perfusion using changes in the cerebral saturation (rSo2) measured by near-infrared spectroscopy. Our goal is to remove 10–20% of the total circulating blood volume prior to surgical incision.
The Use of Tranexamic Acid and Dexamethasone
Due to differences in surgeon's preference, some patients received tranexamic acid (100 mg/kg preincision on CPB and after protamine) and dexamethasone (1 mg/kg).
Cardiopulmonary Bypass
Retrograde autologous prime and/or venous antegrade prime were attempted prior to the initiation of CPB with each patient. Hemofiltration or zero-balance ultrafiltration (ZBUF) during CPB and modified ultrafiltration (MUF) after separation from CPB were attempted on all patients.
Blood Collection and Storage
Three milliliters of blood was collected at five time points, including after anesthetic induction (baseline), after sternotomy, after initiation of CPB, at the conclusion of surgery (separation from CPB and administration of protamine and prior to skin closure), and at 24 hours postoperatively. In addition, a sample of MUF fluid was collected. Blood samples were collected in tubes with EDTA preservative and centrifuged at 1,500 × g, at 4°C, for 10 minutes. The plasma was aliquoted and stored at −80°C until analysis. Plasma was thawed on ice and centrifuged at 14,000 × g for 1 minute prior to analysis. Arterial blood gases, glucose, and lactate levels were recorded at each of the blood draw time points.
Hormone Assays
ACTH and cortisol were assayed by enzyme-linked immunosorbent assay (ELISA) (Cal Biotech, Spring Valley, CA). Epinephrine and norepinephrine were also assayed by ELISA (2-CAT; Rocky Mountain Scientific, Centennial, CO). All assays were performed according to manufacturer's instructions.
Cytokine Assays
Cytokine levels in plasma and MUF samples were measured in the Immune Surveillance Laboratory at The Research Institute at Nationwide Children's Hospital using the Immulite automated chemiluminometer (Siemens Healthcare Diagnostics, Deerfeld, IL). Measured cytokines include interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor-α. Manufacturer-provided standards and controls were run prior to each batch determination to insure proper calibration.
Extubation Criteria
Assuming that there were no preoperative contraindications for early tracheal extubation, we prepared for extubation of patients at the conclusion of the procedure according to set criteria. After separation from CPB, our extubation inclusion criteria includes hemodynamic stability without the need for vasopressor administration and absence of arrhythmias. After reversal of heparin with protamine and achievement of hemostasis, the chest is closed. If the patient continues to maintain stable hemodynamics after chest closure, a trial of spontaneous ventilation is allowed after reversal of muscle relaxants. If the patient continues to maintain stable hemodynamics with acceptable respiratory rate, tidal volume, and expected arterial oxygen saturation at the conclusion of the procedure, we proceed with extubation.
Intraoperative Data Collection
Age, weight, gender, diagnosis, induction time (from start of induction to first blood gas), BIS value, baseline hemoglobin, baseline platelet count, ANH volume prior to CPB, CPB circuit prime constituents, RBC administration, additional blood products (platelet, cryoprecipitate, and plasma) and total volume of each given, ultrafiltration and MUF volume, CPB time, aortic cross clamp time, and the time of extubation, if applicable, were included in the intraoperative data collection.
Postoperative CTICU Data Collection
Length of ventilatory support, total chest tube output during the first 24 hours postoperatively, postoperative platelet count, postoperative absolute neutrophil count (ANC), and postoperative coagulation profile, including prothrombin time (PT), partial thromboplastin time, international normalized ratio (INR), and fibrinogen level, were included in the postoperative CTICU data collection. Data also included inotropic score on arrival and at 24 hours postoperatively. The inotropic score was calculated as follows: dopamine (μg/kg/min) times 1 + milrinone (μg/kg/min) times 10 + epinephrine (μg/kg/min) times 100 (36). Other data collected include total urine output during the first 24 hours postoperatively, blood urea nitrogen and creatinine on arrival and at 24 hours postoperatively, and the use of nurse-controlled analgesia, including starting opioid, the need to change the medication used, and the total dose of opioids for the first 24 hours. Other variables collected include cardiac arrest requiring resuscitation, ventricular or atrial arrhythmia causing hemodynamic disturbances that require treatment, nosocomial infection during the hospitalization, disseminated intravascular coagulation, clinical evidence of seizures, the need for reintubation, length of ICU stay, the need for transfusing blood or blood products and total volume given, length of hospital stay, and postoperative mortality.
Statistical Analysis
Comparisons between groups were made using one-way analyses of variance (ANOVA) with pairwise comparisons made using Mann-Whitney tests. Evaluations of changes in stress markers over time were made using two-way repeated-measures ANOVA. Categorical data were evaluated using Fisher exact test or chi-square test as appropriate. Descriptive data are presented as median (range) for continuous data. Holm's procedure was used to correct for multiple comparisons when it was needed. Type I error was strongly controlled at α = 0.05 for single comparison and with adjustment for multiple comparisons. The data were analyzed using the statistical software SAS version 9.2 (SAS Institute, Cary, NC) and Prism5 (GraphPad, La Jolla, CA).
