Abstract
Secondary analyses of data from two studies were used to assess the effects of protein intake and gender on diet-induced changes in body composition. The primary hypothesis was that the changes of body composition via energy restriction (i.e. lean mass, fat mass, and bone) would be gender and diet specific. For 12 weeks, 43 male (study 1) and 45 female (study 2) overweight and obese adults consumed an energy deficit diet (750 kcal/d less than energy needs) containing either 0.8 (NP, 21 men, 23 women), or 1.4 g protein·kg-1·d-1 (HP, 22 men, 22 women). Body composition measurements were performed at pre and post intervention. Over time, all research participants lost weight, lean body mass (LBM) and fat mass (FM). Independent of protein intake, the men lost more LBM in the trunk (−0.9 vs.−0.5 kg) and less in the legs (−1.5 vs. −1.1 kg) compared with the women (P < 0.05). Independent of gender, the HP group lost less LBM in the trunk and legs than the NP group. These gender and protein intake responses resulted in the NP men losing the most LBM in the legs and the NP women losing the most LBM in the trunk. Over time, men lost more FM (−5.0 vs. −3.9 kg) from the trunk and less from legs (−1.7 vs. −2.1 kg) than women (P < 0.05), which resulted in a greater decrease of the android to gynoid fat ratio for the men. Protein intake did not influence these gender-specific responses or have any independent effects on changes in FM. Also, protein intake did not influence bone mineral density (BMD) responses over time; BMD was reduced in women, but not in men. These findings indicate that higher protein intake during weight loss promotes the retention of lean mass in both the trunk and legs despite the gender-specific changes in these body regions.
Keywords: body composition, weight loss, dietary protein, energy restriction, bone mineral density
1. Introduction
According to the 2007-2008 National Health and Nutrition Examination Survey, about two-thirds of adults in the United States are overweight (BMI between 25 and 29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). Although, overweight and obesity are associated with the development of heart disease, diabetes and premature morality [1], weight loss can effectively improve obesity related complications [2]. Weight loss via moderate energy restriction (500-750 kcal/d energy deficit) and increased dietary protein (>22% of energy from protein) are recommended due to documented improvements in insulin sensitivity [3-5] and increased preservation of lean body mass during weight loss [6-8].
Previously, our research group reported that overweight and obese women consuming an energy deficit diet (which was 750 kcal/d less than energy needs) containing 30% energy from protein (1.4 g protein·kg-1·d-1) for 12 weeks lost less lean body mass than women consuming isocaloric diets with 18% energy from protein (0.8 g protein·kg-1·d-1) [9]. A follow-up study was recently completed in overweight and obese men with similar findings that high-protein diets help preserve lean body mass [10]. Although it is well-documented that higher-protein diets contribute to lean mass retention, the distribution of changes in lean body mass and fat mass are less well studied. One area of interest regarding the preservation of lean mass includes the appendicular region (arms and legs) which is the site containing the majority of bodily muscle tissue. Alternately, fat loss from the abdominal region (i.e. trunk) is more beneficial in improving health status because abdominal fat is independently associated with increased risk of cardiovascular disease and diabetes [11]. In addition, men have more appendicular lean mass and are more likely to store fat in the trunk region than women [12] and may respond differently to weight loss compared with women in terms of body composition changes [13]. Thus, the primary aim of this study is to assess the effects of dietary protein and gender on regional body composition changes and in overweight and obese men and women during weight loss using data collected from previous research [9]. We hypothesized that the changes of body composition via energy restriction (i.e. lean mass, fat mass, and bone) would be gender and diet specific.
Weight loss may accelerate bone mineral density (BMD) loss which is associated with increased risk of osteoporosis. Protein is suggested to be beneficial to bone at weight stable conditions due to stimulation of insulin-like growth factor 1 (IGF-1) and intestinal calcium absorption [14, 15]; however, the effect of the dietary protein on bone during weight loss is not well characterized. Our research group has reported findings from two separate studies [16] that postmenopausal women who consumed a high-protein (30 or 26% energy), energy restricted diet lost more BMD than women consuming a normal-protein (18 or 16% energy) diet during weight loss (12 or 9 weeks). To the contrary, recent research [17] found that postmenopausal women who consumed a higher-protein (24% energy) diet attenuated BMD loss after 12 months of weight loss compared with women who consumed a normal-protein (18% energy) diet. The secondary aim of the current research is to assess the influence of dietary protein on bone during weight loss in overweight and obese men and women. Our second hypothesis was that dietary protein would accelerate bone loss during weight loss in these participants.
