Abstract
Background
Cardiac allograft vasculopathy (CAV) is the major cause of late allograft loss following heart transplantation. CAV lesions contain alloreactive T cells that secrete IFN-γ, a vasculopathic cytokine, and occur more frequently in patients with donor specific antibody (DSA). Pathologic interactions between these immune effectors, representing cellular and humoral immunity, respectively, remain largely unexplored.
Methods and Results
We used human panel reactive antibody (PRA) to form membrane attack complexes (MAC) on allogeneic endothelial cells in vitro and in vivo. Rather than inducing cytolysis, MAC upregulated inflammatory genes, enhancing the capacity of EC to recruit and activate allogeneic IFN-γ-producing CD4+ T cells in a manner dependent upon activation of non-canonical NF-κB signaling. Non-canonical NF-κB signaling was detected in situ within EC both in renal biopsies from transplant patients with chronic antibody-mediated rejection and in PRA-treated human coronary artery xenografts in immunodeficient mice. Upon re-transplantation into immunodeficient hosts engrafted with human T cells, PRA-treated grafts recruited more IFN-γ-producing T cells and enhanced CAV lesion formation.
Conclusions
Alloantibody and complement deposition on graft EC activate non-canonical NF-κB signaling, initiating a pro-inflammatory gene program that enhances alloreactive T cell activation and development of CAV. Non-canonical NF-κB signaling in EC, observed in human allograft specimens and implicated in lesion pathogenesis, may represent a target for new pharmacotherapies to halt the progression of CAV.
Keywords: transplantation, arteriosclerosis, endothelium
Cardiac allograft vasculopathy (CAV), characterized by diffuse concentric stenosis of graft vessels, is a highly prevalent complication of heart transplantation, accounting for a majority of late allograft loss.1 There are no effective medical therapies for CAV and once initiated, its progression is inexorable.2,3 Affected vessels lack signs of acute injury but contain infiltrating host T cells, largely subjacent to luminal endothelium, and a diffusely expanded intima formed by smooth muscle-like cells4 that, in humans, are of graft origin.5–7 Infiltrating intimal T cells elaborate IFN-γ,8 and human artery segments implanted into immunodeficient mice respond to human IFN-γ by smooth muscle cell (SMC) proliferation, resulting in stenotic lesions resembling CAV.9,10 Human coronary artery endothelial cells (EC) express class I and class II HLA in situ4,9 and cultured human EC, treated with IFN-γ to restore HLA expression, efficiently activate allogeneic memory T cells to secrete IFN-γ.11 These observations have suggested that IFN-γproducing T cells, activated by allogeneic HLA molecules expressed on graft EC, cause CAV.12 Donor-specific antibodies (DSA) reactive with graft HLA molecules are a strong independent risk factor for CAV.13–15 Since coronary artery SMC only weakly express HLA molecules in situ,10 DSA is believed to target graft EC. In chronic antibody-mediated rejection (CAMR), DSA deposits on microvascular EC, activating complement without causing cell lysis.16,17 We hypothesized that DSA binding to and complement activation upon graft arterial EC could enhance their capacity to recruit and activate allogeneic host T cells, leading to increased local production of IFN-γ.
Since each acquired DSA typically recognizes one HLA specificity, opportunities to obtain EC expressing the relevant HLA allele are very limited. However, a significant subset of transplant candidates are highly-sensitized prior to transplantation, having high-titer polyclonal mixtures of panel reactive antibodies (PRA) in their serum that recognize many different non-self HLA specificities.18 PRA, like DSA, can activate complement and cause antibody-mediated rejection,19 serving as a useful tool to model effects of DSA on EC.20
Here we report that, PRA activates cultured human EC that had been pretreated with IFN-γ, enhancing the capacity of EC to recruit and activate allogeneic T cells. Complement membrane attack complex (MAC), formed in response to binding of PRA, activates non-canonical (but not canonical) NF-κB signaling in EC, a pathway necessary for enhancing EC-mediated T cell responses. Non-canonical NF-κB signaling is activated in both the EC of allograft biopsies from renal transplant patients with CAMR, but not control biopsies, and in EC lining human coronary arteries implanted as xenografts into immunodeficient mice when exposed to PRA in vivo. Furthermore, human artery graft EC activated by binding of PRA and deposition of non-lytic mouse MAC in vivo show augmented T cell-mediated CAV-like lesions when re-transplanted into recipient mice previously engrafted with T cells allogeneic to the artery segment.
METHODS
Detailed experimental protocols are reported in the Expanded Materials and Methods section in the online supplement. All experiments using human materials were approved by the relevant Institutional Review Boards and those involving animals by the Yale Institutional Animal Care and Use Committee. In vitro studies of human EC responses were conducted using multiple different isolates of serially passaged HUVEC pretreated with IFN-γ to restore in situ levels of class I and class II HLA molecule expression. De-identified high titer PRA sera were obtained from the Yale-New Haven Hospital HLA typing lab. HUVEC responses to PRA sera, control sera, components of these sera, isolated complement components or other agents were assessed by flow cytometry, immunofluorescence microscopy, Western blotting, reporter genes, expression microarrays, or real time quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR). CD4+ memory T cells were isolated from peripheral blood mononuclear cells collected by leukapheresis and interactions with EC were analyzed for adhesion under flow or for activation in response to direct recognition of non-self HLA molecules by flow cytometry or ELISA. De-identified human renal allograft biopsies were analyzed by immunofluorescence microscopy. Responses of human artery xenografts in immunodeficient mice were analyzed by histology, morphometric analyses, immunofluorescence microscopy, and qRT-PCR. Student’s t-test, ANOVA, and Mann-Whiteney analyses were performed using Origin computer software (Northampton, MA). Two-sided p-values are presented in the text with p-values <0.05 considered significant.
