Abstract
Background
Component separation (CS) is an effective technique for reconstructing complex abdominal wall defects. Violation of the rectus abdominis complex is considered a contraindication for CS, but we hypothesized that patients have similar outcomes with or without rectus complex violation.
Study Design
We retrospectively studied all consecutive patients who underwent CS for abdominal wall reconstruction over 8 years and compared outcomes of patients with and without rectus violation. Primary outcome measures included complications and hernia recurrence. Logistic regression analysis identified potential associations between patient, defect, and reconstructive characteristics and surgical outcomes.
Results
One hundred and sixty-nine patients were included: 115 (68%) with and 54 (32%) without rectus violation. Mean follow-up was 21.3 ± 14.5 months. Patient and defect characteristics were similar except for the rectus violation group having a higher body mass index (BMI). The overall complication rates were similar in the violation (24.3%) and the non-violation (24.0%) groups, as were the respective rates of recurrent hernia (7.8% vs. 9.2%, p=0.79), abdominal bulge (3.5% vs. 5.6%, p=0.71), skin dehiscence (20.0% vs. 22.2%, p=0.74), skin necrosis (6.1% vs. 3.7%, p=0.72), cellulitis (7.8% vs. 9.2%, p=0.75), and abscess (12.3% vs. 9.2%, p=0.58). Regression analysis demonstrated BMI to be the only factor predictive of complications.
Conclusions
CS surgical outcomes were similar whether or not the rectus complex was violated. To our knowledge, this study is the first to evaluate the effects of rectus violation on surgical outcomes in CS patients. Surgeons should not routinely avoid CS when the rectus complex is violated.
INTRODUCTION
The component separation (CS) technique has proven to be effective for reconstructing large, midline abdominal wall defects.(1-8) Unilateral or bilateral release of the external oblique aponeurosis from the linea semilunaris enlarges the abdominal wall musculofascial surface area and centralizes the rectus abdominis musculofascial complexes. The CS technique facilitates re-approximation of the rectus abdominis muscles in the midline and primary fascial coaptation for defects too large for primary repair, providing dynamic stability and improved strength to the abdominal wall, with limited surgical site morbidity.(6, 9)
Despite the advantages of CS, it has been suggested that CS be avoided in patients with extensive scarring of the rectus muscles from previous or concurrent ostomies or feeding tubes, transection of the rectus complex, or resection of the rectus complex secondary to tumor extirpation.(4, 9) It is believed that violation of one or both of the rectus complexes and overlying skin by extensive scarring makes the dissection more difficult, and many surgeons have suggested that advancement of a compromised rectus complex may result in compromised surgical outcomes.(4, 7) In addition, medial advancement of the skin flaps in relation to the underlying musculofascia during CS is thought to result in sheering of an ostomy, limited skin advancement, and/or uncovering of a contained parastomal hernia sac.(4, 10, 11)
In the cases where CS is avoided, reconstructive options that are potentially less favorable are often used; these include primary fascial coaptation under excessive tension, bridging the fascial defect with surgical mesh, and the use of musculofascial flaps. Primary fascial coaptation without CS and/or prosthetic reinforcement has been shown to result in hernia recurrence rates of up to 46%.(9, 12, 13) Bridging large fascial defects with bioprosthetic meshes has been shown to result in higher rates of hernia recurrence and bulge compared to CS with primary fascial coaptation.(9, 14-17) The use of regional or distant flaps for musculofascial reconstruction involves donor site morbidity and does not restore the dynamic stability, strength, and contour of the anterior abdominal wall.(6, 18-21)
To our knowledge, no published data justify the proposition that rectus violation is a contraindication to CS. CS is a valuable technique that has been shown to improve outcomes for large midline fascial defects; thus, avoiding CS in cases with rectus violation may be unwarranted and preclude optimal reconstruction. On the basis of our clinical experience with CS, we hypothesized that patients who undergo CS have similar outcomes whether or not the rectus complex has been violated. To test this hypothesis, we reviewed our experience with CS for abdominal wall reconstruction in patients with and without violation of the rectus abdominis complex.
METHODS
We evaluated all consecutive patients who underwent abdominal wall reconstructions in which a CS technique was used since adopting the CS technique into our practice at The University of Texas MD Anderson Cancer Center between June 12, 2002 and November 16, 2010. Data were prospectively entered into a departmental database, and the patients’ medical records were retrospectively reviewed. MD Anderson Cancer Center’s Institutional Review Board approved this study. Indications for CS were the presence of a ventral hernia or extirpative oncologic defect for which the fascia could not be primarily closed without undue tension. We included in our analysis only those patients whose abdominal wall reconstruction was completed by either a unilateral or bilateral CS technique and who had at least 6 months of postoperative follow-up. The decision to perform unilateral vs. bilateral CS was at the discretion of the surgeon and recorded for the purpose of analysis in this study.