2. Methods and Materials
2.1 Research participants
Potential research participants were recruited via local newspaper advertisements, posted flyers and campus mail. The Purdue University Biomedical Institutional Review Board approved the study protocol and each research participant signed an informed-consent form before enrollment (clinicaltrial.gov registration ID: NCT00812162). Inclusion criteria were as follows: 1) 21 years and older; 2) BMI between 25.0-39.9 kg/m2; 3) weight stable (< 4.5 kg weight change within last 6 months); 4) non-smoking; 5) constant habitual activity patterns within last 3 months; and 6) clinically normal blood profiles (specifically, normal liver and kidney functions; fasting blood glucose <110 mg/dL). Each research participant received a monetary stipend for participating in the study. Fifty-five men and fifty-four women met the inclusion criteria and started the intervention. Forty-five men and forty-six women completed the trial. Two-men and one woman were excluded from the final analyses due to noncompliance with testing procedures, dietary control and incomplete data collection. Thus, data from 43 men and 45 women were analyzed and reported.
2.2 Study Design
The 13-week protocol included 1 week of baseline and 12 weeks of controlled-feeding intervention with energy restriction. Research participants consumed their usual, unrestricted diets ad libitum during the baseline week and were randomly assigned to a normal-protein (0.8 g protein·kg-1·d-1) or higher-protein (1.4 g protein·kg-1·d-1), energy-restricted diet to consume for 12 weeks. The same set of testing was performed at baseline and the 12th week of the intervention, including two fasting blood samples (one each on separate days), one body composition assessment including muscle, fat and bone mass measurements, and dietary assessments.
2.3 Dietary Intervention
Research participants consumed isocaloric energy-restricted diets for 12 weeks with different macronutrient distributions. The energy content of each research participant's diet was 750 kcal/d less than their energy requirement, which was estimated using the Harris-Benedict equation [18] with an activity factor of 1.5. The NP diet contained the Recommended Dietary Allowance (RDA) of 0.8 g protein·kg-1·d-1 while the HP diet contained 1.4 g protein ·kg-1·d-1, based on the research participant's body weight at screening. Macronutrient distributions of the diets are shown in Table 1. Fat content was kept constant at 25% for both the NP and HP diets while carbohydrate contents varied. The authors recognize that the changes in outcomes might be attributable to increased protein or decreased carbohydrate. For descriptive purposes, the dietary interventions are described with respect to protein (NP vs. HP) because the relative difference between groups of protein intake is greater than of carbohydrate (60% vs. 21%, respectively). Research participants consumed one multivitamin/mineral supplement (Centrum; Wyeth Consumer Healthcare, Madison, NJ) and two calcium citrate tablets (400 mg calcium/tablet; total 800 mg calcium/d) daily.
Table 1. Macronutrient distributions of prescribed diets consumed during the 12-week energy restriction (750 kcal/day energy deficit) in overweight and obese men (n=43) and women (n-45)a.
| Parameter | Group | Gender | Prescribed diet |
|---|---|---|---|
| Protein (g·kg-1·d-1) | NP | Men | 0.8 |
| NP | Women | 0.8 | |
| HP | Men | 1.4 | |
| HP | Women | 1.4 | |
| Protein (%) | NP | Men | 15 |
| NP | Women | 18 | |
| HP | Men | 25 | |
| HP | Women | 30 | |
| Carbohydrate (%) | NP | Men | 60 |
| NP | Women | 57 | |
| HP | Men | 50 | |
| HP | Women | 45 | |
| Fat (%) | NP | Men | 25 |
| NP | Women | 25 | |
| HP | Men | 25 | |
| HP | Women | 25 |
NP: normal protein diet group; HP: higher-protein diet group.