RESULTS
High-titer PRA deposits alloantibody and sub-lytic complement on endothelial cells, potentiating EC-mediated recruitment and activation of alloimmune CD4+ T cells
High titer PRA sera from transplant candidates with >80% class I and/or II HLA reactivity have a similar IgG subtype distribution as control non-PRA sera (n=4, Fig 1a, top graph). All four human IgG subtypes, including both IgG1 and IgG3, subtypes that efficiently fix complement, bind to allogeneic human umbilical vein endothelial cells (HUVEC), indicative of the polyclonal nature of PRA (n=4, Fig 1a, bottom histograms). IFN-γ pretreatment, which increases expression of class I and induces class II HLA antigens on cultured EC to in situ levels of expression, increased IgG binding and permitted efficient early and terminal complement binding to HUVEC as assessed by C4d and polyC9 staining, respectively (Fig 1b). EC express several different complement regulatory proteins that contribute to resistance to lysis. We observed no significant change in surface expression of any of these several complement regulatory molecules as a consequence of PRA treatment (Fig 1c, top row), but EC viability was similar following treatment with PRA sera for 4 hours compared to control sera lacking anti-HLA antibodies (Fig 1d). Viability remained comparable, to that of EC exposed to non-PRA sera or to EC treated with complement inactivated (CI)PRA sera for at least 72 h after treatment (Fig 1e, left graph), consistent with prior reports demonstrating EC survival following in vitro assembly of MAC.21–25 EC viability is reduced during incubation with allogeneic CD4+ T cells. To assess if treatment with PRA sera affected EC viability in this setting, HUVEC were pre-treated with vehicle, CIPRA, or PRA for 6 h and then co-cultured with allogeneic memory CD4+ T cells for up to 7 days. PRA-treated EC showed slightly increased viability compared to controls (Fig 1e, right graph).
Figure 1.
High-titer PRA deposits alloantibody and sub-lytic complement on endothelial cells, potentiating EC-mediated recruitment and activation of alloimmune CD4+ T cells. Sera were assayed for antibody isotype (a, top graph, n=4), and isotype binding to HUVEC was quantified by flow cytometry (a, bottom histograms). Human IgG, C4d, and polyC9 were detected by flow cytometry in HUVEC pre-treated with or without IFN-γ (50ng/mL) for 48–72 hours and treated with PRA sera in gelatin veronal buffer for 2 hours (b). Error bars indicate standard deviations. CD46, CD55, and CD59 expression was assessed by flow cytometry at the indicated times (c). Annexin V and propidium iodide staining in CD31+ gated cells was assessed by flow cytometry (d, left and right graphs). Viability was measured by Trypan blue exclusion at various times in HUVEC pre-treated with IFN-γ for 48–72 hours prior to addition of PRA (e, left graph). HUVEC were pre-treated with vehicle, CIPRA, or PRA for 6 hours and co-cultured with allogeneic CD4+CD45RA−HLA-DR− T cells. Annexin V staining in CD31+ gated cells was assessed by flow cytometry at the indicated times (e, right graph). Asterisks indicate p<0.05 in pairwise comparisons with vehicle-treated EC. CD4+CD45RA−HLA-DR− T cells were flowed over confluent HUVEC monolayers pre-treated with vehicle, complement-inactivated PRA, or PRA for 24 hours. Captured T cells were quantified by immunostaining (f). CD4+CD45RA−HLA-DR− T cell proliferative responses (g, top row) and intracellular cytokine production (g, second row) were measured 7 days after co-culture with HUVEC given the indicated pre-treatments for 4 hours. Elaborated Th1-like cytokine in culture supernatants was measured by ELISA 24 hours after the addition of T cells (g, bottom row). All assays were conducted at least three times with similar results; representative data are shown.
We next assessed the effect of PRA and MAC deposition, on the ability of EC to recruit and activate CD4+ T cells. For T cell recruitment, IFN-γ-pre-treated EC were incubated with PRA, CIPRA, or vehicle, and human memory CD4+ T cells were flowed over the monolayer. PRA-treated EC captured significantly more CD4+ T cells compared to CIPRA- or vehicle-treated EC (Fig 1f). To examine whether PRA treatment altered immunogenicity of EC, IFN-γ-pretreated EC were incubated with PRA, CIPRA, or vehicle and then co-cultured with human allogeneic memory CD4+ T cells for 7 days. Incubation with PRA significantly increased the proliferative responses of CD4+ T cells, assessed by CFSE dilution (Fig 1g, top row), and IFN-γ synthesis, assessed by intracellular cytokine staining (Fig 1g, second row). Increased Th1-like cytokine elaboration was confirmed by ELISA of culture supernatants (Fig 1g, bottom row). We concurrently observed increased production of IL-4 and IL-17 by CD4+ T cells (data not shown), indicating activation of multiple memory T helper subpopulations. Complement inactivation significantly reduced but did not completely abolish the ability of PRA to potentiate the immunogenicity of EC, suggesting some effect of alloantibody binding that may be independent of complement (Fig 1f and 1g).
Alloantibody plus complement initiates a pro-inflammatory gene program in PRA-treated EC
To further characterize PRA-induced EC changes, we performed microarray analyses of HUVEC treated with PRA sera (alloantibody plus complement activation), CIPRA sera (alloantibody but no complement activation), non-PRA sera (serum factors only), or vehicle. Principal component analysis showed that PRA sera initiated a gene expression program in EC that was globally distinct from other treatment groups (Fig 2a, left, Supplementary Fig 1a–1c). CIPRA also had some effect on gene expression relative to non-PRA sera (NPRA, Supplementary Table 1) confirming that alloantibody can alter gene expression in EC.26 By unsupervised hierarchical clustering we observed that, compared to CIPRA, PRA reproducibly induced multiple inflammatory genes including chemokines, cytokines, cytokine receptors, and adhesion molecules (Fig 2a, right, Supplementary Table 2, and Supplementary Table 3. We selected CCL5, CCL20, IL-6, SELE (E-selectin), and vascular cell adhesion molecule (VCAM)-1 for validation based on their reproducible and significant upregulation in microarray analyses and on their previously characterized roles in inflammation. We confirmed that PRA sera increased mRNA expression of this subset of inflammatory genes assessed by real time qRT-PCR (Fig 2b, left) and increased protein expression for the latter two molecules assessed by flow cytometry (Fig 2b, right).