Patient, treatment, and surgical outcome data were analyzed and directly compared between patient groups with and without rectus violation. The primary outcome measure was the relationship between prior or concurrent violation of the rectus complex and the development of complications. Secondary outcome measures included the relationship between the specific type and laterality of CS relative to the rectus violation and the development of complications. Specific complications evaluated included recurrent hernia, abdominal bulging, skin dehiscence, skin necrosis, abscess, cellulitis, hematoma, and seroma. Abdominal hernia and bulging were defined as contour deformities noted on physical examination with or without a fascial defect, respectively. Skin dehiscence was defined as a separation of at least 0.5 cm requiring debridement and healing by secondary intention. Skin necrosis was full-thickness skin loss requiring debridement. An abscess was defined as a purulent fluid collection requiring incision and drainage. Cellulitis included erythema at the wound site that resolved with intravenous or oral antibiotics alone. Hematoma and seroma were defined as subcutaneous collections of blood or serous fluid, respectively, that required percutaneous or operative drainage. An active smoker was defined as a patient who smoked within one month of surgery.
We also evaluated the association between specific types of rectus violation and postoperative complications. Rectus violation was sub-classified into four specific subtypes: (1) prior or concurrent ostomy, (2) transversely divided rectus abdominis muscle, (3) prior or concurrent gastrostomy/jejunostomy tube (GT/JT) placement, and (4) resected rectus abdominis muscle. Prior or concurrent ostomy included any type of ostomy through the rectus complex (e.g., colostomy, ileostomy, or ileal conduit). A transversely divided rectus abdominis muscle resulted from any incision that transected the full width of the rectus complex and linea semilunaris (e.g., subcostal incisions). Prior or concurrent GT/JT placement included any GT/JT surgically, radiologically, or endoscopically placed through the rectus complex. The resected rectus abdominis muscle subgroup included patients who had at least half of their rectus abdominis muscles resected during a prior or concurrent surgery. Patients with resected rectus muscles due to previously or concurrently harvested rectus abdominis–based flaps (i.e., vertical rectus abdominis musculocutaneous or transverse rectus abdominis musculocutaneous flaps) were excluded from the study.
Figure 1 illustrates how we stratified the patients to control for variations in the laterality of the rectus violation and the CS. Subset comparisons evaluated differences in complications with respect to unilateral vs. bilateral rectus violation, unilateral vs. bilateral CS, and unilateral CS with ipsilateral vs. contralateral violation of the rectus muscle complex (Table 1). Bilateral CS patients included those with release of the right and left external obliques. Unilateral involved only a single external oblique release. Unilateral CS patients were further analyzed according to whether the CS was ipsilateral or contralateral to the violated rectus complex.
Figure 1.
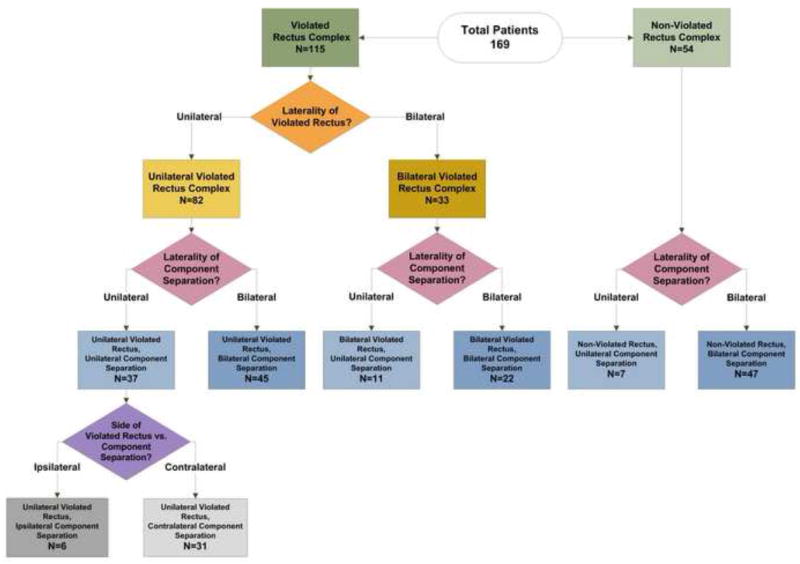
Distribution of study patients according to laterality of rectus violation and component separation.
Table 1.
Summary of Subset Analysis Comparisons of Complication Rates
| Experimental Group | Control Group | p Value |
|---|---|---|
| Overall Comparisons | ||
|
| ||
| Any violated RC, any CS | Non-violated RC, any CS | 0.97 |
| Any violated RC, any CS (excluding unilateral violated RC, contralateral CS) | Non-violated RC, any CS | 0.67 |
|
| ||
| Bilateral CS Comparisons | ||
|
| ||
| Any violated RC, bilateral CS | Non-violated RC, bilateral CS | 0.93 |
| Bilateral violated RC, bilateral CS | Non-violated RC, bilateral CS | 0.46 |
| Unilateral violated RC, bilateral CS | Non-violated RC, bilateral CS | 0.73 |
|
| ||
| Unilateral CS Comparisons | ||
|
| ||
| Any violated RC, unilateral CS | Non-violated RC, unilateral CS | 0.58 |
| Unilateral violated RC, ipsilateral CS | Non-violated RC, unilateral CS | 0.28 |
| Unilateral violated RC, contralateral CS | Non-violated RC, unilateral CS | 0.56 |
RC, Rectus Complex; CS, Component Separation.