Research participants were counseled by a registered dietitian to follow 7-d menus with specified quantities of typical and brand-specific food items to purchase and consume. The NP group menus were void of animal flesh foods (i.e., striated tissues) and egg products. The NP research participants were provided with portioned quantities of milk comprising 13% of their total protein intake and the HP research participants were provided with portioned quantities of cooked lean pork (loin, ham and Canadian bacon) and egg products (men only) comprising 40% of their total protein intake. Thus, the NP diet was lacto-vegetarian and the HP diet was omnivorous. This study was not designed to compare sources of protein (dairy –vs. meat/egg). Milk was given to the NP group to equalize the interactions and contact time with the study coordinator that the HP group received. A more detailed dietary intervention is published [9]. Research participants' actual dietary intakes were analyzed using weekly food check-offs that research participant's completed documenting their dietary compliance. Energy and macronutrient composition were calculated using ProNutra (Release 3.2, Viocare Technologies, Inc. Princeton, NJ) for men and Nutritionist Pro Version 2.0 (First Data Bank, San Bruno, CA) for women.
2.4 Body Composition and Bone Measurements
Each research participant's fasting-state body mass (ES200L; Mettler, Toledo, OH) and height were measured and body mass index was calculated. Whole body and regional body composition including fat mass and lean body mass were measured using dual-energy x-ray absorptiometry (DXA; GE Medical Systems/LUNAR Prodigy™) at baseline and post-intervention. Bone mineral content (g) and bone area (cm2) were also measured by DXA. Bone mineral density (g/cm2) was calculated as bone mineral content (g)/bone area (cm2).
2.5 Blood and Urine Sampling and Analyses
Fasting state blood samples were collected on 2 days at baseline and 2 days post-intervention, into serum-separator tubes, centrifuged for 10 min at 4000 g and 4 °C to obtain serum, and stored at -80 °C until analyzed. Blood urea nitrogen (BUN) was measured using a photometric assay (Chemistry Immuno Analyzer AU5700; Olympus) performed by MidAmerica Clinical Laboratories (Indianapolis, IN). BUN was used to crudely document differences in protein consumption between groups [19].
2.6 Statistical Analyses
Data were analyzed using SAS (version 9.1.2; SAS Institute Inc, Cary, NC). Group data are presented as means ± SEM. Student's t test was used to compare differences between the NP and HP groups and between men and women at baseline. Repeated measures ANOVA was performed to access the main effects of group, gender and time, and their interactions. Post hoc analyses used Tukey's multiple comparisons and Student's t test, as appropriate. P < 0.05 was considered statistically significant.
3. Results
3.1 Research Participant Characteristics
There were no differences between the NP and HP groups in height, weight, BMI, or body composition at baseline. Men had a greater height, weight, lean body mass and BMD than women (Table 2).
Table 2. Research participant characteristics at baselinea.
| Parameter | Group | Gender | Baseline |
|---|---|---|---|
| Age (years) | NP | Men | 45 ± 4 |
| NP | Women | 53 ± 3 | |
| HP | Men | 51 ± 3 | |
| HP | Women | 46 ± 2 | |
| Heightb (cm) | NP | Men | 179 ± 1 |
| NP | Women | 165 ± 2 | |
| HP | Men | 180 ± 1 | |
| HP | Women | 163 ± 2 | |
| Weightb (kg) | NP | Men | 103 ± 3 |
| NP | Women | 84 ± 2 | |
| HP | Men | 101 ± 3 | |
| HP | Women | 83 ± 2 | |
| BMI (kg/m2) | NP | Men | 32 ± 0.7 |
| NP | Women | 31 ± 0.9 | |
| HP | Men | 31 ± 0.7 | |
| HP | Women | 31 ± 0.6 | |
| Fat mass (kg) | NP | Men | 37 ± 1.5 |
| NP | Women | 37 ± 1.3 | |
| HP | Men | 35 ± 1.7 | |
| HP | Women | 37± 1.3 | |
| Lean body massb (kg) | NP | Men | 63 ± 1.5 |
| NP | Women | 43 ± 1.0 | |
| HP | Men | 60 ± 1.4 | |
| NP | Women | 43 ± 1.4 | |
| BMDb (g/cm2) | NP | Men | 1.265 ± 0.086 |
| NP | Women | 1.185 ± 0.101 | |
| HP | Men | 1.294 ± 0.109 | |
| HP | Women | 1.204 ± 0.095 |
Means ± SEM, NP: normal-protein diet group, HP: high-protein diet group
Different in men vs. women (P < 0.05)
3.2 Dietary Intake and Compliance
Table 3 shows the actual macronutrient and energy intakes from the research participants at the beginning and end of the intervention (week 2 and week 13 of the trial while week 1 is baseline). There was no change in energy or macronutrient intakes (kcal/d and g/d, respectively) between week 1 and 12. As designed, the NP group consumed less protein and more carbohydrate compared with the HP group (P < 0.001). Both groups consumed similar quantities of fat. Men consumed more energy and macronutrients in grams compared with women. Protein intake expressed as g·kg-1·d-1 increased from week 2 to week 13 in both NP and HP groups because of weight loss, independent of gender.