Figure 2.
Alloantibody plus complement initiates a pro-inflammatory gene program in PRA-treated EC. Principal component analysis of HUVEC treated with PRA sera, CIPRA sera, non-PRA sera, or vehicle (a, left). Expression of genes by microarray analysis of HUVEC treated with PRA sera vs CIPRA sera and non-PRA sera (a, right). Upregulation of selected genes by PRA sera compared to non-PRA sera was confirmed by RT-PCR (b, left) and by flow cytometry (b, right). Asterisks indicate p<0.05 in non-PRA- vs PRA-treated EC. Microarray analyses were performed 3 times analyzing triplicate samples for each treatment.
Membrane attack complex formation is necessary for pro-inflammatory gene expression in PRA-treated EC
To analyze the contributions of alloantibody and complement to EC activation, PRA sera were separated into IgG+ fractions containing alloantibody and IgG− fractions containing complement. Neither fraction alone could deposit C4d on EC (Fig 3a, middle and bottom scatterplots) compared to intact PRA sera (top scatterplot, gated cells). However, the IgG+ fraction of PRA serum could deposit C4d when combined with non-PRA serum (NPRA) containing complement (Fig 3b, bottom scatterplot, gated cells), and the IgG− fraction of PRA serum could deposit C4d when combined with complement-inactivated serum (CIPRA) containing alloantibody (Fig 3c, bottom scatterplot, gated cells). Neither the IgG+ (Fig 3a, middle graph) or IgG− fractions (Fig 3a, bottom graph) of PRA serum could significantly match the degree of inflammatory gene induction caused by intact PRA (Fig 3a, top graph), but the purified IgG+ fraction from PRA serum could do so when combined with NPRA serum containing complement (Fig 3b, bottom graph). Similarly CIPRA serum strongly induced inflammatory genes when combined with a complement-containing IgG− fraction of PRA serum (Fig 3c, bottom graph), confirming that complement activation was required for full induction of this subset of proinflammatory genes in PRA sera-treated HUVEC. Neutralizing antibodies to C3a and C5a did not inhibit the response to PRA when added prior to or after PRA addition (Fig 3d, left and right graphs). However, compared to the PRA IgG+ fraction combined with NPRA serum, the response was markedly diminished when combined with C6- or C9-deficient sera (Fig 3e, left graph), suggesting that MAC formation is necessary for full induction of inflammatory gene expression. To determine if alloantibody is necessary for the alterations induced in HUVEC by PRA, we assembled MAC on the surface of EC using isolated terminal complement components. In vitro formation of MAC elicited some inflammatory gene expression compared to untreated controls, but at significantly lower levels than intact PRA (Fig 3e, right graph) likely because MAC assembled from purified components was expressed at lower levels than MAC assembled from PRA sera on EC (Supplementary Fig 2). Neither MAC formation nor inflammatory gene expression were increased by adding PRA IgG+ fractions after assembly of MAC with late complement components (Supplementary Fig 2, Fig 3e, right graph). Thus alloantibody seems to function primarily to generate high levels of MAC, though a lesser direct effect of alloantibody was also observed.
Figure 3.
Membrane attack complex formation is necessary for pro-inflammatory gene expression in PRA-treated EC. PRA serum (a, top row) was fractionated into IgG+ and IgG− fractions, and added to HUVEC cultures either alone (a, middle and bottom rows) or in combination with non-PRA (b, bottom row) or complement-inactivated PRA (c, bottom row) for 4 hours. HUVEC were subsequently stained for surface deposition of IgG and C4d, and gene expression was assessed by RT-PCR. Statistical comparisons for each respective gene were made comparing PRA serum to either PRA IgG(+) and PRA IgG(−) fractions for Fig 3a, NPRA + PRA IgG(+) fraction to NPRA serum for Fig 3b, and CIPRA + PRA IgG(−) fraction to CIPRA serum for Fig 3c. Asterisks indicate p<0.05. HUVEC were pre-treated with blocking Ab as indicated for 30 minutes prior to (d, left graph) or following (d, right graph) addition of PRA. N.S. indicates p>0.05. The IgG+ fraction of PRA serum was added to NPRA, C6−, or C9− sera (e, left graph). Asterisks indicate p<0.05 comparing PRA IgG(+) fraction + NPRA with either PRA IgG(+) fraction + C6− serum or PRA IgG(+) fraction + C9− serum. Gene expression was measured by RT-PCR following in vitro assembly of membrane attack complexes (C5b-9) for four hours. In some groups, PRA IgG (+) fractions were added after MAC assembly (e, right graph). Asterisks indicate p<0.05 and N.S. indicate p>0.05 for the indicated comparisons. In all experiments RT-PCR data is reported as normalized threshold cycle where a relative decrease in cycle number by x indicates gene induction by 2x. Experiments were repeated at least three times using duplicate wells per sample.