Surgical Technique
The abdominal reconstructions were performed by 14 plastic surgeons at MD Anderson during the study period. When indicated, we generally did not avoid CS in patients with rectus complex violation such as ostomies, feeding tubes, or incisions that violated the rectus complex. All CS procedures were performed in conjunction with an extirpative surgeon (e.g., surgical oncologist, oncologic gynecologist, or colorectal surgeon) who performed the exploratory laparotomy, adhesiolysis, and/or intraperitoneal tumor extirpation, if required. Briefly, CS was performed by releasing the external oblique aponeurosis from above the costal margin to near the pubis, with complete lateral dissection between the internal and external oblique muscles.(1, 22) Inlay surgical mesh (synthetic or bioprosthetic) was used to reinforce the midline fascial closure with at least 3-5 cm of underlay by securing the mesh to the lateral rectus border and semilunar line with interpreted #1 polypropylene full-thickness musculofascial sutures. Synthetic (polypropylene) vs. bioprosthetic (acellular dermal matrix) mesh for musculofascial reinforcement was utilized at the discretion of the reconstructive surgeon and was recorded for the purposes of analysis. Indications for bioprosthetic rather than permanent synthetic mesh included bacterial contamination of the wound, unreliable skin coverage with a high risk of wound breakdown, and placement of the mesh in direct contact with the bowel. Primary, midline fascia coaptation was achieved in most cases; all others underwent a bridged repair in which the underlay mesh spanned the area of the separated fascia.
Patients were generally followed up in the outpatient clinic weekly for 2 months after discharge, every 3 months for 6 months, and then yearly thereafter. Postoperative oncologic surveillance with abdominal computed tomography and/or magnetic resonance imaging when performed were used in our evaluation of surgical outcomes, in addition to serial physical examination.
Statistical Analysis
The associations between patients with bilateral or unilateral rectus violation and postoperative complications were evaluated overall and in subgroup analyses using univariate regression analysis. Means and standard deviations were used to summarize continuous variables. Frequencies and proportions were used to present the categorical clinical characteristics. Fisher’s exact test was used to test associations between complications and categorical variables. Univariate and multivariate logistic regression models were used to analyze associations between complications and prior rectus violation vs. no rectus violation. Univariate analyses evaluated the associations between complications and other potential predictive factors (sex, age, body mass index [BMI], smoking status, chemotherapy, defect size, prior abdominal surgery, CS laterality, and CS technique). A stepwise model selection method was used to fit a multivariate logistic regression model after screening variables by univariate analyses. A post hoc power analysis was performed. Chi-square testing was performed to evaluate if rectus violation biased surgeons’ decision making to employ CS and to compare complication rates between early and late CS performed to control for the effect of surgeon experience on surgical outcomes. A senior biostatistician (JL) performed the analyses using SAS 9.2 (SAS Institute Inc., Cary, NC) and R (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 237 patients who underwent complex abdominal wall reconstruction during the study period. Of the total of 167 patients who underwent abdominal wall reconstruction in the setting of rectus violation during the study period, our surgeons chose to add a CS 69% of the time. Among the overall 70 non-violated rectus patients, surgeons added a CS 77% of the time. The rates at which our surgeons performed CS in the setting of rectus violation vs. non-rectus violation was statistically equivalent (p=0.26). We excluded the 68 abdominal wall reconstruction patient who did not undergo CS (52 with and 16 without rectus violation). Therefore, we included in this study the 169 consecutive patients (83 males)who had undergone 55 (32%) unilateral and 114 (68%) bilateral CS procedures. Mean follow-up was 21.3 ± 14.5 months during which eighty-three percent of the underwent postoperative oncologic surveillance with abdominal computed tomography and/or magnetic resonance imaging. Patient and defect characteristics are listed in Table 2. Complex ventral hernia repair was the predominant indication for CS in these patients. The distribution of CS performed to repair ventral hernias vs. extirpative oncologic defects differed between the violated and non-violated rectus groups (p<0.01). Patients with one or more prior abdominal surgeries had significantly higher rates of rectus violation than patients without prior surgery (p<0.03). Prior hernia repair was likewise significantly associated with the incidence of rectus violation (p=0.04).
Table 2.
