Summary
Background
In determining whether clinical decision support (CDS) should be interruptive or non-interruptive, CDS designers need more guidance to balance the potential for interruptive CDS to overburden clinicians and the potential for non-interruptive CDS to be overlooked by clinicians.
Objective
(1)To compare performance achieved by clinicians using interruptive CDS versus using similar, non-interruptive CDS. (2)To compare performance achieved using non-interruptive CDS among clinicians exposed to interruptive CDS versus clinicians not exposed to interruptive CDS.
Methods
We studied 42 emergency medicine physicians working in a large hospital where an interruptive CDS to help identify patients requiring contact isolation was replaced by a similar, but non-interruptive CDS. The first primary outcome was the change in sensitivity in identifying these patients associated with the conversion from an interruptive to a non-interruptive CDS. The second primary outcome was the difference in sensitivities yielded by the non-interruptive CDS when used by providers who had and who had not been exposed to the interruptive CDS. The reference standard was an epidemiologist-designed, structured, objective assessment.
Results
In identifying patients needing contact isolation, the interruptive CDS-physician dyad had sensitivity of 24% (95% CI: 17%-32%), versus sensitivity of 14% (95% CI: 9%-21%) for the non-interruptive CDS-physician dyad (p = 0.04). Users of the non-interruptive CDS with prior exposure to the interruptive CDS were more sensitive than those without exposure (14% [95% CI: 9%-21%] versus 7% [95% CI: 3%-13%], p = 0.05).
Limitations
As with all observational studies, we cannot confirm that our analysis controlled for every important difference between time periods and physician groups.
Conclusions
Interruptive CDS affected clinicians more than non-interruptive CDS. Designers of CDS might explicitly weigh the benefits of interruptive CDS versus its associated increased clinician burden. Further research should study longer term effects of clinician exposure to interruptive CDS, including whether it may improve clinician performance when using a similar, subsequent non-interruptive CDS.
Key words: Clinical decision support systems, computer-assisted decision making, infection control
1. Introduction
There is good evidence that clinical decision support (CDS) can improve healthcare processes and outcomes [1]. One critical challenge involves engaging providers to consistently use the CDS as intended [2]. This challenge has been appreciated since the earliest studies of CDS, which reported physician usage rates as low as 36% [3]. More recently, multiple studies have shown that CDS alerts are often overridden [4, 5]. In our study, rather than focusing on physician usage rates, we measured the more patient-relevant outcome of whether the physician ultimately behaved in a manner consistent with an accepted reference standard.
In an era when Meaningful Use incentives aim to encourage widespread CDS implementation [6], it will be important to identify methods of increasing appropriate clinician usage of CDS. Because providers have been sensitive to the burden of health information technologies [7], one element of such customization may include tailoring CDS so as to maximize clinical benefit while minimizing the clinician’s burden [8].
Other researchers have adjusted the ‘interruptiveness’ of CDS user interface with mixed results [9-13]but only a few successful strategies to optimize CDS performance have emerged[14]. Two recent reviews thoroughly examined several characteristics of the CDS user interface, but did not examine interruptiveness in detail [15, 16].To better understand how adjusting the user interface of a CDS system ultimately affects the success of a CDS tool and the clinician using it (clinician-CDS dyad), we studied an interruptive CDS that was made non-interruptive for operational reasons by removal of a soft stop. The CDS, which was triggered when physicians admitted a patient, helped physicians identify patients requiring contact isolation. To quantify how the user interface affected the accuracy of this identification, the sensitivity of the initial interruptive CDS was compared with a subsequent non-interruptive CDS. We hypothesized that the initial interruptive CDS, while more burdensome to the physician users, would be more sensitive. Because the subsequent non-interruptive CDS was designed to resemble the initial interruptive CDS, we also hypothesized that providers exposed to the initial interruptive CDS might have better sensitivity with the subsequent non-interruptive CDS than those physicians seeing the CDS for the first time. Thus, we compared sensitivity for the subsequent non-interruptive CDS between providers who had and who had not been exposed to the prior interruptive CDS.
1.1 Clinical Background
CDC guidelines recommend the use of contact isolation to reduce the transmission of multi-drug resistant organisms (MDROs) between patients, other healthcare workers, and the hospital environment [17]. However, evidence suggests that health care providers frequently underuse contact isolation [18, 19]. One contributing factor may be that the CDC recommendations are complex, and not easy to memorize. In an academic institution staffed by employee physicians and a long history of successful CDS use [20], computerized CDS improved appropriate contact isolation rates [21]. This project looks at two different implementations of similar CDS systems and the response among contracted emergency department (ED) physicians.