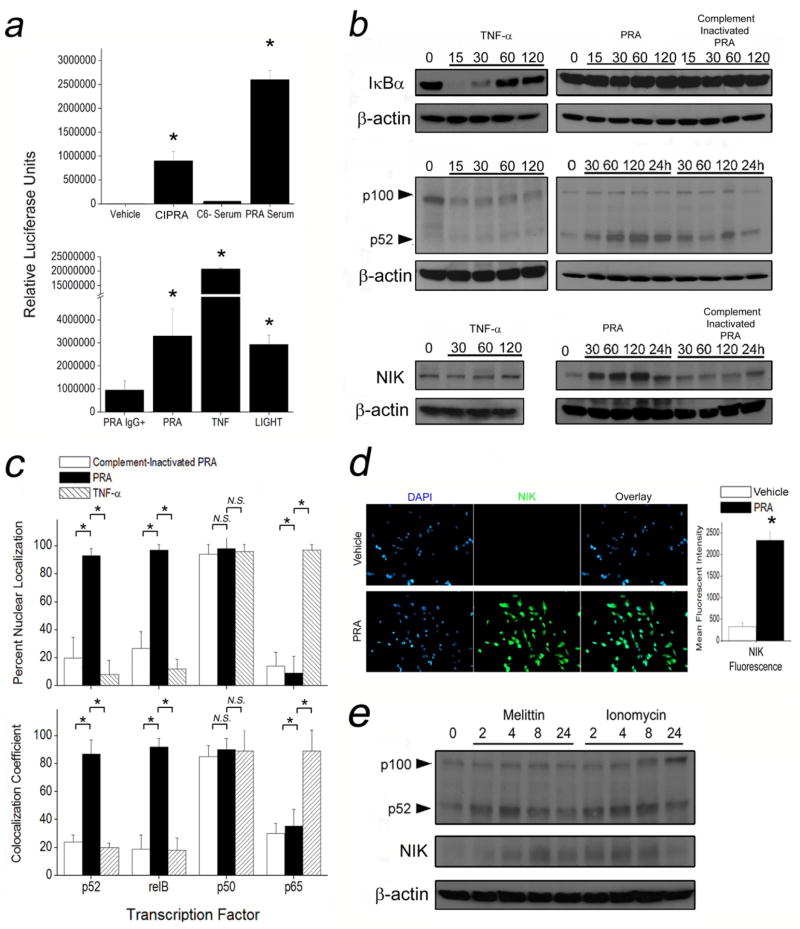
MAC and pore-forming compounds selectively activate non-canonical NFκB signaling in EC
Pathway analysis and cross-referencing of public gene databases indicated that a significant subset of genes upregulated by PRA in EC are regulated by NF-κB (Supplementary Table 4). We therefore analyzed NF-κB activation using HUVEC transfected with an NF-κB luciferase reporter that responds to both TNF-α or LIGHT, cytokine activators of canonical and non-canonical NF-κB signaling, respectively (Fig 4 a, bottom). EC treated with PRA showed increased luciferase activity compared to vehicle-, CIPRA-, and complement deficient sera-treated controls (Fig 4a, top), suggesting complement-induced NF-κB activation. To test which pathway was activated by PRA, we compared EC treated with PRA to EC treated with TNF-α. TNF-α, but not PRA, induced IκBα degradation (Fig 4b, top, Supplementary Fig 3). PRA, but not TNF-α, induced partial processing of p100 to p52 (Fig 4b, middle) and increased levels of NF-κB-inducing kinase (NIK) (Fig 4b, bottom). No changes in IκBα, p52, or NIK levels in vehicle-treated controls were observed (data not shown). Immunofluorescence microscopy further revealed that PRA induced nuclear translocation of p52 and relB but not of p65 subunits of NF-κB (Fig 4c, Supplementary Fig 4) and confirmed that PRA increased NIK expression (Fig 4d). These responses indicate that PRA selectively activates the non-canonical NF-κB pathway.
Figure 4.
MAC and pore-forming compounds selectively activate non-canonical NF-κB signaling in EC. HUVEC transfected with an NF-κB promoter-luciferase reporter were treated with vehicle, CIPRA, C6− sera, and PRA for 6 hours (a, top graph) or with TNF-α, LIGHT, and PRA for 6 hours (a, bottom graph). Following the indicated treatments, HUVEC were probed by Western blot for IκBα (b, top panels), p100/p52 (middle panels), or NIK (b, bottom panels) for the indicated times. HUVEC were treated with CIPRA (white bars), PRA (black bars), or TNF-α (hatched bars), and immunostained to localize NFκB signaling components. Nuclear staining was measured by percent nuclear localization (c, top) and co-localization coefficients (c, bottom). HUVEC were treated with vehicle (white bars) or PRA (black bars) for 4 hours and immunostained for NIK (d). Asterisks indicate p<0.05. HUVEC, in the presence of sub-lytic concentrations of melittin and ionomycin, were probed at various times as indicated by Western blot for p100/p52 and NIK (e). Asterisks indicate p<0.05 comparing PRA-treated samples to vehicle-treated controls. All experiments were repeated at least three times with different sources of PRA and HUVEC with similar results.
To investigate whether MAC activated non-canonical NF-κB signaling through pore formation, sub-lytic concentrations of melittin and ionomycin, alternative pore-forming compounds, were added to HUVEC cultures. Both melittin and ionomycin induced p52 processing and increased NIK expression (Fig 4e), suggesting that non-canonical NF-κB activation may indeed occur in response to sublytic surface pore formation on EC.
