Abstract
We have previously reported the isolation from E. coli of a specific inhibitor of polypeptide chain initiation that is rendered ineffective when active aminoacylation of transfer RNA is taking place; this is normally the case during natural messenger RNA translation. Surprisingly, the inhibitory activity appears to be a hitherto unrecognized property of the chain elongation factor G. The following hold for preparations purified for either translocase or inhibitor activity: (1) equal electrophoretic mobility on polyacrylamide gels; (2) equal specific activities for (a) inhibition of initiation, (b) translocation, and (c) ribosome-dependent, uncoupled GTPase; and (3) similar heat sensitivity of translocase and inhibitor activities in a temperature-sensitive E. coli mutant with an altered elongation factor G. Different sites are apparently involved in translocation and inhibition because the former, but not the latter, is sensitive to p-chloromercuribenzoate and fusidic acid.
Keywords: initiation complex, translocase activity
Full text
PDF
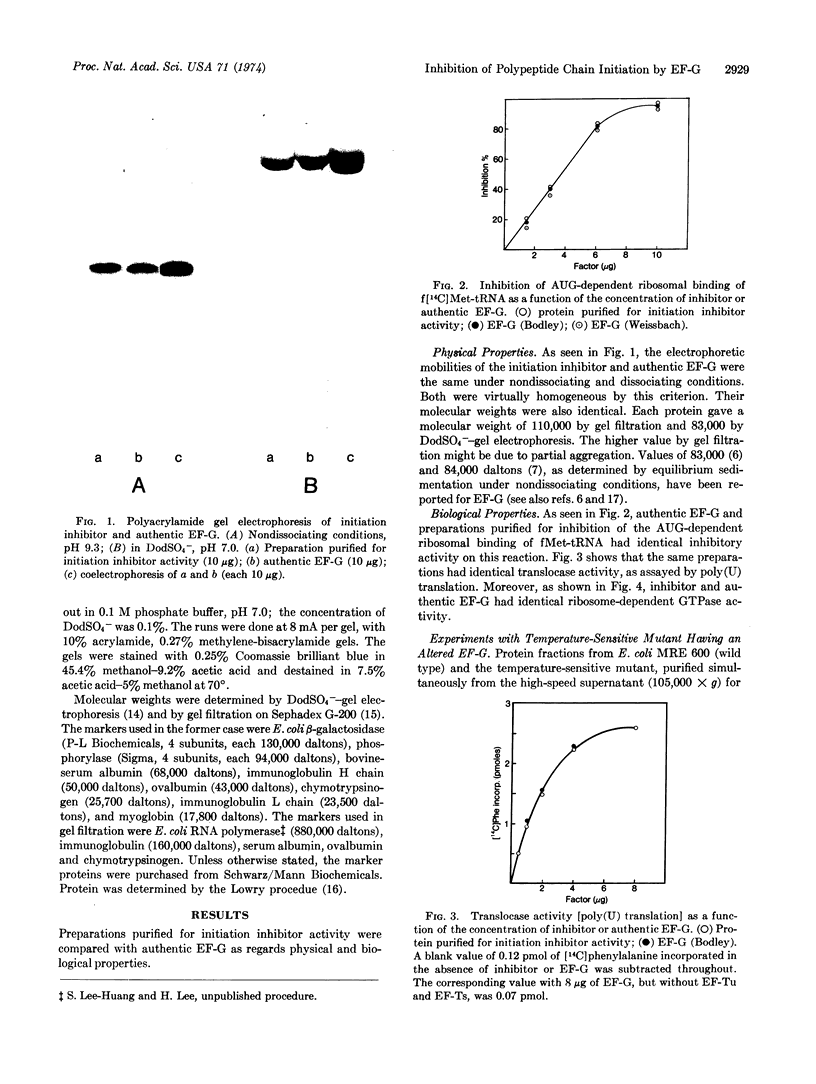
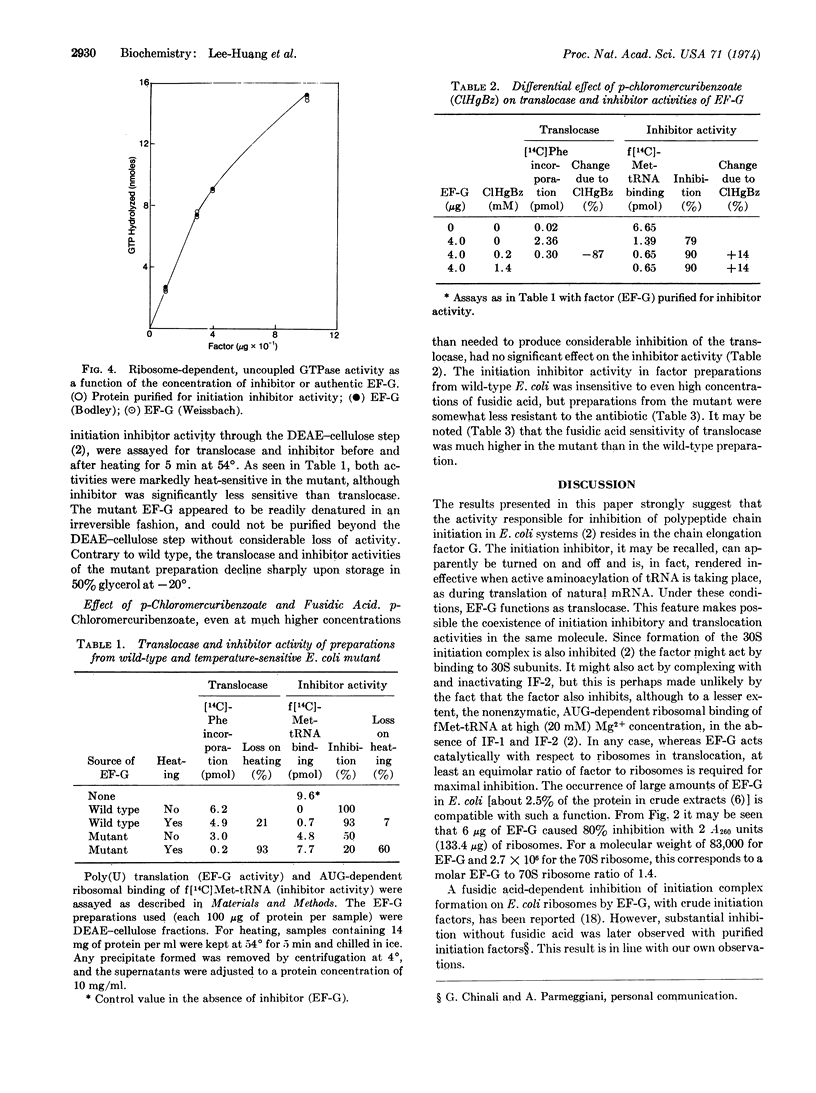

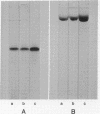
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey T., Leder P., Moldave K., Schlessinger D. Translation: its mechanism and control. Science. 1972 Apr 14;176(4031):195–197. doi: 10.1126/science.176.4031.195. [DOI] [PubMed] [Google Scholar]
- Chinali G., Parmeggiani A. Properties of elongation factor G: its interaction with the ribosomal peptidyl-site. Biochem Biophys Res Commun. 1973 Sep 5;54(1):33–39. doi: 10.1016/0006-291x(73)90884-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Felicetti L., Tocchini-Valentini G. P., Di Matteo G. F. The role of G factor in protein synthesis. Studies on a temperature-sensitive Escherichia coli mutant with an altered G factor. Biochemistry. 1969 Aug;8(8):3428–3432. doi: 10.1021/bi00836a044. [DOI] [PubMed] [Google Scholar]
- Groner Y., Pollack Y., Berissi H., Revel M. Cistron specific translation control protein in Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):16–19. doi: 10.1038/newbio239016a0. [DOI] [PubMed] [Google Scholar]