Results
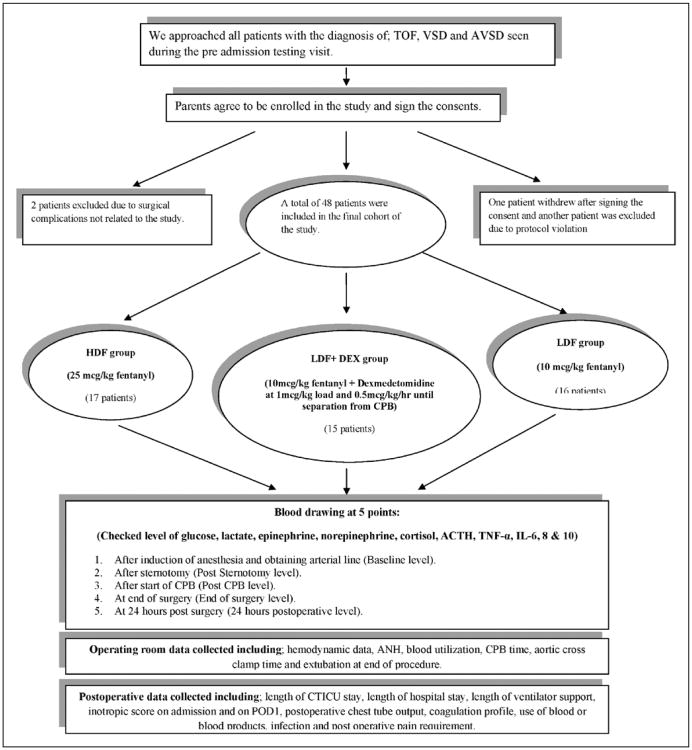
A total of 52 subjects were enrolled, one patient withdrew and one patient was excluded due to a study protocol deviation. Finally, two patients were excluded due to surgical complications unrelated to study drug administration, leaving 48 subjects for analysis (Fig. 1).
Figure 1.
Flow chart of the study design. TOF = Tetralogy of Fallot; VSD = ventricular septal defect; AVSD = atrioventricular septal defect; HDF = high-dose fentanyl plus placebo; LDF + DEX = low-dose fentanyl plus dexmedetomidine; LDF = low-dose fentanyl plus placebo; TNF-α = tumor necrosis factor-α; IL = interleukin; CPB = cardiopulmonary bypass; ANH = acute normovolemic hemodilution; CTICU = cardiothoracic intensive care unit; POD1 = postoperative day 1.
Of the 48 subjects, 16 patients were randomized to the LDF group, 17 patients to the HDF group, and 15 patients to the LDF + DEX group. There were no statistically significant differences between the three groups with regard to age, weight, or the distribution of diagnoses (Table 1). Also, there were no significant differences between the three groups when considering the number of subjects who received tranexamic acid and dexamethasone (seven subjects in the LDF group, six subjects in the LDF + DEX group, and eight subjects in the HDF group) (Table 2). There were no significant differences between the three groups in the time of anesthetic induction, the starting hemoglobin, CPB time, or the aortic cross clamp time (Table 2). Although there was no difference between groups in terms of the number of subjects undergoing ANH (p = 0.29, ANOVA), the subgroup of children who underwent ANH in the HDF and LDF + DEX groups had greater ANH volume removal compared with the LDF group. Children in the HDF group received significantly less blood in the operating room compared with the other two groups (p = 0.004, ANOVA). There was no difference in the frequency of extubation in the operating room between groups (p = 0.39). There were no statistically significant differences between the three groups relative to the intraoperative hemodynamics (Supplemental data, Supplemental Digital Content 1, http://links.lww.com/PCC/A55). The patients in the LDF + DEX group did demonstrate a significantly lower BIS value at the end of the procedure (p = 0.02) when compared with the other two groups. There was no mortality during the hospital course in any of the three groups.
Table 1. Data for Age and Weight Represent Median (Range). Anatomical Defects Data Represents Number of Subjects.
| Variable | Low-Dose Fentanyl Plus Placebo (n = 16) | Low-Dose Fentanyl Plus Dexmedetomidine (n = 15) | High-Dose Fentanyl Plus Placebo (n = 17) |
|---|---|---|---|
| Age (mo) | 4 (3–7) | 5 (2–21) | 4 (1–35) |
|
| |||
| Gender | 12 males/4 females | 11 males/4 females | 9 males/8 females |
|
| |||
| Weight (kg) | 5.82 (3.7–7.6) | 6.1 (3.9–12.1) | 6.5 (3–13.9) |
|
| |||
| Anatomical defect | |||
| Tetralogy of Fallot | 6 | 6 | 6 |
| Ventricular septal defect | 7 | 7 | 8 |
| Atrioventricular septal defect | 3 | 2 | 3 |
Data represent median (range) unless otherwise specified.