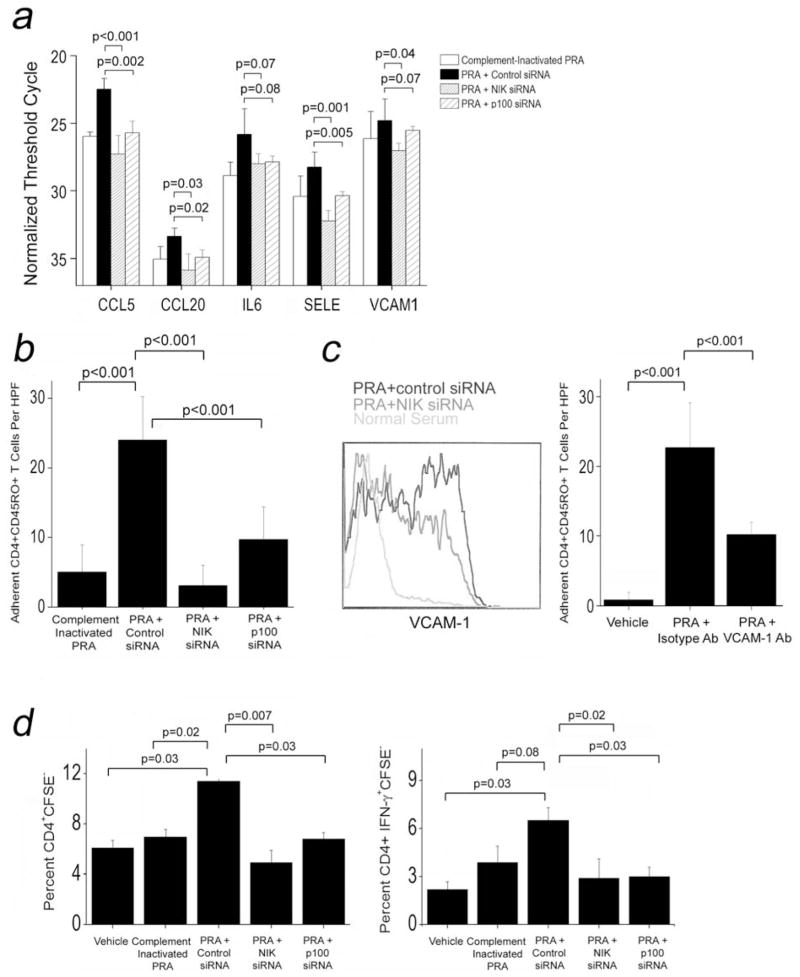
Non-canonical NF-κB signaling in EC is required for PRA-mediated activation of allogeneic T cells
To explore the relationship of non-canonical NF-κB signaling to EC activation in response to PRA, we performed siRNA-mediated knockdown of NIK or p100, essential components of non-canonical NF-κB signaling and observed inhibition of the increase in mRNA expression of five pro-inflammatory genes (CCL5, CCL20, IL-6, E-selectin and VCAM-1) induced by PRA (Fig 5a). NIK or p100 knockdown also decreased the number of CD4+ T cells captured by PRA-treated HUVEC under flow conditions (Fig 5b). To investigate the significance of PRA-induced VCAM-1 expression, we confirmed that PRA treatment induced VCAM-1 protein and demonstrated that NIK knockdown reduced VCAM-1 protein expression induced by PRA treatment (Fig 5c, left). Treatment of PRA-activated HUVEC with blocking antibody against VCAM-1 significantly abrogated T cell adhesion to the EC monolayer compared to treatment with isotype antibody control under flow conditions (Fig 5c, right graph), identifying VCAM-1 as a PRA-induced molecule that can functionally alter EC interactions with T cells. Additionally, knockdown of NIK or p100 significantly abrogated the enhanced proliferation of (Fig 5d, left) and cytokine secretion by (Fig 5d, right) allogeneic memory CD4+ T cells co-cultured with PRA-treated EC. Thus the enhanced immunological functions of EC following PRA treatment and MAC formation are significantly dependent on non-canonical NF-κB signaling.
Figure 5.
Non-canonical NF-κB signaling in EC is required for PRA-mediated activation of allogeneic T cells. HUVEC were transfected with NIK, p100, or control siRNA and treated with vehicle, CIPRA or PRA sera. siRNA-transfected HUVEC were then assessed for pro-inflammatory genes by RT-PCR (a). Adhesion of CD4+CD45RA−HLA-DR− T cells under flow conditions were measured following transfection with the indicated siRNAs (b). HUVEC were transfected with the indicated siRNAs and VCAM-1 expression was assessed by flow cytometry (c, left). T cell adhesion under flow conditions was measured following pre-treatment with the indicated antibodies (c, right graph). HUVEC were transfected with the indicated siRNAs and assessed for capability to elicit T cell proliferative (d, left graph) and IFN-γ cytokine responses (d, right graph). Representative data from 2 to 6 experiments shown.
Non-canonical NF-κB activation in peritubular capillaries in CAMR patients and in human arterial intima in vivo
To test whether non-canonical NF-κB activation occurs in human graft EC we used immunofluorescence to compare, renal allograft biopsies from three patients who had developed graft dysfunction in the setting of a DSA and who also showed C4d deposition on peritubular capillary EC, indicative of CAMR, to biopsies from three transplant patients with preserved renal function lacking DSA and C4d positivity. We found both significantly increased p52 nuclear staining (Fig 6a) and NIK expression (Fig 6b) in EC in all three CAMR+ patients compared to CAMR− patients (Fig 6c).
Figure 6.
Non-canonical NF-κB activation in peritubular capillaries in CAMR patients and in human arterial intima in vivo. p52 nuclear staining (a) and NIK expression (b) were assessed in renal biopsies from CAMR negative and CAMR positive patients (scale bars indicate 100μm). In CAMR negative (n=3) and CAMR positive (n=3) biopsies p52 nuclear staining was quantified using colocalization coefficients, and NIK staining was calculated by percent staining of C4d+ microvessels (c). SCID/beige mice bearing human arterial segments were injected with PRA or IgG depleted PRA sera. Arterial grafts were harvested 18–24 hours later and immunostained (d, n=6 pairs). Nuclei within regions costained with ULEX and C4d were quantified in five high power fields (e, top row scale bars indicate 400μm and bottom row scale bars indicate 200μm). Arterial grafts were harvested 18–24 hours after injection with intact PRA sera or IgG depleted PRA sera and immunostained (f, top), intra-graft gene transcripts were quantified by RT-PCR normalized to CD31 (f, bottom graph), and p52 nuclear staining and NIK cell staining were assessed (g, n=5 pairs). Following treatment with PRA sera or IgG depleted PRA sera for 18–24 hours, human arterial segments were retransplanted into naive SCID/beige recipients. Grafts were harvested at three weeks and stained and quantified for IgG, polyC9, and NIK (h, scale bars indicate 200μm). Asterisks indicate p<0.05 comparing CAMR patients to healthy patient controls or PRA sera-treated samples to IgG− PRA sera-treated controls.