Table 3. Dietary intakes at the first and twelfth weeks of dietary intervention a.
| Parameter | Group | Gender | Intervention Week 1 | Intervention Week 12 |
|---|---|---|---|---|
| Energyb (kcal/d) | NP | Men | 2263 ± 75 | 2273 ± 78 |
| NP | Women | 1530 ± 40 | 1500 ± 40 | |
| HP | Men | 2234 ± 70 | 2254 ± 75 | |
| HP | Women | 1560 ± 60 | 1540 ± 60 | |
| Proteinc (g·kg-1·d-1) | NP | Men | 0.80 ± 0.02 | 0.88 ± 0.02 |
| NP | Women | 0.82 ± 0.02 | 0.92 ± 0.02 | |
| HP | Men | 1.40 ± 0.02 | 1.55 ± 0.03 | |
| HP | Women | 1.41 ± 0.02 | 1.52 ± 0.02 | |
| Proteind (g/d) | NP | Men | 82 ± 2 | 82 ± 2 |
| NP | Women | 68 ± 3 | 67 ± 3 | |
| HP | Men | 141 ± 4 | 143 ± 4 | |
| HP | Women | 116 ± 4 | 115 ± 4 | |
| Carbohydrated (g/d) | NP | Men | 354 ± 12 | 355 ± 13 |
| NP | Women | 218 ± 6 | 214 ± 6 | |
| HP | Men | 282 ± 10 | 284 ± 11 | |
| HP | Women | 174 ± 6 | 172 ± 6 | |
| Fatb (g/d) | NP | Men | 64 ± 2 | 65 ± 2 |
| NP | Women | 43 ± 1 | 36 ± 2 | |
| HP | Men | 62 ± 2 | 63 ± 2 | |
| HP | Women | 45 ± 2 | 42 ± 2 |
Means ± SEM, NP: normal-protein diet group, HP: high-protein diet group. Intervention weeks 1 and 12 occurred on the 2nd and 13th weeks of the study, since study week 1 was used for baseline data collection.
Different in men vs. women (P < 0.05)
Different in week 12 vs. 1 & NP vs. HP (P < 0.05, no interactions)
Different in men vs. women & NP vs. HP (P < 0.05, no interactions)
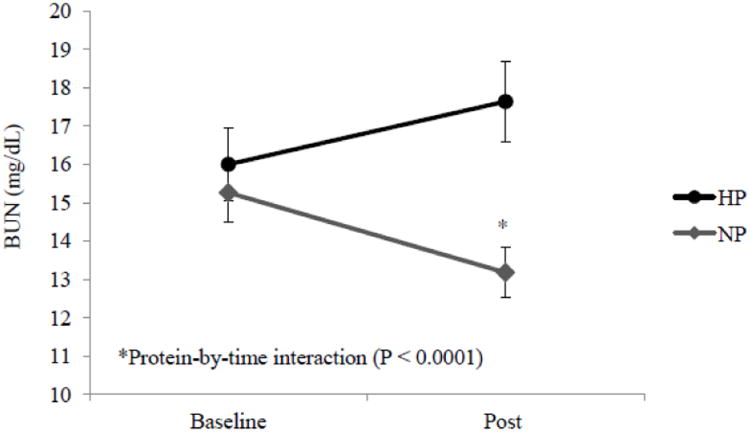
BUN concentration, which reflects dietary protein consumption, increased over time in the HP group (Δ1.6 ± 0.5 mg/dL) and decreased in the NP group (Δ-2.1 ± 0.4 mg/dL), consistent with different protein intakes between groups during the intervention (protein-by-time P < 0.0001). There was no effect of gender on BUN changes (Figure 1). The increase of BUN in the HP group and decrease of BUN in the NP group indicate that the research participants' habitual protein consumption was between 0.8 and 1.4 g ·kg-1·d-1.
Figure 1.
Changes of blood urea nitrogen (BUN) from baseline to post intervention in the normal-protein (NP) and higher-protein (HP) groups. Values are means and error bars.