Table 2. Intraoperative Anesthetic and Cardiopulmonary Bypass Data.
| Patients | Low-Dose Fentanyl Plus Placebo (n = 16) | Low-Dose Fentanyl Plus Dexmedetomidine (n = 15) | High-Dose Fentanyl Plus Placebo (n = 17) | p |
|---|---|---|---|---|
| Induction time (min) | 41 (13–229) | 39 (11–99) | 43 (21–152) | 1.0 |
| Starting hemoglobin (gm/dL) | 10.5 (8.8–13.6) | 11.2 (9.2–16) | 11.2 (8.5–15.3) | 0.45 |
| No. of patients receiving dexamethasone and tranexamic acid | 7 | 6 | 8 | 0.92 |
| ANH (No. of patients) (n, %) | 7 (43.8) | 9 (60) | 12 (70.6) | 0.29 |
| ANH volumea (mL/kg) | 7. 6 (2.2–14) | 12.2 (3.2–17)* | 9.8 (7.8–17)* | 0.0276 |
| CPB time (min) | 124 (83–173) | 113 (74–225) | 100 (60–140) | 0.31 |
| Aortic cross clamp time (min) | 86 (50–133) | 82 (41–128) | 59 (37–104) | 0.29 |
| CPB primed with blood (n, %) | 2 (12.5) | 4 (26.7) | 3 (17.6) | 0.59 |
| Blood given in OR (mL/kg) | 40 (0–72) | 45 (0–100) | 22 (0–43)*# | 0.0044 |
| Extubation in OR (n, %) | 12 (75) | 14 (93.3) | 14 (82.4) | 0.44 |
| No. of bloodless cases (n, %) | 0 (0) | 0 (0) | 4 (23.5) | 0.0286 |
ANH = acute normovolemic hemodilution, CPB = cardiopulmonary bypass, OR = operating room.
For subjects undergoing ANH.
p ≤ 0.03 versus low-dose fentanyl plus placebo.
p = 0.003 versus low-dose fentanyl plus dexmedetomidine.
Data represent median (range) unless otherwise specified.
There were no statistically significant differences between the three groups relative to the length of ICU stay, length of hospital stay, length of time on the ventilator, and inotropic score on arrival to the ICU or on postoperative day 1 (Table 3).
Table 3. Postoperative Outcomes and Hematologic Data.
| Patients | Low-Dose Fentanyl Plus Placebo | Low-Dose Fentanyl Plus Dexmedetomidine | High-Dose Fentanyl Plus Placebo | p |
|---|---|---|---|---|
| Length of CTICU stay (d) | 1 (1–4) | 2 (1–5) | 1 (1–8) | 0.5995 |
| Length of hospital stay (d) | 5 (3–15) | 5 (3–23) | 5.5 (3–32) | 0.5561 |
| Time on ventilator (hr) | 3.79 (0–16) | 2.4 (0–2.4) | 10.75 (0–27.32) | 0.43 |
| Inotropic score on CTICU admission | 2.5 (0–2.5) | 2.5 (0–5) | 2.5 (0–5) | 0.2541 |
| Inotropic score on postoperative day 1 | 2.5 (0–2.5) | 2.5 (0–7.5) | 2.5 (0–5) | 0.1728 |
| Bloodless throughout hospitalization (n, %) | 0 (0) | 0 (0) | 4 (23.5) | 0.0286 |
| Preoperative platelet count (K/cu mm) | 345 (216–511) | 357 (187–521) | 326 (201–696) | 0.9681 |
| Postoperative platelet count (K/cu mm) | 106 (48–250) | 116 (48–250) | 132 (75–287) | 0.5500 |
| Postoperative prothrombin time (s) | 20 (17.5–24.7) | 19.2 (15.9–24.7) | 18.8 (15.9–21.5)* | 0.0466 |
| Postoperative partial thromboplastin time (s) | 42.5 (32–86) | 44 (30–250) | 40 (28–61) | 0.3365 |
| Postoperative international normalized ratio | 1.7 (1.5–2.3) | 1.6 (1.3–2.3) | 1.6 (1.3–1.9)* | 0.1018 |
| Postoperative fibrinogen (mg/dL) | 118 (68–165) | 135 (68–222) | 143 (93–222)# | 0.1882 |
| Postoperative absolute neutrophil count (cells/cu mm) | 7,260 (3,100–16,184) | 8,643 (4,514–13,272) | 9,380 (3,690–16,960)# | 0.1343 |
CTICU = cardiothoracic ICU.
p ≤ 0.03 versus low-dose fentanyl plus placebo.
p = 0.07 versus low-dose fentanyl plus placebo.
Data represent median (range) unless otherwise specified.