Patient Characteristics
| Characteristic | Total n=169 | Non-Violated Rectus Complex n=54 | Violated Rectus Complex n=115 | P-value |
|---|---|---|---|---|
| Age, y, mean ± SD | 59.6 ± 12.0 | 59.5 ± 12.4 | 59.7 ± 11.4 | 0.92 |
| Sex, n (%) | ||||
| Female | 86 (50.9) | 33 (38.4) | 53 (61.6) | 0.07 |
| Male | 83 (49.1) | 21 (25.3) | 62 (74.7) | |
| BMI, kg/m2, mean ± SD | 30.5 ± 7.8 | 29.5 ± 7.5 | 32.5 ± 8.0 | 0.02 |
| BMI >35, n (%) | 34 (20.7) | 16 (47.1) | 18 (52.9) | 0.04 |
| Preoperative chemotherapy, n (%) | 106 (62.7) | 32 (30.2) | 74 (69.8) | 0.52 |
| Postoperative chemotherapy, n (%) | 34 (20.1) | 4 (11.8) | 30 (88.2) | <0.01 |
| Any medical comorbidity, n (%) | 140 (82.84) | 44 (31.4) | 96 (68.6) | 0.83 |
| Active or former smoker, n (%) | 64 (37.9) | 17 (26.6) | 47 (73.4) | 0.24 |
| Hernia repairs, n (%) | 143 (84.6) | 54 (100) | 89 (77.4) | |
| Extirpative oncologic defects, n (%) | 26 (15.4) | 0 (0) | 26 (22.6) | <0.01 |
| Defect Size, cm2, mean ± SD | 291.5 ± 255.2 | 254.6 ± 247.2 | 308.8 ± 258.1 | 0.20 |
| Prior abdominal operations, n (%) | 166 (98.2) | 53 (31.9) | 113 (68.1) | >0.99 |
| 1 | 39 (23.5) | 18 (46.2) | 21 (53.8) | |
| 2 | 58 (34.9) | 20 (34.5) | 38 (65.5) | |
| ≥3 | 69 (41.6) | 15 (21.7) | 54 (78.3) | 0.03 |
| Prior hernia repairs, n (%) | 45 (26.6) | 20 (44.4) | 25 (55.6) | 0.04 |
| 1 | 32 (71.1) | 16 (50) | 16 (50) | |
| 2 | 8 (17.8) | 3 (37.5) | 5 (62.5) | |
| ≥3 | 5 (11.1) | 1 (20) | 4 (80) | 0.51 |
BMI, body mass index;SD, standard deviation.
Table 3 further outlines the clinical details of the component separation procedures. Mesh was used in 94% of repairs. Of these, bioprosthetic mesh was used in 97% and synthetic mesh in 3%. Of the 115 patients with rectus complex violation, 82 (71%) had a unilateral rectus violation compared to 33 (29%) with a bilateral rectus violation (Figure 1). Patients who underwent bilateral CS were more likely to have had a violated rectus complex compared to patients with unilateral CS (87% vs. 59%, p<0.01). The use of mesh and type of mesh were not associated with rectus violation status (p=0.47 and p=0.31, respectively). As demonstrated in Table 2, fascial defects were large for both groups and larger in the violated vs. the non-violated rectus group. Despite this, we were able to achieve primary fascial coaptation in 90% of the patients. (Table 3) More of the violated rectus patients underwent a bridged repair (13%) than the non-violated rectus patients (4%), although this difference was not statistically significant (p=0.10).
Table 3.
Component Separation Characteristics
| Characteristic | Total n=169 | Non-violated rectus complex n=54 | Violated rectus complex n=115 | p Value |
|---|---|---|---|---|
| Laterality of CS: | ||||
| Bilateral CS | 114 (67.5%) | 47 (41.2%) | 67 (58.8%) | |
| Unilateral CS | 55 (71.3%) | 7 (12.7%) | 48 (87.3%) | <0.01 |
| Mesh use: | ||||
| Non-mesh closure | 9 (5.9%) | 4 (44.4%) | 5 (55.6%) | |
| Mesh closure | 160 (94.1%) | 50 (31.2%) | 110 (68.8%) | 0.47 |
| Bioprosthetic mesh | 156 (97.5%) | 50 (32.1%) | 106 (67.9%) | |
| Synthetic mesh | 4 (2.5%) | 0 (0%) | 4 (100%) | 0.31 |
| Bridged fascia repair | 17 (10.2%) | 2 (3.7%) | 15 (13.4%) | 0.10 |
CS, component separation.
The overall complication rate was identical in the violated and non-violated groups (24%). Post hoc power analysis demonstrated that the study was adequately powered (data not shown). There were also no differences in specific complication rates between the groups (Table 4). When we controlled for the effect of surgeon experience on patient outcomes by comparing the first half of the cases performed to the second half of the cases performed in each group, we found the overall complication rate was statistically equivalent between the early and late non-rectus violation reconstructions (26% vs. 22%, p.0.99). However, the overall complication rate was significantly higher in earlier compared to later rectus violation patients (33% vs. 16%, p=0.03). After evaluating all potential patient, defect, and treatment factors with univariate logistic regression, BMI was the only factor that was significantly associated with the development of a complication (p<0.001). There were no differences in complication rates between patients who underwent bilateral vs. unilateral CS in either group.
Table 4.