3.3 Body Composition Changes
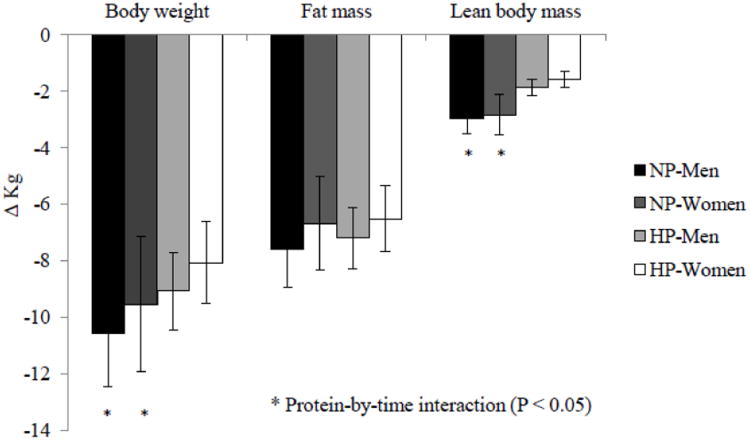
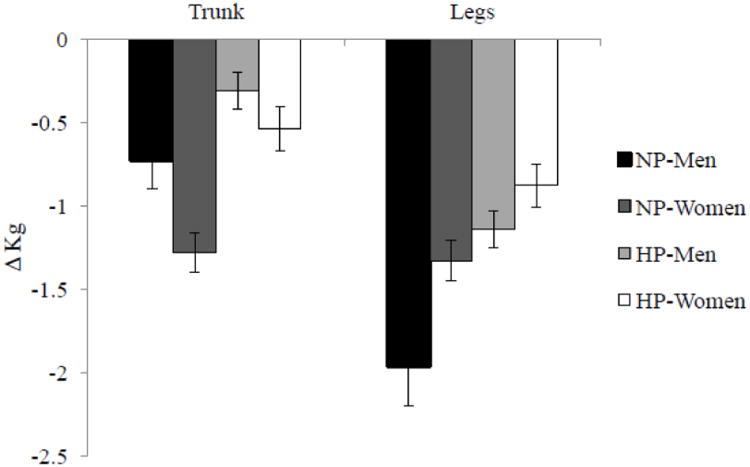
After 12 weeks of energy restriction, all research participants lost significant amounts of body weight, fat mass and lean body mass (Figure 2). The NP group lost more body weight than the HP group, independent of gender (−10.1 ± 0.6 vs. −8.6 ± 0.4 kg; protein-by-time P < 0.05). With respect to lean body mass, the NP group lost more lean body mass than the HP group, independent of gender (−2.9 ± 0.3 vs. −1.7 ± 0.2 kg; protein-by-time P < 0.05). Appendicular lean mass loss, which was primarily muscle, accounted for 65% of total lean mass loss among all research participants. Appendicular lean mass loss occurred in the legs, not the arms. This loss was affected by protein and gender such that the men in the NP group lost the most lean mass, whereas the women in the HP group lost the least (Figure 3), after accounting for baseline differences between men and women. Both protein and gender affected lean mass loss in the trunk region without interactions: women (−1.0 ± 0.2 kg) lost more than men (− 0.5 ± 0.2 kg, P < 0.001) and the NP group (−1.0 ± 0.4 kg) lost more than the HP group (−0.4 ± 0.3 kg, P < 0.05) after baseline difference being adjusted (Figure 3).
Figure 2.
Changes in body weight, fat mass and lean body mass after a 12-week peiiod of energy restriction (750 kcal/day energy deficit) in men (n=43) and women (n=45) in the normal-protein (NP) and higher-protein (HP) groups. Values are means and error bars.
Figure 3. Regional lean mass losses.
Changes in lean mass in the trunk and legs after a 12-week period of energy restriction (750 kcal/day energy deficit) in overweight and obese men (n=43) and women (n=45). There were protein-by-time (P < 0.001) and gender-by-time (P < 0.05) interactions. The main effects of protein and gender were additive. NP: normal-protein diet: HP: higher-protein diet. Values are means and error bars.