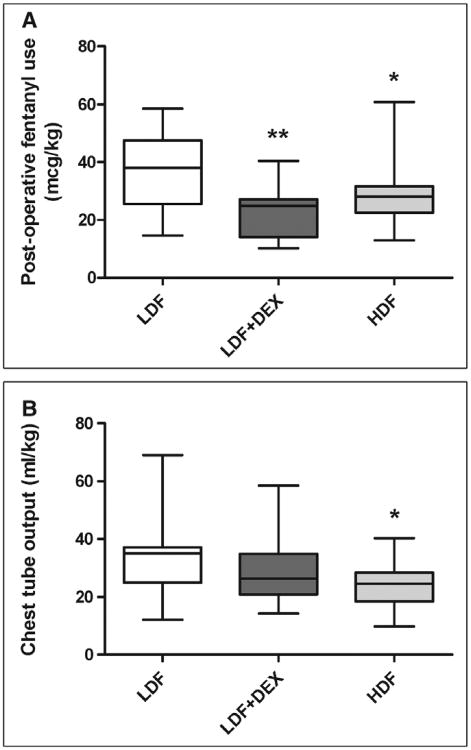
The experimental group was highly associated with narcotic requirement over the first 24 hours postoperatively (p = 0.004), with patients in the LDF group having significantly higher postoperative fentanyl use compared with both HDF and LDF + DEX groups (Fig. 2A). Similarly, chest tube output over the same time frame was highest in the LDF group with the HDF group being significantly lower (Fig. 2B). There was no significant difference in the level of starting hemoglobin at the time of admission to the CTICU, at 24 hours postoperatively, or the change in hemoglobin during the first 24 hours. Four patients in the HDF group (23.5%), compared with none in the other two groups, received no blood or blood products during the entire hospitalization (p = 0.0286). Although there were no differences in platelet counts between groups, subjects in the LDF group had longer PT time, higher INR, and lower fibrinogen levels (Table 3). Although there were no differences in preoperative ANC between groups, there was a trend toward lower postoperative ANC in the LDF group.
Figure 2.
Fentanyl use and chest tube output in the first 24 hr after surgery. Subjects receiving low-dose fentanyl plus placebo (LDF) demonstrated higher use of fentanyl in the first 24 hr after surgery (A) compared with subjects receiving high-dose fentanyl plus placebo (HDF) or low-dose fentanyl plus dexmedetomidine (LDF + DEX) (p = 0.004, two-way analyses of variance). Subjects receiving LDF demonstrated higher chest tube output (B) compared with the HDF group over the same period. *p < 0.05 HDF versus LDF; **p < 0.01 LDF-DEX versus LDF. Boxes represent median and interquartile range, and whiskers represent minimum-maximum.
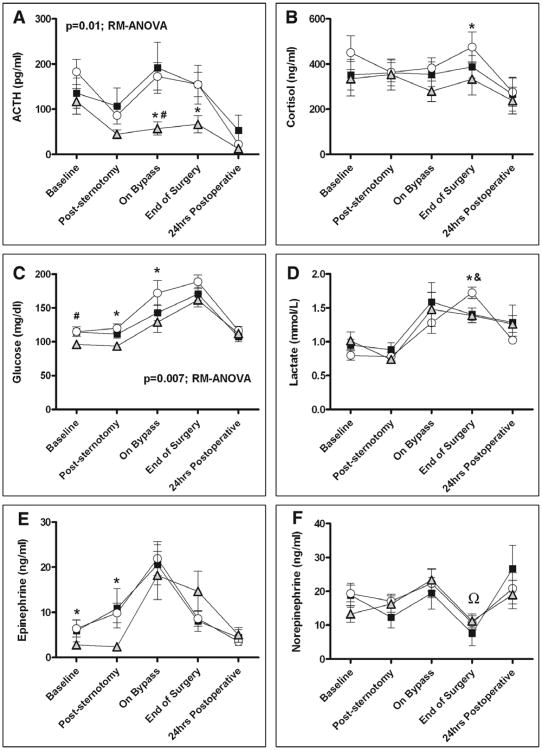
When evaluating hormonal and metabolic markers of stress, the HDF group demonstrated the lowest levels of plasma ACTH (Fig. 3A) over time, with the greatest differences seen on bypass and at the end of surgery. Similarly, plasma cortisol levels in the HDF group were lower than those in the LDF group at the end of surgery (Fig. 3B). Plasma glucose levels (Fig. 3C) were highest in the LDF group over time, particularly poststernotomy and during bypass. Plasma lactate levels were similar between groups at early time points, but they were significantly higher at the end of surgery in the LDF group compared with both the HDF and LDF + DEX groups (Fig. 3D). Plasma epinephrine levels (Fig. 3E) were significantly lower over time in the HDF group. Post hoc pairwise comparisons also showed that norepinephrine level at the end of surgery in the LDF + DEX group was significantly lower than HDF and LDF groups (p < 0.05) (Fig. 3F). In addition, subjects receiving LDF + DEX demonstrated a statistically significant reduction in norepinephrine levels from baseline to poststernotomy, after start of CPB, and at end of surgery (p ≤ 0.05). There was no difference in postoperative arrhythmia among the three groups. Only four patients had a documented rhythm other than sinus rhythm (two in the HDF and two in the LDF + DEX) (Table 4). In addition, there was no significant difference in the prevalence of postoperative infection between the three groups with a total of three patients (two patients in the LDF + DEX group and one patient in the HDF group) demonstrating positive blood culture on postoperative days 4, 9, and 11, respectively.