2. Methods
2.1 Study Design
We conducted a single-institution retrospective analysis of medical record data.
2.2 Setting
The Cedars-Sinai ED is a Level I trauma center that services more than 77,000 patient visits annually. An ED electronic medical record (EMR) with computerized physician order entry was initially implemented in 1996 [22]. On November 15, 2009, the ED changed to a different EMR (Epic, Verona, WI, USA).
2.3 Study Population
All ED orders placed to admit a patient during the time periods studied were included in the analysis. Attending physicians sometimes supervise resident physicians in the ED on a 1:1 basis, but it is exceedingly rare for a resident physician to enter an ED order to admit a patient. All attending physicians were either board certified or board eligible in emergency medicine.
2.4 Intervention
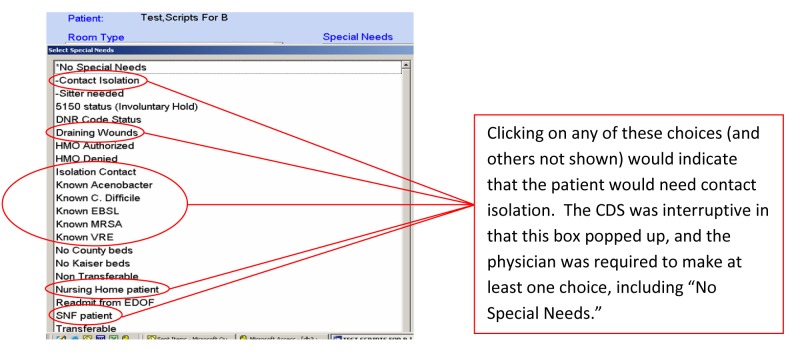
As part of a system-wide initiative to reduce healthcare associated infections, the ED implemented an interruptive CDS that helped physicians to identify patients needing isolation precautions in 2005. Entering an ED order to admit a patient triggered the CDS. The initial interruptive CDS (► Figure 1) required the ED physician to consider various ‘Special Needs,’ including contact isolation, which a patient might have at admission. The interruptive aspect of the CDS was a pop-up box with a stop that required the physician to select at least one special need or a ‘No Special Needs’ button.
Fig. 1.
Screen shot of the Interruptive CDS (Pop-up Window) used in Physician Assessment of Need for Contact Isolation
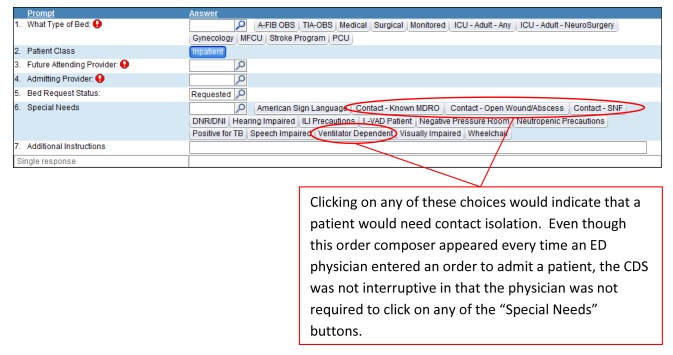
The change to a new EMR was motivated by a health system-wide change to a new enterprise information system, and was not related to the CDS studied. To minimize physician burden during the transition period, most workflow interruptions were eliminated. In this case, the interruptive CDS was replaced by a subsequent, non-interruptive CDS (► Figure 2) that lacked the prior popup box and soft stop. It was designed to resemble the initial interruptive CDS by including similar wording, with the intention that clinicians would be more likely to respond to a familiar interface. Nonetheless, the physicians leading the transition hypothesized that physicians using the non-interruptive CDS would be less sensitive in identifying patients needing contact isolation.
Fig. 2.
Screen shot of Non-interruptive CDS (part of the ED Admission Order Workflow for all patients) used in Physician Assessment of Need for Contact Isolation
Although the CDS was designed to increase identification of patients needing contact isolation, this isolation had not yet been operationalized at the time of this study. Nonetheless, because early identification of patients needing contact isolation was part of a long term system-wide plan to reduce healthcare associated infections (HAI), ED physicians were trained on the CDS functionality, and encouraged to identify patients needing contact isolation.