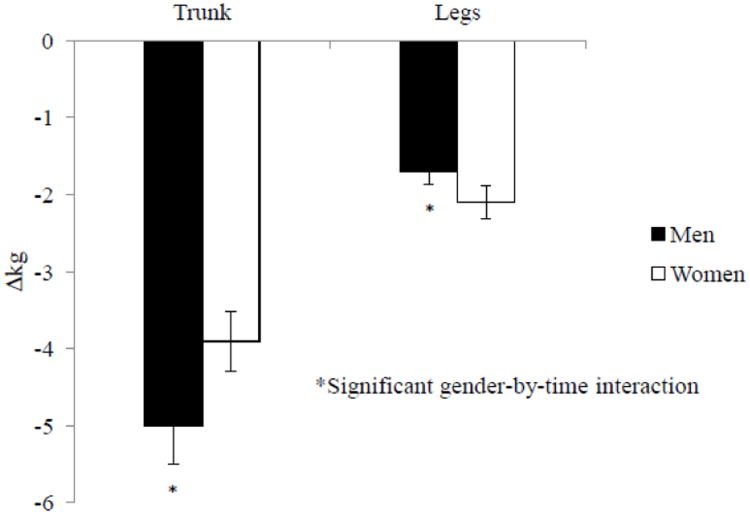
Total fat loss was not different between the NP and HP groups or between men and women. However, regardless of the diets, men lost more fat from trunk (−5.0 ± 0.2 vs. −3.9 ± 0.3 kg, P < 0.05) and less from the legs (−1.7 ± 0.1 vs. −2.1 ± 0.1 kg, P < 0.001) than women after baseline difference between genders were adjusted for (Figure 4). In addition, men (−0.07 ± 0.02) also had a greater decrease of android/gynoid fat ratio than women (−0.04 ± 0.02, gender-by-time interaction P < 0.05).
Figure 4. Regional fat mass losses.
Changes in fat mass in the trunk and legs after a 12-week period of energy restriction (750 kcal/day energy deficit) in overweight and obese men (n=43) and women (n-45). There was an effect of gender-by-time (P < 0.05) in both the trunk and legs, but no effect of dietary protein groups. Values are means and error bars.
3.4 Bone Mineral Density Changes
After adjusting for baseline difference in BMD between men and women, BMD decreased over time in the women, but not the men (−0.011 ± 0.003 vs. −0.003 ± 0.002 g/cm2, gender-by-time interaction P < 0.01), independent of diet. No change in BMC was observed.
4. Discussion
Research participant's compliance was closely monitored in both subjective and objective ways. Twice weekly during the intervention, the research participant came to the research laboratory to self record his/her body weight and return the weekly food check-off list. This regimen was very effective to monitor the research participant's weight change pattern and dietary compliance as well as keep the research participant motivated.
A 750 kcal daily energy deficit should, theoretically, lead to approximately 9.2 kg weight loss over 12 weeks, assuming 25% loss is lean body mass and 75% is fat mass [2]. The average weight loss among men and women was 9.3 ± 3.7 kg and fat loss was 7.0 ± 2.6 kg (75%). It is important to note that during the period of energy deficiency, the human body tends to reserve energy via lowing basal metabolic rate and increasing energy utilization efficiency. Thus, achieving a net 750 energy deficiency would either require a dietary change higher than the prescribed diet allowed or an increase in energy expenditure. In this study, energy requirement was estimated by an activity factor of 1.5. Giving that the actual weight loss was extremely close to the theoretical value, it is possible that subjects initially lost body water or the average physical activity level of these participants were higher than 1.5. Because body composition was measured by DXA scan, it is also possible that fat mass change was a little bit over estimated during active weight loss [20]. Nonetheless, these issues should not detract from the overall success of the diet interventions to achieve significant weight loss and body composition changes. Results from the study support our hypothesis that body composition changes via energy restriction are diet and gender specific. Findings from numerous studies suggest that a higher protein intake can help preserve lean body mass during weight loss [6-8]. A recent meta-regression [8] showed that protein consumption is strongly associated with lean body mass changes and the quantity of lean mass retention increases with the increase of protein consumption. Maintaining lean body mass is critical for preventing weight regain and maintain physical functions [21]. In the current study, a high protein intake helps to preserve lean mass from both the trunk and the legs, suggesting a HP diet may be beneficial for attenuating the losses of both organ mass and muscle mass [22]. This conclusion should be viewed cautiously since the trunk region includes both organ and muscle mass. Within the appendicular region, the higher protein intake reduced the loss of lean mass in the legs, but not the arms, suggesting that protein intake impacts weight-bearing muscles more than non-weight-bearing muscles.