Surgical Outcomes of Patients with Non-Violated vs Violated Rectus Complex
| Complication | Total n=169 | Non-violated rectus complex n=54 | Violated rectus complex n=115 | p Value |
|---|---|---|---|---|
| Any Complication | 41 (24.3%) | 13 (24.1%) | 28 (24.3%) | 0.97 |
| Recurrent hernia | 14 (8.3%) | 5 (9.2%) | 9 (7.8%) | 0.79 |
| Abdominal bulging | 7 (4.1%) | 3 (5.6%) | 4 (3.5%) | 0.71 |
| Skin dehiscence | 35 (20.7%) | 12 (22.2%) | 23 (20.0%) | 0.74 |
| Skin necrosis | 9 (5.3%) | 2 (3.7%) | 7 (6.1%) | 0.72 |
| Infection abscess | 19 (11.2%) | 5 (9.2%) | 14 (12.3%) | 0.58 |
| Infection cellulitis | 14 (8.3%) | 5 (9.2%) | 9 (7.8%) | 0.75 |
| Hematoma | 5 (3.0%) | 1 (1.9%) | 4 (3.5%) | >0.99 |
| Seroma | 8 (4.7%) | 2 (3.7%) | 6 (5.2%) | >0.99 |
Multivariate regression analysis identified BMI as an independent risk factor associated with an approximately 6-fold increased risk of postoperative complications (odds ratio [OR]=5.89, 95% confidence interval [CI]=2.55-13.57, p<0.001). The presence of rectus muscle complex violation was not found to be an independent risk factor for development of a postoperative complication (OR=1.40, 95% CI=0.61-3.22, p=0.427).
The univariate analysis stratified by the type of rectus violation, with prior/concurrent ostomy status serving as the reference, showed that the ostomy patients had the highest complication rate (32%), followed by the resected rectus abdominis muscle patients (27%), the transversely-divided rectus abdominis muscle patients (20%), and the GT/JT patients (14%); however, none of these differences were significant (Table 5).
Table 5.
Univariate Logistic Regression Analysis of Complications by Rectus Violation Type
| Type of violation | n (%) | No Complication | Complication | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Ostomy | 34 (29.6%) | 23 (67.6%) | 11 (32.4%) | Ref | |
| Divided rectus | 31 (27.0%) | 25 (80.1%) | 6 (19.9%) | 0.46 (0.15 - 1.45) | 0.19 |
| Resected rectus | 30 (26.1%) | 22 (73.3%) | 8 (26.7%) | 0.73 (0.25 -2.16) | 0.57 |
| GT/JT | 21 (18.3%) | 18 (85.7%) | 3 (14.3%) | 0.35 (0.09 -1.47) | 0.15 |
GT/JT, gastrostomy tube/jejunostomy tube; OR, odds ratio; CI, confidence interval.
We conducted subgroup analyses of the rectus violation group to evaluate overall and specific complications associated with the sidedness of the CS and rectus violation. A summary of the various subset analyses performed demonstrates no significant differences in the overall or specific complication rates for any of these comparisons (Table 1). Three of these subset comparisons warrant specific discussion. First, the overall comparison between the violated and non-violated rectus groups included 31 patients with a unilateral rectus violation who underwent a unilateral CS performed on the opposite side of the violation. We thought that performing CS only on the non-violated side might result in less morbidity and, thus, fewer complications. Therefore, we excluded these 31 unilateral violated rectus/contralateral CS patients, and we again saw no association between rectus violation and complications. Second, when directly comparing patients with bilateral violation of the rectus complex who underwent bilateral CS to non-violated rectus patients with bilateral CS, we found no significant difference in the overall rate of complications (Table 1). Third, we specifically evaluated patients with a unilateral violated rectus who underwent an ipsilateral CS on the same side as the violation and found no significant difference in the complication rate compared to that of unilateral CS patients without rectus violation. Complications were also similar between the non-violated unilateral CS patients and the unilateral violated rectus patients with contralateral CS.
DISCUSSION
We found that overall and specific complication rates were similar in patients with or without violation of the rectus complex who underwent CS. This was true despite patients in the rectus violation group having more adverse characteristics potentially associated with complications, such as higher BMI, greater incidence of postoperative chemotherapy, larger defect size, more patients with 2 or more previous surgeries, and more frequent use of bilateral rather than unilateral CS. In addition, the specific type of rectus violation did not appear to influence complication rates. Finally, bilateral CS was shown to have outcomes similar to unilateral CS.
Some surgeons have suggested that prior violation of the rectus complex represents a contraindication to component separation, and in such situations, alternative reconstructive strategies should be considered, such as the use of interposition bridging mesh reconstruction without an attempt to approximate the midline fascia together.(6, 9, 10) In our practice, we have not avoided the use of CS in patients with prior rectus violation, as we believe this technique is safe, effective, and preferable to an interposition bridging mesh repair that does not facilitate primary fascial coaptation.(22, 23) This is evidenced by the fact that we saw no difference in the rates in which our surgeons performed CS in the violated vs. non-violated rectus groups. Prior to this study, the effects of prior rectus violation on the outcomes of abdominal wall reconstruction with CS were unknown. This study is the first to confirm our hypothesis that the presence or absence of prior rectus violation does not affect the outcomes achieved with unilateral or bilateral CS. Despite the potential adverse impact of prior rectus violation on surgical outcomes, it does not appear to increase the overall complication rate following CS.