Figure 3.
Plasma hormonal and metabolic stress markers. Subjects receiving high-dose fentanyl plus placebo (HDF) (shaded triangle) exhibited lower plasma levels of ACTH (A) and glucose (C) over the study period compared with subjects receiving low-dose fentanyl plus placebo (LDF, open circle) or low-dose fentanyl plus dexmedetomidine (LDF + DEX, closed square) (p ≤ 0.01, repeated-measures analyses of variance [RM-ANOVA] for both markers). Subjects receiving HDF had lower cortisol levels (B) at the end of surgery and lower epinephrine levels (E) after sternotomy compared with the LDF group. Subjects in both the HDF and LFD + DEX groups had lower lactate levels (D) at the end of surgery compared with subjects in the LDF group. For norepinephrine at the end of surgery (F)(double closed circle), LDF + DEX gave significantly lower level than HDF and LDF groups (p < 0.05). *p < 0.05 HDF versus LDF, #p < 0.05 HDF versus LDF + DEX, &p < 0.05 LDF + DEX versus LDF, Ωp < 0.05 LDF + DEX versus LDF and HDF. Data represent mean (SEM).
Table 4. Documented Arrhythmias in the Postoperative Period.
| Patient | Rhythm on Arrival to Cardiothoracic ICU | Rhythm at 24 Hr | Drug Group | Diagnosis |
|---|---|---|---|---|
| 118 | Sinus rhythm | Junctional rhythm with ectopic atrial beats | HDF | Ventricular septal defect |
|
| ||||
| 207 | Paced rhythm | Complete heart block | LDF + DEX | Tetralogy of Fallot |
|
| ||||
| 306 | Junctional rhythm | Junctional rhythm | LDF + DEX | AVSD |
|
| ||||
| 307 | Paced with complete heart block | Complete heart block | HDF | AVSD |
HDF = high-dose fentanyl plus placebo, LDF + DEX = low-dose fentanyl plus dexmedetomidine, AVSD = atrioventricular septal defect.
There were no statistically significant differences in plasma cytokine levels between groups over time nor were there statistically significant differences between groups at individual time points (supplemental data, Supplemental Digital Content 2, http://links.lww.com/PCC/A56). Although there were no significant differences between the three groups with regard to the IL-6 and IL-10 levels, there was significant difference between the three groups when comparing the ratio of IL-6 with IL-10 at 24 hours after surgery (p < 0.0001 ANOVA). The IL-6:IL-10 ratio was the highest in the LDF group at an average of 19.66 ± 1.26 pg/mL, compared with the HDF group with an average ratio of 2.83 ± 0.16 pg/mL and the LDF + DEX group with an average ratio of 2.19 ± 0.14 pg/mL.
Within study medication groups, children who received dexamethasone and tranexamic acid demonstrated lower plasma IL-6 levels on postoperative day 1 (LDF [50.38 ± 24.6 vs 170.7 ± 98.5 pg/mL, p = 0.01], LDF + DEX [50.65 ± 41.8 vs 190.1 ± 137 pg/mL, p = 0.006]). There was no difference in the IL-6 level within the HDF group based on the use of dexamethasone and tranexamic acid. In addition, there was no difference in the level of hormonal stress markers within each arm of the study based on the use of these medications.
Discussion
Our data show that the LDF group consistently had significantly higher levels of metabolic and hormonal stress markers when compared with the HDF or the LDF + DEX group. These statistically significant changes in the stress response were associated with improvements in some clinical outcomes. The LDF group demonstrated an increase in postoperative coagulopathy with significantly higher PT when compared with the HDF group. This was associated with an accompanying increase in perioperative bleeding as evidenced by the significantly higher chest tube output over the first 24 hours.
Although there were no significant differences between the three groups with regard to the IL-6 and IL-10 levels, there was significant difference between the three groups when comparing the ratio of IL-6 with IL-10 at 24 hours after surgery. This would suggest predominance of the proinflammatory response in children in the LDF group. Some studies have shown that a shift toward the proinflammatory response is associated with adverse cardiac outcomes (37, 38).
HDF appears to have the most blunting effect on metabolic and hormonal stress response. This was associated with lower prevalence and volume of intraoperative transfusion, better coagulation profile, lower chest tube output, and significantly higher prevalence of bloodless procedures throughout the hospitalization period. This was achieved while being able to extubate 82% of HDF patients in the operating room.