Improving body composition, especially body fat distribution, is essential for improving health status after weight loss. Abdominal adipose tissue is directly associated with metabolic abnormalities such as diabetes, hypertension and cardiovascular disease [23] due to chronic inflammation associated with central obesity [24]. Very limited research exists that assess changes of regional fat distribution with different dietary protein intakes during weight loss. In the current study, all research participants lost significant amounts of fat from the trunk, independent of dietary protein. These findings are consistent with a previous study [25] in which research participants consumed either a normal-protein (15% energy from protein) or a high-protein weight-loss diet (30% energy from protein) for 5 months and displayed comparable fat mass loss from the trunk. Some studies [6, 21, 26] suggest high-protein diets induce more fat mass loss compared with a normal-protein diet during weight loss, but none specified changes in fat distribution. An interesting and novel observation of the current study is that it appears that men benefit more from the HP diet due to a greater fat mass loss from the trunk and decrease of android/gynoid fat ratio than women. With the same energy deficiency, men tended to utilize more fat from the trunk region than women, although the mechanism for this differential response is not known. It is also important to note that losing fat from the peripheral region (appendicular fat) may be adverse for women since it is an independent protective factor of cardiovascular diseases [27]. Although dietary protein is shown to have anabolic effects on bone with weight stable conditions [15, 28], debates continue on whether these effects still hold during weight loss. Previously, our research group reported in two separate interventions (12 weeks and 9 weeks) that postmenopausal women lost more total body BMD when consuming a high-protein diet (BMD change: −1.4 ± 0.4% and −1.1 ± 0.3%) compared with women consuming a normal-protein diet (BMD change: −0.3 ± 0.2% and 0%) during weight loss [16]. Research participants from one of the two interventions (Study 1) were a subgroup of women from the current study who are postmenopausal. In the current study, the effect of dietary protein is no longer detectable when including premenopausal women and men in the analysis because there was no time or protein effect on BMD in premenopausal women or men. The differential response was driven by the postmenopausal women on the HP diet. Postmenopausal women have a much higher risk of developing osteoporosis and fracture occurrence due to lack of estrogen [29]. Very limited research is conducted assessing the impact of dietary protein on BMD during weight loss and results are inconclusive. Some research shows dietary protein attenuates BMD during weight loss [17] and some suggests dietary protein is detrimental [16], while others studies found no effect of dietary protein on bone [30, 31]. Only one study directly assessed the effect of menopausal status on the results. Two high-protein (1.2 g protein·kg-1·d-1) diets from difference sources (mixed or predominately dairy) were consumed by obese men and women for 12-weeks. There was no change of BMD from baseline and no interactions with menopausal status or weight loss [32]. Note that the sample size is relatively small with a total of 25 women and 17 were postmenopausal. Although results from the present study did not support our hypothesis, it is possible that there may not be enough postmenopausal women included to show a differential response. Other studies that found no effect of dietary protein included both men and women [30, 31], like the current study, but did not assess menopausal status. In fact, research participants from studies that showed changes of BMD from baseline when consuming a high-protein, energy-restricted diet, were all postmenopausal women [16, 17]. Collectively, although data are limited, it appears that postmenopausal women are more prone to BMD changes than premenopausal women and men, when consuming a high-protein, energy-restricted diet. Of course, duration of intervention, quantity and predominant source of protein are all important contributing variables other than menopausal status.
The fact that these results were generated from secondary analyses of data from separate studies conducted with men and women [10, 33] using the same study design is a limitation. The original studies were not designed to test the potential gender difference in body composition changes during weight loss, but the retrospective analysis suggests the sample size did give enough power to detect a gender difference. However, as discussed above, the subgroup of postmenopausal women may not be large enough to show a differential response in BMD changes.
In conclusion, the consumption of a high-protein, moderate energy-restricted diet for a short period of time (12 weeks) in overweight and obese men and women attenuate lean body mass loss from both trunk and appendicular regions and does not affect fat mass loss. It appears that men benefit more from energy-restriction induced weight loss as men exhibited a greater improvement of fat distribution than women, and maintained BMD during weight loss.
Acknowledgments
This study was funded by the National Pork Board, American Egg Board-Egg Nutrition Center, with support from the Indiana Clinical and Translational Sciences Institute, grant # TR000006.