Since CAV is most evident in epicardial and intramyocardial arteries, vessels not typically sampled in endomyocardial biopsies of cardiac allografts, we used a humanized mouse model of CAV to determine if antibody and complement-induced non-canonical NF-κB activation can occur in human arterial EC in vivo.10 Adjacent segments of human artery branches were interposed into the infra-renal aortae of pairs of immunodeficient mice and allowed to quiesce for 30 days. Hosts were then injected with PRA sera or PRA sera depleted of IgG, and the grafts were harvested 24 hours later and examined by immunofluorescence. Grafts exposed to intact PRA sera but not IgG-depleted PRA sera showed binding of human IgG as well as mouse C4d and poly C9 on human EC, the latter identified by Ulex Europeous agglutinin I (ULEX, Fig 6d, Supplementary Fig 5a). Despite deposition of MAC, PRA treatment did not cause intimal denudation (Fig 6e, left), and the number of DAPI-stained nuclei within cells co-stained with ULEX did not significantly differ between these treatment groups (Fig 6e, right graph). PRA activated the EC as assessed by induction of E-selectin and VCAM-1 in the PRA-treated but not the control-treated vessels (Fig 6f, top, Supplementary Fig 5b). qRT-PCR analysis further revealed significant increases in inflammatory gene expression in grafts treated with intact PRA vs. controls (Fig 6f, bottom graph). Graft EC with bound IgG and C4d also showed significantly elevated expression of p52+ nuclei (p=0.004) and NIK (p=0.000002) compared to the controls (Fig 6g), consistent with non-canonical NF-κB activation. To assess durability of non-canonical activation, hosts bearing arterial grafts were injected with PRA or IgG depleted PRA sera. Twenty-four hours later grafts were re-transplanted into naive immunodeficient hosts and harvested one, two, or three weeks later. Grafts treated with IgG-depleted PRA sera showed no detectable IgG, C4d, polyC9, or NIK staining at any time point. PRA-treated grafts showed C4d staining that was retained for 3 weeks whereas IgG and polyC9 expression was significantly diminished at 1 week following re-transplantation (data not shown) and was gone by 2 weeks following re-transplantation. Like C4d staining, increased NIK expression persisted in PRA sera-treated grafts for the full 3 weeks (Fig 6h).
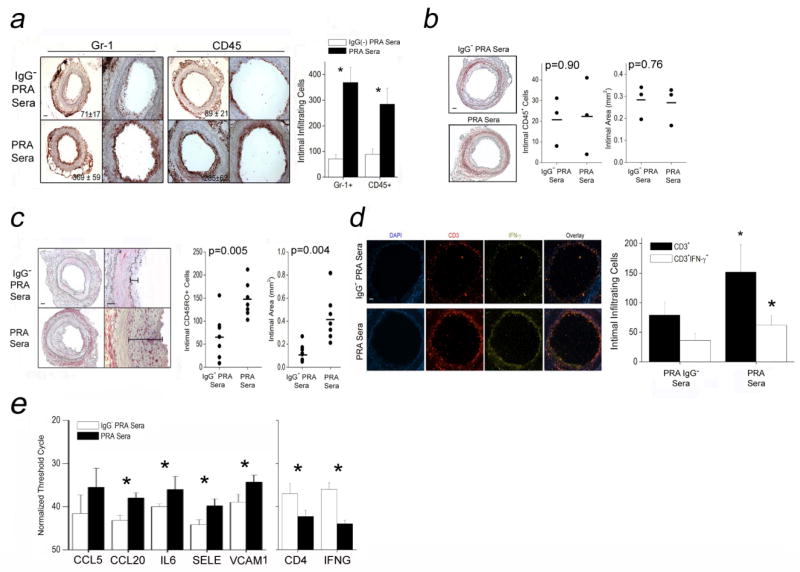
PRA elicits CAV-like lesions in a T cell-dependent manner
To examine the effect of PRA serum-treated EC on intimal T cell responses, we injected PRA sera or IgG-depleted PRA sera into immunodeficient paired hosts bearing quiesced human arterial segments. Each graft was harvested and re-transplanted twenty-four hours later into a second immunodeficient host which, in some cases, had been previously engrafted with human T cells allogeneic to the artery donor. In this experimental design, circulating leukocytes in the second mouse host are not directly exposed to the PRA sera, circumventing potential confounding effects of PRA on FcR-bearing mouse leukocytes as well as on adoptively transferred human T cells expressing HLA molecules. Grafts exposed to PRA sera and implanted into mice lacking human T cells showed significantly enhanced recruitment of murine Gr-1+ myeloid cells (p=0.02) or CD45+ leukocytes (p=0.04) one week after transplantation compared to controls treated with IgG-depleted PRA sera (Fig 7a, n=6 pairs). However, by three weeks after re-transplantation, no morphological differences could be discerned between arteries that had been exposed to intact PRA sera vs. IgG-depleted PRA sera (Fig 7b, n=3 pairs). In contrast, arterial segments re-implanted into hosts previously engrafted with human T cells (n=7 pairs) showed greater numbers of intimal-infiltrating T cells (Fig 7c, left graph) and increased intimal areas (Fig 7c, right graph) at 3 weeks post-implantation compared to control treated grafts. PRA pre-treatment also increased the numbers of intimal-infiltrating human IFN-γ producing T cells (Fig 7d) with accordingly increased intra-graft transcripts of pro-inflammatory genes (Fig 7e, left graph) and T cell-associated transcripts (Fig 7e, right graph). These data indicate that PRA-treated human arterial xenografts develop enhanced CAV-like changes associated with increased infiltration of allogeneic T cells and intra-graft IFN-γ expression.