Although the overall effect of rectus violation on the outcomes of CS appears to be minimal, it is important to discuss the impact of particular patient and reconstructive factors such as BMI, laterality of the CS, and specific types of rectus violation. Increased BMI was the only patient comorbidity shown to be a significant independent predictor of developing a complication. BMI has been demonstrated in other studies to be associated with increased surgical morbidity.(24-32) What is particularly interesting is that we also found that patients with rectus violation had a significantly higher BMI compared to the non-violated rectus group (Table 2). However, despite the higher prevalence of this significant predictor of compromised outcomes among the violated rectus group, surgical outcomes were still equivalent to those of the non-violated rectus group. Knowledge of the effects of BMI on CS outcomes is a useful finding for reconstructive surgeons when selecting patients for CS. While obese patients with a resectable malignancy typically undergo tumor extirpation and reconstruction, obese patients with a ventral hernia requesting elective CS may benefit from referral to a medically supervised weight loss program before being offered elective CS.
Although elevated BMI was the only factor found to be significantly associated with adverse surgical outcomes, other factors warrant specific discussion. It is our practice to employ a unilateral CS rather than a bilateral CS when unilateral CS alone will suffice to achieve a minimal tension primary fascial coaptation. When a unilateral CS is insufficient to achieve midline fascial coaptation, we believe our data support a bilateral CS, even in the presence of a violated rectus complex. Indeed, bilateral vs. unilateral CS did not affect the outcomes of this study, and our results demonstrate that our surgeons tended to not avoid the use of CS in the face of rectus violation. This comes as no surprise, as our surgeons believe that the benefits of primary fascial coaptation outweigh any potential consequences of using CS for patients with a violated rectus complex.(22, 33) Furthermore, the patients with bilateral rectus violation did not have a significantly higher rate of complications compared to the unilateral and non-violated rectus complex groups. Thus, we believe our estimation of the benefits of CS to achieve primary midline fascial coaptation over a bridged fascial repair to be justified, as performance of CS appears to be safe, even in cases of unilateral or bilateral rectus complex violation.(9, 22, 33)
Despite both groups having large defect sizes, we were able to achieve primary fascial coaptation in 90% of these patients. We believe that considerable previous experience and specific technical factors contributed to this, including a properly performed CS to maximize musculofacial medialization. This included complete release of the external oblique aponeurosis from the pubis to at least 12 cm superior to the costal margin and complete dissection between the internal and external oblique muscles. The use of adjunctive mesh in 94% of these reconstructions further reflects our belief that a reinforced CS is superior to a CS alone. This belief is based on our experience with complex abdominal wall reconstruction as well as the experience of others who have shown hernia recurrence rates to be higher in CS patients who do not receive mesh reinforcement.(34-36) Inlay prosthetic or bioprosthetic mesh likely offloads tension from the midline to create a load-sharing closure set to physiologic tension. It is also our belief that performing a CS to decrease the fascial defect is favorable, even if the medialization of the rectus complexes is incomplete. The CS positions the rectus abdominis muscles into a more centralized location, resulting in a more anatomic and potentially physiologic origin/insertion relationship of the muscles. It also creates a greater ratio of innervated muscle to adynamic mesh bridge, which may improve the dynamic function of the abdominal wall and decrease the incidence of postoperative bulge.
All of our surgeons are experienced with CS and perform a high volume of complex abdominal wall reconstructions. Achieving optimal outcomes with CS requires experience, particularly in the setting of rectus violation due to changes in abdominal wall anatomy and scarring that interferes with development of the dissection planes necessary to complete a CS. The challenges presented by CS in the face of rectus violation were demonstrated in this study when we controlled for the variable of time’s effect on outcomes. We saw no difference in complications between our early and late experience with CS in the non-rectus violation patients, however, we saw a significantly higher rate of complications in our early violated rectus CS experience compared to our later experience. The overall equivalent outcomes between CS in violated vs. non-violated rectus patients seen in our study may reflect what can be achieved by surgeons highly experienced in complex abdominal wall reconstruction and CS techniques. Equivalent outcomes for CS in the setting of rectus violation may not be achieved by surgeons who are still on the CS learning curve, and we therefore alert surgeons unfamiliar with complex abdominal wall reconstruction to exercise caution when considering CS in the setting of rectus violation.
Due to the retrospective design of our study, we were not able to determine the exact locations of the ostomies with respect to the semilunar line, but it has been our practice to avoid CS when the ostomy is lateral to the semilunar line rather than through the rectus complex. However, should a CS be indicated in the case of a lateral ostomy, an ipsilateral CS can still be successfully performed by releasing the external oblique through the muscle (not aponeurosis) at least 3 cm lateral to the ostomy. In conjunction with this, avoiding skin flap elevation near the existing ostomy preserves the integrity of the ostomy-musculofascial interface. Such maneuvers leave the ostomy site with all 3 lateral muscle layers undisrupted and able to be centralized together with the contiguous rectus complex. A similar strategy of dividing the external oblique muscle lateral to a transversely divided rectus muscle incision extending through the linea semilunaris also may be employed to perform CS in the setting of a violated external oblique aponeurosis. However, a very wide transverse scar through the lateral oblique muscles or a lateral muscle resection would not be amenable to such modifications, making CS truly contraindicated in such situations.