The addition of dexmedetomidine to the LDF regimen reduced the stress response when compared with LDF alone, as evidenced by lower lactate levels at the end of surgery, lower nor-epinephrine levels at the end of surgery and blunting of the nor-epinephrine response after sternotomy, at the start of CPB, and at the end of surgery. Although it did not reach statistical significance, the greatest proportion of patients were successfully extubated in the operating room in this group (93%). Additionally, the use of LDF + DEX was associated with a significantly lower fentanyl requirement during the first 24 hours postoperatively.
Advances in CPB technique have resulted in reducing the stress response inherent to cardiac surgery in children (12, 13, 39–42). Together, smaller circuits and hemofiltration or ZBUF combine to reduce circulating levels of activated leukocytes, decrease transfusion requirements, decrease renal dysfunction, and decrease pulmonary inflammation, assisting with early extubation and improved neurological outcomes.
Another strategy that has improved the stress response to CPB is the utilization of MUF. MUF further results in reversal of the hemodilution that occurs before and during CPB and promotes decreased tissue edema, improved lung function (and an associated decrease in the duration of ventilatory support postoperatively), improved left ventricular function, and decreased postoperative bleeding (43–45).
Although these advances in the technical aspects of CPB may have resulted in reduction of the stress response to pediatric cardiac surgery, our results suggest that the differences in the levels of stress response markers may have been due to the effect of the different arms of the study on the stress response rather than the CPB technique as all patients were managed using a similar CPB technique. The use of ZBUF or MUF does not reduce the production of the cytokines but rather extracts these cytokines from the blood. This is evident by the steep rise in the level of these different cytokines after separation from CPB.
Our study is the first to look at the stress response in the setting of early extubation/fast tracking after cardiac surgery in children, which is currently being adopted by many institutions. Our data clearly point out the fact that low-dose fentanyl in combination with inhalational agents is not sufficient to blunt the metabolic and hormonal stress response in children undergoing cardiac surgery.
Although our data may show a correlation between the use of a higher dose fentanyl with the associated blunting in some stress response markers with the possible improvement of the postoperative coagulation profile, this association has to be investigated further. Many studies have failed to show a correlation between blunting the stress response, while using much higher doses of fentanyl, and an improved postoperative clinical outcome (3, 18).
One of the limitations of this study is the small sample size in each arm of the study. There are some emerging animal data that suggest the possible anti-inflammatory effect of dexmedetomidine (46–48). Although we reached a statistical significance between the three arms for the metabolic and hormonal responses, a larger sample size could have resulted in showing a significant difference when it comes to the cytokine levels among the three groups.
Another possible limitation to this study is that some of our patients (equally distributed among the three arms) received dexamethasone and tranexamic acid. Although the use of these medications was associated with differences in plasma IL-6 levels on postoperative day 1 within some groups, the fact that equal number of patients within each group received steroids and tranexamic acid suggests that the difference in the hormonal, cytokines, and clinical data presented in this study is likely to be attributed to the study drugs and not the use of tranexamic acid and dexamethasone.
In conclusion, the addition of dexmedetomidine offered advantages over low-dose fentanyl alone. Dexmedetomidine offered early extubation, more blunting of some of the metabolic and hormonal markers and better postoperative pain control as evident by the lower total narcotic requirements of the patients who received dexmedetomidine. The combination of a dose higher than the 10 mcg/kg of fentanyl in addition to dexmedetomidine may offer more blunting of the stress response while achieving a safe fast tracking of pediatric patients undergoing cardiac surgery.
Supplementary Material
Acknowledgments
We thank the cardiac anesthesia team at Nationwide Children's Hospital for their help in taking care of the patients in this study. We also thank Tracy Heard, CPNP, and Lauren Dumm, RN, for their help in collecting data that were used in this study.
Supported, in part, by an intramural grant from Heart Center Translational Research Fund (grant #231708) at Nationwide Children's Hospital.
Footnotes
For information regarding this article, aymen.naguib@nationwide-childrens.org
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/pccmjournal).