List of Abbreviations
- NP
normal-protein diet
- HP
high-protein diet
- FM
fat mass
- LBM
lean body mass
- BMD
bone mineral density
- BMI
body mass index
- IGF-1
insulin-like growth factor 1
- DXA
dual-energy x-ray absorptiometry
- BUN
blood urea nitrogen
Footnotes
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orpana HM, Berthelot JM, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity (Silver Spring) 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 3.Johnston CS, Tjonn SL, Swan PD. High-protein, low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults. J Nutr. 2004;134:586–591. doi: 10.1093/jn/134.3.586. [DOI] [PubMed] [Google Scholar]
- 4.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502. doi: 10.1038/sj.ejcn.1600782. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. doi: 10.1038/sj.ijo.0802461. [DOI] [PubMed] [Google Scholar]
- 6.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 7.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a metaregression 1. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 9.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 10.Tang M, Armstrong CL, Leidy HJ, Campbell WW. Normal vs high-protein weight loss diets in men: Effects on body composition and indices of metabolic syndrome. Obesity. 2013;21:E204–210. doi: 10.1002/oby.20078. [DOI] [PubMed] [Google Scholar]
- 11.Emery EM, Schmid TL, Kahn HS, Filozof PP. A review of the association between abdominal fat distribution, health outcome measures, and modifiable risk factors. Am J Health Promot. 1993;7:342–353. doi: 10.4278/0890-1171-7.5.342. [DOI] [PubMed] [Google Scholar]
- 12.Dionne I, Despres JP, Bouchard C, Tremblay A. Gender difference in the effect of body composition on energy metabolism. Int J Obes Relat Metab Disord. 1999;23:312–319. doi: 10.1038/sj.ijo.0800820. [DOI] [PubMed] [Google Scholar]
- 13.Volek J, Sharman M, Gomez A, Judelson D, Rubin M, Watson G, Sokmen B, Silvestre R, French D, Kraemer W. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab (Lond) 2004;1:13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68:859–865. doi: 10.1093/ajcn/68.4.859. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP, McCarron DA, Dawson-Hughes B, Oparil S, Berga SL, Stern JS, Barr SI, Rosen CJ. Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc. 1999;99:1228–1233. doi: 10.1016/S0002-8223(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 16.Campbell WW, Tang M. Protein intake, weight loss, and bone mineral density in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2010;65:1115–1122. doi: 10.1093/gerona/glq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011;26:1339–1348. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morse MH, Haub MD, Evans WJ, Campbell WW. Protein requirement of elderly women: nitrogen balance responses to three levels of protein intake. J Gerontol A Biol Sci Med Sci. 2001;56:M724–730. doi: 10.1093/gerona/56.11.m724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. The American journal of clinical nutrition. 1998;67:734–738. doi: 10.1093/ajcn/67.4.734. [DOI] [PubMed] [Google Scholar]
- 21.Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, Griel A, Psota T, Kris-Etherton P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139:514–521. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 22.Byrne NM, Weinsier RL, Hunter GR, Desmond R, Patterson MA, Darnell BE, Zuckerman PA. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: comparison of responses in white and black women. Am J Clin Nutr. 2003;77:1368–1373. doi: 10.1093/ajcn/77.6.1368. [DOI] [PubMed] [Google Scholar]
- 23.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 24.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- 25.Aldrich ND, Reicks MM, Sibley SD, Redmon JB, Thomas W, Raatz SK. Varying protein source and quantity do not significantly improve weight loss, fat loss, or satiety in reduced energy diets among midlife adults. Nutr Res. 2011;31:104–112. doi: 10.1016/j.nutres.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 27.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. The American journal of clinical nutrition. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 28.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Jr, Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–1050. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Treyzon L, Chen S, Yan E, Thames G, Carpenter CL. Protein-enriched meal replacements do not adversely affect liver, kidney or bone density: an outpatient randomized controlled trial. Nutr J. 2010;9:72. doi: 10.1186/1475-2891-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–1306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 32.Bowen J, Noakes M, Clifton PM. A high dairy protein, high-calcium diet minimizes bone turnover in overweight adults during weight loss. J Nutr. 2004;134:568–573. doi: 10.1093/jn/134.3.568. [DOI] [PubMed] [Google Scholar]
- 33.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity. 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]