Another factor that has been suggested to be a contraindication to CS is prior radiation therapy.(9) We originally classified prior abdominal wall radiation therapy as a type of rectus violation for the purposes of this study. However, when the data from our study were analyzed, prior radiation therapy was shown to have no effect on surgical outcomes (data not shown). We believe that this likely represented selection bias in this retrospective analysis, as patients with heavy radiation damage to their abdominal walls tend to not be offered CS in our practice. We believe that a more comprehensive review of the effects of radiation on abdominal wall reconstruction using all available techniques is warranted to better explore this variable, but this is beyond the scope of the current study.
The strengths of this study include a large cumulative experience with abdominal wall reconstruction by multiple surgeons using similar techniques at a single center, careful study design to isolate and compare abdominal wall donor site morbidity between strict rectus violation groups and non-violation groups, data obtained from a prospectively maintained patient database, and use of multiple regression analysis models. Limitations of this study include its retrospective design, potential selection bias introduced by surgeons potentially avoiding CS in the presence of rectus violation or preferentially offering CS to selected patients, and follow-up of only 21 months. As hernia recurrences continue to develop in patients following CS for years following complex abdominal wall reconstruction with CS, longer follow-up is needed to more accurately determine the long-term recurrence rates of the patients in this study. A prospective study would also be useful to identify potential specific complications that might be associated with CS and ipsilateral rectus violation and help further identify specific patient selection criteria. Nevertheless, this study represents the only study to date that specifically evaluates the effect of rectus complex violation on CS outcomes.
Based on the findings of this study, experienced surgeons should not be reluctant to perform CS in patients with violation of the rectus complex who would otherwise benefit from the CS procedure. Acceptable outcomes can be achieved using CS in abdominal wall reconstruction, even in cases of violation of the rectus abdominis complex. When considering the risks and benefits of a unilateral or bilateral CS in the face of unilateral or bilateral rectus complex violation, surgeons should not consider rectus complex violation a contraindication to CS.
Acknowledgments
The authors thank Dawn Chalaire from The University of Texas MD Anderson Cancer Center, Department of Scientific Publications for assistance with scientific editing.
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CI
confidence interval
- CS
component separation
- GT/JT
gastrostomy/jejunostomy tube
- OR
odds ratio
- SD
standard deviation
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 97th Annual Clinical Congress, San Francisco, CA, October 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–527. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Levine JP, Karp NS. Restoration of abdominal wall integrity as a salvage procedure in difficult recurrent abdominal wall hernias using a method of wide myofascial release. Plast Reconstr Surg. 2001;107:707–716. doi: 10.1097/00006534-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 3.DiBello JN, Jr, Moore JH., Jr Sliding myofascial flap of the rectus abdominis muscles for the closure of recurrent ventral hernias. Plast Reconstr Surg. 1996;98:464–469. doi: 10.1097/00006534-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 4.de Vries Reilingh TS, van Goor H, Rosman C, et al. “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg. 2003;196:32–37. doi: 10.1016/s1072-7515(02)01478-3. [DOI] [PubMed] [Google Scholar]
- 5.Girotto JA, Ko MJ, Redett R, et al. Closure of chronic abdominal wall defects: a long-term evaluation of the components separation method. Ann Plast Surg. 1999;42:385–395. doi: 10.1097/00000637-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shestak KC, Edington HJ, Johnson RR. The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg. 2000;105:731–740. doi: 10.1097/00006534-200002000-00041. [DOI] [PubMed] [Google Scholar]
- 7.Lowe JB, Garza JR, Bowman JL, et al. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg. 2000;105:720–729. doi: 10.1097/00006534-200002000-00039. [DOI] [PubMed] [Google Scholar]
- 8.Maas SM, de Vries RS, van Goor H, et al. Endoscopically assisted “components separation technique” for the repair of complicated ventral hernias. J Am Coll Surg. 2002;194:388–390. doi: 10.1016/s1072-7515(01)01140-1. [DOI] [PubMed] [Google Scholar]
- 9.Ventral Hernia Working Group. Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Maas SM, van Engeland M, Leeksma NG, Bleichrodt RP. A modification of the “components separation” technique for closure of abdominal wall defects in the presence of an enterostomy. J Am Coll Surg. 1999;189:138–140. doi: 10.1016/s1072-7515(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 11.Cobb WS, Harris JB, Lokey JS, McGill ES, Klove KL. Incisional herniorrhaphy with intraperitoneal composite mesh: A report of 95 cases. Am Surg. 2003:784–787. [PubMed] [Google Scholar]