Figure 7.
PRA elicits CAV-like lesions in a T cell-dependent manner. Grafts treated with PRA sera or IgG-depleted PRA sera for 18–24 hours were retransplanted into naive SCID/beige hosts. Hosts with PRA-treated xenografts and without circulating human CD3+ T cells at one week showed significantly increased intimal infiltration of Gr-1+ and CD45+ myeloid cells compared to hosts treated with IgG-depleted PRA sera (a, scale bars indicate 400μm). However, three weeks after re-transplantation, xenografts treated with PRA sera showed no morphological differences compared to hosts receiving xenografts treated with IgG-depleted PRA sera (b, scale bars indicate 400μm). Arterial xenografts treated with PRA or IgG-depleted PRA sera (scale bars indicate 400μm) and reimplanted into naive SCID/beige hosts engrafted with human CD3+ T cells were harvested three weeks later and assessed for intimal infiltrating CD45RO+ T cells (c, left graph) and intimal areas (c, right graph). At two weeks after re-transplantation, intimal CD3+ and CD3+IFN-γ+ T cells were quantified by immunofluorescence (d, scale bars indicate 400μm), intra-graft pro-inflammatory gene transcripts were normalized to CD31 (e, left graph), and T cell-associated transcripts, CD4 and IFN-γ, were normalized to GAPDH and CD4, respectively (right graph). Asterisks indicate p<0.05 comparing PRA sera-treated samples to IgG− PRA sera-treated controls.
DISCUSSION
CAV is the major cause of late cardiac allograft loss and, unlike acute rejection, is pharmacologically untreatable. Development of new therapies has been hindered by a lack of relevant models to explore pathogenic mechanisms.2,3 We used PRA to model the complement-fixing effects of DSA and identified an alloantibody- and complement-induced signaling pathway involving non-canonical NF-κB signaling components in graft EC that results in enhanced EC-mediated recruitment and activation of host alloreactive T cells. Non-canonical NF-κB signaling thus represents a novel and attractive target for the development of pharmacotherapeutics.
Distinct lines of evidence have favored pathogenic roles for either T cell infiltration and IFN-γ secretion4–10,12 or development of a DSA and activation of complement13–15 in CAV. Specifically, the pathogenic role of T cells has been investigated using analysis of human tissues and by experiments with various murine knockout models or with humanized mice.12 The pathogenic effects of alloantibodies on EC have been studied using in vitro incubation27–30 or in vivo injection of monoclonal murine anti-HLA antibody.31,32 The pro-inflammatory effects of MAC have been modeled using in vitro assembly of MAC.21–25 Our results support the conclusion that these elements can act in concert. Alloantibody is required for high-level deposition of terminal complement on EC (Fig 3e, Supplementary Fig 2). Terminal complement, in turn, initiates a durable activation signal to affected EC that can last for weeks in vivo (Fig 6h), driving pro-inflammatory genes that enable the EC to exacerbate T cell-mediated changes in the artery wall characteristic of CAV (Fig 7c and 7d). Our findings do not rule out a role for T cells, antibody or complement acting independently, but instead suggest that graft EC, the major cell population expressing graft HLA antigens in the vessel wall, may serve to integrate these signals.
PRA upregulated pro-inflammatory genes without a significant increase in cell death in human EC in vitro (Fig 1e) and in vivo. Indeed, PRA-treated EC may actually better resist cell death when co-cultured with T cells. Resistance to death by PRA may in part depend on the expression of complement regulatory proteins by EC (Fig 1c). The survival of EC under these circumstances may also depend upon induction of the many pro-survival genes known to be regulated by NF-κB.22 If pro-survival genes are induced in vivo, rather than reduce vasculopathy, they may contribute to persistent stimulation of host alloimmunity by graft EC in patients with CAMR.16,17
Alloantibody and complement have been previously shown to modulate function of leukocytes. Fc receptor bearing macrophages31 and NK cells32 can respond to IgG bound to EC surfaces. Moreover, locally synthesized anaphylatoxins by EC can enhance alloimmune T cell responses.33–35 By using a re-transplantation strategy whereby host immune cells were not directly exposed to PRA, we avoided potentially confounding effects of direct alloantibody and/or complement binding to leukocytes. These results therefore do not contradict prior reports showing enhanced immune function due to locally synthesized anaphylatoxins34,35 but rather support an additional mechanism of pathology whereby EC-bound alloantibody and complement potentiate the capacity of EC to promote alloreactive T cell recruitment and activation.
Non-canonical NF-κB signaling has been associated with signaling through TNF receptor superfamily members, such as LIGHT, or toll-like receptors utilizing TRIF.36 As in our study, other pathways of non-canonical signaling are driven by increased protein levels of NIK, leading to partial processing of p100 to p52. The increase in NIK results from protein stabilization rather than increased transcription and depends on recruitment of c-IAP1, cIAP-2, and TRAF3 to the activated receptor signaling complex. In this setting, TRAF3 is ubiquitinylated by cIAPs and degraded while basal ubiquitinylation and proteolytic degradation of NIK, normally mediated by the same cIAPs, declines, leading to a rise in NIK expression levels. The rise in NIK levels is generally slow, requiring several hours, and contributes mostly to lymphoid organogenesis or the late augmentation of inflammation mediated at earlier times by canonical signaling. NIK levels induced by MAC also correlate with protein stabilization rather than new transcription, but are rapid, do not correlate with TRAF3 degradation and remain durably elevated up to three weeks after a single PRA injection (Fig 6e and unpublished observations, D.J.). The rapidly inducible, and durable qualities of non-canonical NF-κB signaling caused by MAC may underlie its role in the slow and chronic progression of CAV.