References
- 1.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: Effects on the stress response. Lancet. 1987;1:243–248. doi: 10.1016/s0140-6736(87)90065-1. [DOI] [PubMed] [Google Scholar]
- 2.Gajarski RJ, Stefanelli CB, Graziano JN, et al. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr Crit Care Med. 2010;11:44–51. doi: 10.1097/PCC.0b013e3181a64743. [DOI] [PubMed] [Google Scholar]
- 3.Gruber EM, Laussen PC, Casta A, et al. Stress response in infants undergoing cardiac surgery: A randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion. Anesth Analg. 2001;92:882–890. doi: 10.1097/00000539-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Roth-Isigkeit A, Brechmann J, Dibbelt L, et al. Persistent endocrine stress response in patients undergoing cardiac surgery. J Endocrinol Invest. 1998;21:12–19. doi: 10.1007/BF03347280. [DOI] [PubMed] [Google Scholar]
- 5.Anand BK, Raghunath P, Dua S, et al. Hypothalamic control of the pituitary adrenocortical response to stress stimuli. Indian J Med Res. 1954;42:231–248. [PubMed] [Google Scholar]
- 6.Anand KJ. The stress response to surgical trauma: From physiological basis to therapeutic implications. Prog Food Nutr Sci. 1986;10:67–132. [PubMed] [Google Scholar]
- 7.Anand KJ, Maze M. Fetuses, fentanyl, and the stress response: Signals from the beginnings of pain? Anesthesiology. 2001;95:823–825. doi: 10.1097/00000542-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: Effects on the stress response. Lancet. 1987;1:62–66. doi: 10.1016/s0140-6736(87)91907-6. [DOI] [PubMed] [Google Scholar]
- 9.Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JC., Jr What initiates the systemic inflammatory response in cardiac surgery patients? Chest. 1997;112:1153–1154. doi: 10.1378/chest.112.5.1153-a. [DOI] [PubMed] [Google Scholar]
- 11.Hirai S, Hamanaka Y, Mitsui N, et al. Prospective study of systemic inflammatory response syndrome after cardiac surgery as a effective indicator. Kyobu Geka. 2004;57:455–458. [PubMed] [Google Scholar]
- 12.Raja SG, Dreyfus GD. Modulation of systemic inflammatory response after cardiac surgery. Asian Cardiovasc Thorac Ann. 2005;13:382–395. doi: 10.1177/021849230501300422. [DOI] [PubMed] [Google Scholar]
- 13.Soares LC, Ribas D, Spring R, et al. Clinical profile of systemic inflammatory response after pediatric cardiac surgery with cardiopulmonary bypass. Arq Bras Cardiol. 2010;94:127–133. doi: 10.1590/s0066-782x2010000100019. [DOI] [PubMed] [Google Scholar]
- 14.Lespron Robles MC. Systemic inflammatory response in pediatric cardiac surgery. Arch Cardiol Mex. 2006;76(Suppl 2):S92–S99. [PubMed] [Google Scholar]
- 15.Sendasgupta C, Makhija N, Kiran U, et al. Caudal epidural sufentanil and bupivacaine decreases stress response in paediatric cardiac surgery. Ann Card Anaesth. 2009;12:27–33. doi: 10.4103/0971-9784.45010. [DOI] [PubMed] [Google Scholar]
- 16.Prakanrattana U, Suksompong S. Comparison of sufentanil and fentanyl for surgical repair of congenital cardiac defects. J Med Assoc Thai. 2002;85(Suppl 3):S807–S814. [PubMed] [Google Scholar]
- 17.Bichel T, Rouge JC, Schlegel S, et al. Epidural sufentanil during paediatric cardiac surgery: Effects on metabolic response and postoperative outcome. Paediatr Anaesth. 2000;10:609–617. doi: 10.1111/j.1460-9592.2000.00557.x. [DOI] [PubMed] [Google Scholar]
- 18.Allan CK, Newburger JW, McGrath E, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244–1251. doi: 10.1213/ANE.0b013e3181f333aa. [DOI] [PubMed] [Google Scholar]
- 19.Peterson KL, DeCampli WM, Pike NA, et al. A report of two hundred twenty cases of regional anesthesia in pediatric cardiac surgery. Anesth Analg. 2000;90:1014–1019. doi: 10.1097/00000539-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kloth RL, Baum VC. Very early extubation in children after cardiac surgery. Crit Care Med. 2002;30:787–791. doi: 10.1097/00003246-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Worley S, Mee RB, et al. Factors associated with early extubation after cardiac surgery in young children. Pediatr Crit Care Med. 2004;5:63–68. doi: 10.1097/01.PCC.0000102386.96434.46. [DOI] [PubMed] [Google Scholar]
- 22.Vida VL, Leon-Wyss J, Rojas M, et al. Pulmonary artery hypertension: Is it really a contraindicating factor for early extubation in children after cardiac surgery? Ann Thorac Surg. 2006;81:1460–1465. doi: 10.1016/j.athoracsur.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Meissner U, Scharf J, Dötsch J, et al. Very early extubation after open-heart surgery in children does not influence cardiac function. Pediatr Cardiol. 2008;29:317–320. doi: 10.1007/s00246-007-9023-0. [DOI] [PubMed] [Google Scholar]
- 24.Winch PD, Nicholson L, Isaacs J, et al. Predictors of successful early extubation following congenital cardiac surgery in neonates and infants. Heart Lung Circ. 2009;18:271–276. doi: 10.1016/j.hlc.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Preisman S, Lembersky H, Yusim Y, et al. A randomized trial of outcomes of anesthetic management directed to very early extubation after cardiac surgery in children. J Cardiothorac Vasc Anesth. 2009;23:348–357. doi: 10.1053/j.jvca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Abuchaim DC, Bervanger S, Medeiros SA, et al. Early extubation in the operating room in children after cardiac heart surgery. Rev Bras Cir Cardiovasc. 2010;25:103–108. doi: 10.1590/s0102-76382010000100020. [DOI] [PubMed] [Google Scholar]