- 12.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 13.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–585. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries R, v G H, Charbon JA, Rosman C, Hesselink EJ, van der Wilt GJ, Bleichrodt RP. Repair of giant midline abdominal wall hernias: “Components separation technique” versus prosthetic repair. World J Surg. 2007;31:756–763. doi: 10.1007/s00268-006-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voyles CR, Richardson JD, Bland KI, et al. Emergency abdominal wall reconstruction with polypropylene mesh: Short-term benefits versus long-term complications. Ann Surg. 1981;194:219–223. doi: 10.1097/00000658-198108000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–382. doi: 10.1001/archsurg.133.4.378. [DOI] [PubMed] [Google Scholar]
- 17.Awad ZT, Puri V, LeBlanc K, et al. Mechanisms of ventral hernia recurrence after mesh repair and a new proposed classification. J Am Coll Surg. 2005;201:132–140. doi: 10.1016/j.jamcollsurg.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Wangensteen O. Repair of large abdominal defects by pedicled fascial flaps. Surg Gynecol Obstet. 1946;82:144. [PubMed] [Google Scholar]
- 19.Ger R, Duboys E. The prevention and repair of large abdominal-wall defects by muscle transposition: a preliminary communication. Plast Reconstr Surg. 1983;72:170–176. doi: 10.1097/00006534-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Williams JK, Carlson GW, deChalain T, et al. Role of tensor fasciae latae in abdominal wall reconstruction. Plast Reconstr Surg. 1998;101:713–718. doi: 10.1097/00006534-199803000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Ninkovic M, Kronberger P, Harpf C, et al. Free innervated latissiums dorsi muscle flap for reconstruction of full-thickness abdominal wall defects. Plast Reconstr Surg. 1998;101:971–978. doi: 10.1097/00006534-199804040-00013. [DOI] [PubMed] [Google Scholar]
- 22.Butler CE, Campbell K. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg. 2011;128:698–709. doi: 10.1097/PRS.0b013e318221dcce. [DOI] [PubMed] [Google Scholar]
- 23.Sacks JM, Butler CE. Outcomes of complex abdominal wall reconstruction with bioprosthetic mesh in cancer patients. Plast Reconstr Surg. 2008;121:39. [Google Scholar]
- 24.Garvey PB, Buchel EW, Pockaj BA, et al. The deep inferior epigastric perforator flap for breast reconstruction in overweight and obese patients. Plast Reconstr Surg. 2005;115:447–457. doi: 10.1097/01.prs.0000149588.09148.53. [DOI] [PubMed] [Google Scholar]
- 25.Kroll SS, Netscher DT. Complications of TRAM flap breast reconstruction in obese patients. Plast Reconstr Surg. 1989;84:886–893. doi: 10.1097/00006534-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Spear SL, Ducic I, Cuoco F, Taylor N. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2007;119:788–795. doi: 10.1097/01.prs.0000252003.14537.d2. [DOI] [PubMed] [Google Scholar]
- 27.Vyas RM, Dickinson BP, Fastekjian JH, et al. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519–1526. doi: 10.1097/PRS.0b013e31816b1458. [DOI] [PubMed] [Google Scholar]
- 28.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179:275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 29.Choban PS, Flancbaum L. The impact of obesity on surgical outcomes: a review. J Am Coll Surg. 1997;185:593–603. doi: 10.1016/s1072-7515(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 30.Jiganti JJ, Goldstein WM, Williams CS. A comparison of the perioperative morbidity in total joint arthroplasty in the obese and nonobese patient. Clin Orthop Relat Res. 1993;289:175–179. [PubMed] [Google Scholar]
- 31.Prasad US, Walker WS, Sang CT, et al. Influence of obesity on the early and long term results of surgery for coronary artery disease. Eur J Cardiothorac Surg. 1991;5:67–73. doi: 10.1016/1010-7940(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 32.Jandali S, Nelson JA, Sonnad SS, et al. Breast reconstruction with free tissue transfer from the abdomen in the morbidly obese. Plast Reconstr Surg. 2011;127:2206–2213. doi: 10.1097/PRS.0b013e3182131c93. [DOI] [PubMed] [Google Scholar]
- 33.Itani KM, Rosen M, Vargo D, et al. Prospective multicenter clinical study of single-stage repair of infected or contaminated abdominal incisional hernias using Strattice reconstructive tissue matrix. American College of Surgeons 96th Clinical Congress; Washington D.C.: Oct 5, 2010. Abstract. [Google Scholar]
- 34.Espinosa-de-los-Monteros A, de la Torre JI, Marrero I, Andrades P, Davis MR, Vasconez LO. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann Plast Surg. 2007;58:264–267. doi: 10.1097/01.sap.0000254410.91132.a8. [DOI] [PubMed] [Google Scholar]
- 35.Ko JH, Salvay DM, Paul BC, Wang EC, Dumanian GA. Soft polypropylene mesh, but not cadaveric dermis, significantly improves outcomes in midline hernia repairs using the components separation technique. Plast Reconstr Surg. 2009;124:836–848. doi: 10.1097/PRS.0b013e3181b0380e. [DOI] [PubMed] [Google Scholar]
- 36.Ko JH, Wang EC, Salvay DM, Paul BC, Dumanian GA. Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg. 2009;144:1047–1055. doi: 10.1001/archsurg.2009.192. [DOI] [PubMed] [Google Scholar]