- Highland J. H., Lin L., Bodley J. W. Protection of ribosomes from thiostrepton inactivation by the binding of G factor and guanosine diphosphate. Biochemistry. 1971 Nov 23;10(24):4404–4409. doi: 10.1021/bi00800a009. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Inoue-Yokosawa N., Kawakita M. Studies on polypeptide elongation factor from E. coli. I. Crystalline factor G. J Biochem. 1972 Oct;72(4):853–863. doi: 10.1093/oxfordjournals.jbchem.a129980. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Inoue N. Crystalline G factor from Escherichia coli. J Biochem. 1968 Sep;64(3):423–425. doi: 10.1093/oxfordjournals.jbchem.a128913. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee-Huang S., Lee H., Ochoa S. A specific inhibitor of polypeptide-chain initiation in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2874–2878. doi: 10.1073/pnas.70.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Purification and properties of two messenger-discriminating species of E. coli initiation factor 3. Arch Biochem Biophys. 1973 May;156(1):84–96. doi: 10.1016/0003-9861(73)90344-5. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Specific inhibitors of MS2 and late T4 RNA translation in E. coli. Biochem Biophys Res Commun. 1972 Oct 17;49(2):371–376. doi: 10.1016/0006-291x(72)90420-2. [DOI] [PubMed] [Google Scholar]
- Meier D., Lee-Huang S., Ochoa S. Factor requirements for initiation complex formation with natural and synthetic messengers in Escherichia coli systems. J Biol Chem. 1973 Dec 25;248(24):8613–8615. [PubMed] [Google Scholar]
- Parmeggiani A., Gottschalk E. M. Isolation and some properties of the amino acid polymerization factors from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1969;34:377–384. doi: 10.1101/sqb.1969.034.01.044. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Mattoccia E. A mutant of E. coli with an altered supernatant factor. Proc Natl Acad Sci U S A. 1968 Sep;61(1):146–151. doi: 10.1073/pnas.61.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]