While the present study was undertaken to better understand how DSA and complement could increase the incidence of CAV, our findings may be of much more generalized import. Similar changes induced by alloantibody and complement on microvessels of other solid organ transplants may lead to delayed organ-specific complications analogous to CAV, e.g., CAMR and bronchiolitis obliterans in renal and pulmonary allografts, respectively. Further, it is possible that autoantibodies reactive with other EC antigens or immune complexes that form or deposit in a sub-endothelial location may also lead to MAC formation on the EC surface and may exert similar effects, contributing to vascular complications associated with connective tissue disorders such as systemic lupus erythematosus or rheumatoid arthritis. If such an association exists, there may be much more general applicability in pharmacotherapeutics that target MAC-activated non-canonical NF-κB signaling. Patient-derived biopsies and sera from such populations, modeled on our experiments, could be utilized to investigate these possibilities.
Supplementary Material
Acknowledgments
The authors would like to thank Maria Stavropoulos for providing PRA sera and Dr. William Baldwin III for providing anti-mouse C4d antibody.
Funding Sources: This work was supported by National Institutes of Health grants R01-HL051014 and HL109455 (to J.S.P.) and T32-AI089704 (to D.J.)
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009;28:1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Nair N, Ball T, Uber PA, Mehra MR. Current and future challenges in therapy for antibody-mediated rejection. J Heart Lung Transplant. 2011;30:612–617. doi: 10.1016/j.healun.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Salomon RN, Hughes CC, Schoen FJ, Payne DD, Pober JS, Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson C, Horsley J, Rhind-Tutt S, Charman S, Phillpotts CJ, Wallwork J, Goddard MJ. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J Heart Lung Transplant. 2004;23:427–435. doi: 10.1016/S1053-2498(03)00222-5. [DOI] [PubMed] [Google Scholar]
- 6.Normann SJ, Khan SR, Leelachaikul P, Salomon DR. Origin of cells in the coronary intima during acute vascular rejection of the transplanted human heart. J Heart Lung Transplant. 1992;11:492–499. [PubMed] [Google Scholar]
- 7.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 8.Van Hoffen E, Gmelig-Meyling FH, Bosboom-Kalsbeek KC, Hu H, De Jonge N, Tilanus MG, Lahpor JR, De Weger RA. Cytokine messenger RNA expression by donor-specific cytotoxic T-cell clones after allogeneic heart transplantation. J Heart Lung Transplant. 1997;16:216–221. [PubMed] [Google Scholar]
- 9.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdal SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 11.Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397–404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 12.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 13.Petrossian GA, Nichols AB, Marboe CC, Sciacca R, Smith CR, Cannon PJ, Reemtsma K, Powers ER. Relation between survival and development of coronary artery disease and anti-HLA antibodies after cardiac transplantation. Circulation. 1989;80:III122–125. [PubMed] [Google Scholar]
- 14.Suciu-Foca N, Reed E, Marboe C, Harris P, Yu PX, Sun YK, Ho E, Rose E, Reemtsma K, King DW. The role of anti-HLA antibodies in heart transplantation. Transplantation. 1991;51:716–724. doi: 10.1097/00007890-199103000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Cherry R, Nielsen H, Reed E, Reemtsma K, Suciu-Foca N, Marboe CC. Vascular (humoral) rejection in human cardiac allograft biopsies: relation to circulating anti-HLA antibodies. J Heart Lung Transplant. 1992;11:24–29. [PubMed] [Google Scholar]
- 16.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8:1367–73. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 17.Racusen LC, Regele H. The pathology of chronic allograft dysfunction. Kidney Int Suppl. 2010;119:S27–S32. doi: 10.1038/ki.2010.419. [DOI] [PubMed] [Google Scholar]
- 18.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 19.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, Webber S. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannam VK, Lewis RE, Cruse JM. The fate of renal allografts hinges on responses of the microvascular endothelium. Exp Mol Pathol. 2012;94:398–411. doi: 10.1016/j.yexmp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton KK, Hattori R, Esmon CT, Sims PJ. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;265:3809–3814. [PubMed] [Google Scholar]
- 22.Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41:583–597. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- 25.Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 26.Jin YP, Jindra PT, Gong KW, Lepin EJ, Reed EF. Anti-HLA class I antibodies activate endothelial cells and promote chronic rejection. Transplantation. 2005;79:S19–S21. doi: 10.1097/01.tp.0000153293.39132.44. [DOI] [PubMed] [Google Scholar]
- 27.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, Wasawska BA, Baldwin WM, 34d, Pober JS, Lowenstein CJ. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci USA. 2007;104:1301–1306. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihrcke NS, Platt JL. Shedding of heparan sulfate proteoglycan by stimulated endothelial cells: evidence for proteolysis of cell-surface molecules. J Cell Physiol. 1996;168:625–637. doi: 10.1002/(SICI)1097-4652(199609)168:3<625::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Galvani S, Auge N, Calise D, Thiers JC, Canivet C, Kamar N, Rostaing L, Abbal M, Sallusto F, Salvayre R, Bohler T, Zou Y, Stastny P, Negre-Salvayre A, Thomsen M. HLA class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/beige mice. Am J Transplant. 2009;9:2607–2614. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirohashi T, Uehara S, Chase CM, DellaPelle P, Madsen JC, Russell PS, Colvin RB. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant. 2010;10:510–517. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CY, Lotfi-Emran S, Erdinc M, Murata K, Velidedeoglu E, Fox-Talbot K, Liu J, Garyu J, Baldwin WM, 34d, Wasawska BA. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324–1334. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 32.Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 34.Strainic MG, Liu J, Huang D, An F, Lalli PN, Mugim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9:1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 36.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.