- 27.Cray SH, Holtby HM, Kartha VM, et al. Early tracheal extubation after paediatric cardiac surgery: The use of propofol to supplement low-dose opioid anaesthesia. Paediatr Anaesth. 2001;11:465–471. doi: 10.1046/j.1460-9592.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- 28.Friesen RH, Veit AS, Archibald DJ, et al. A comparison of remifentanil and fentanyl for fast track paediatric cardiac anaesthesia. Paediatr Anaesth. 2003;13:122–125. doi: 10.1046/j.1460-9592.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 29.Rosen KR, Rosen DA. Caudal epidural morphine for control of pain following open heart surgery in children. Anesthesiology. 1989;70:418–421. doi: 10.1097/00000542-198903000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Leyvi G, Taylor DG, Reith E, et al. Caudal anesthesia in pediatric cardiac surgery: Does it affect outcome? J Cardiothorac Vasc Anesth. 2005;19:734–738. doi: 10.1053/j.jvca.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Chrysostomou C, Sanchez-de-Toledo J, Wearden P, et al. Perioperative use of dexmedetomidine is associated with decreased incidence of ventricular and supraventricular tachyarrhythmias after congenital cardiac operations. Ann Thorac Surg. 2011;92:964–972. doi: 10.1016/j.athoracsur.2011.04.099. discussion 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrysostomou C, Sanchez De Toledo J, Avolio T, et al. Dexmedetomidine use in a pediatric cardiac intensive care unit: Can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009;10:654–660. doi: 10.1097/PCC.0b013e3181a00b7a. [DOI] [PubMed] [Google Scholar]
- 33.Mukhtar AM, Obayah EM, Hassona AM. The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg. 2006;103:52–56. doi: 10.1213/01.ane.0000217204.92904.76. [DOI] [PubMed] [Google Scholar]
- 34.Naguib A, McKee C, Phillips A, et al. Dexmedetomidine as the primary anesthetic agent during cardiac surgery in an infant with a family history of malignant hyperthermia. Saudi J Anaesth. 2011;5:426–429. doi: 10.4103/1658-354X.87276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobias JD, Gupta P, Naguib A, et al. Dexmedetomidine: Applications for the pediatric patient with congenital heart disease. Pediatr Cardiol. 2011;32:1075–1087. doi: 10.1007/s00246-011-0092-8. [DOI] [PubMed] [Google Scholar]
- 36.Wernovsky G, Kuijpers M, Van Rossem MC, et al. Postoperative course in the cardiac intensive care unit following the first stage of Norwood reconstruction. Cardiol Young. 2007;17:652–665. doi: 10.1017/S1047951107001461. [DOI] [PubMed] [Google Scholar]
- 37.Kilic T, Ural D, Ural E, et al. Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart. 2006;92:1041–1046. doi: 10.1136/hrt.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas S, Ghoshal PK, Mandal SC, et al. Relation of anti- to pro-inflammatory cytokine ratios with acute myocardial infarction. Korean J Intern Med. 2010;25:44–50. doi: 10.3904/kjim.2010.25.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gourlay T, Shedden L. The impact of different biocompatible coated cardiopulmonary bypass circuits on inflammatory response and oxidative stress. Perfusion. 2010;25:427–429. doi: 10.1177/0267659110381453. [DOI] [PubMed] [Google Scholar]
- 40.Caputo M, Mokhtari A, Rogers CA, et al. The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2009;138:206–214. doi: 10.1016/j.jtcvs.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81:S2347–S2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 42.Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. 2001;45:671–679. doi: 10.1034/j.1399-6576.2001.045006671.x. [DOI] [PubMed] [Google Scholar]
- 43.Ootaki Y, Yamaguchi M, Oshima Y, et al. Effects of modified ultrafiltration on coagulation factors in pediatric cardiac surgery. Surg Today. 2002;32:203–206. doi: 10.1007/s005950200021. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi RR, Shore DF, White PA, et al. Modified ultrafiltration improves global left ventricular systolic function after open-heart surgery in infants and children. Eur J Cardiothorac Surg. 1999;15:742–746. doi: 10.1016/s1010-7940(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 45.Davies MJ, Nguyen K, Gaynor JW, et al. Modified ultrafiltration improves left ventricular systolic function in infants after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115:361–399. doi: 10.1016/S0022-5223(98)70280-6. discussion 369. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22:221–228. doi: 10.1007/s00540-008-0611-9. [DOI] [PubMed] [Google Scholar]
- 47.Steppan J, Hofer S, Wagner T, et al. Dexmedetomidine and Clonidine as a novel pre-emptive therapeutic option in the treatment of patients prone to sepsis. Infection. 2009;37(Suppl. III):14. [Google Scholar]
- 48.Can M, Gul S, Bektas S, et al. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.