2.5 Reference Standard
To assess the accuracy of ED physicians’ assessments of the presence of risk factors for transmission of HAI that would require contact isolation, we compared their assessments with the results of an inpatient nursing assessment performed on every patient at the time of admission. This structured, objective nursing assessment was designed by the Cedars-Sinai Epidemiology Department in accordance with CDC recommendations to facilitate targeted screening and isolation of patients at high risk for transmission of HAI (► Figure 3) [15]. The use of such assessments has been shown to reduce transmission of multidrug resistant organisms, and is thus a Category IA recommendation of the Society for Healthcare Epidemiology of America [23]. These reference standard assessments were used for contact isolation during the study period. Conversely, recommendations from the ED physicians had not yet been operationalized during the study period. Thus, information generated by the ED physician-CDS dyad was not being provided to nurses for the nursing assessment.
Fig. 3.

Nursing Assessment of Patient’s Need for Contact Isolation
2.6 Data Collection and Abstraction
ED physicians’ responses to the CDS were retrieved by querying each of the two ED information systems. We manually abstracted nursing assessments from patients’ medical records. ► Figures 1 - 3 demonstrate that the categorizations offered by the different CDS systems and the nursing assessment were not always perfectly congruent. Nonetheless, there were several terms that matched across each of the decision tools. We thus classified a CDS system as indicating a requirement for contact isolation if one of the trigger terms listed in Table I was identified. It should be noted that although some of the terms in the ED physician CDS do not explicitly indicate that the patient needs contact isolation, these terms would cause the patient to receive contact isolation based on the nursing assessment shown in Figure 3. In essence, we assessed the ability of the ED physician-CDS dyad to identify risk factors for transmission of healthcare associated infections that would have triggered contact isolation based on our epidemiology department’s algorithm.
2.7 Primary Outcomes and Power Analysis
A primary outcome was specified to test each of our two hypotheses. The first primary outcome was the change in sensitivity associated with a change from an interruptive to a non-interruptive CDS. In calculating sensitivity, the ED physician’s assessment was compared with the reference standard described above. Based on preliminary data showing interruptive CDS sensitivity of 20%, our power analysis estimated that 906 events in each group of physicians would be necessary to detect a 5% absolute change in sensitivity with 80% power. P values less than 0.05 were considered significant (2-sided).
The primary outcome for the second hypothesis was the sensitivity obtained by the physicians using the non-interruptive CDS who had been exposed to the interruptive CDS, compared to the sensitivity obtained by the physicians using the non-interruptive CDS without such exposure. Based on the measured sensitivity of the non-interruptive CDS among all physicians in the October 1 – 15, 2010 time period, we determined that we would need to have 638 events in each group of physicians to detect a 5% absolute difference in sensitivity with 80% power.
2.8 Study Period and Inclusion/Exclusion Criteria
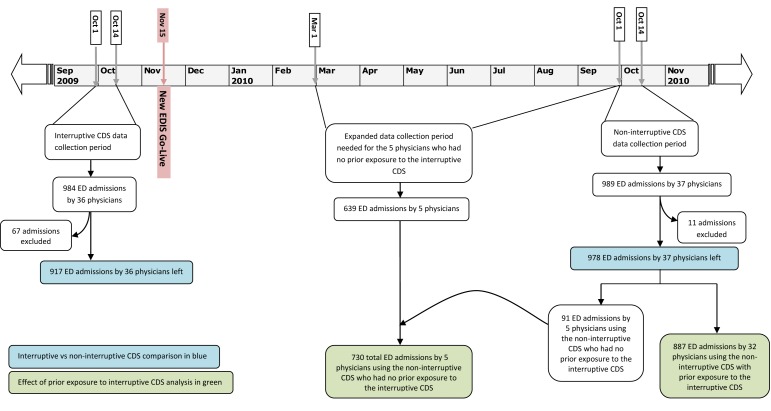
Because our reference standard required time-intensive manual data abstraction, we used these power analyses to limit the length of the time periods analyzed. To minimize seasonal differences in ED physician awareness of infectious illness requiring isolation, we chose two 14-day periods exactly one year apart to compare the effect of interruptive versus non-interruptive CDS: Oct 1-14, 2009 (interruptive CDS) and Oct 1-14, 2010 (non-interruptive CDS). We examined the medical records of all patients admitted through the ED during these time periods.
To evaluate our second hypothesis, we compared sensitivity for the subsequent non-interruptive CDS between providers who had and had not been exposed to the prior interruptive CDS. Because only five physicians were newly hired after the implementation of the subsequent non-interruptive CDS (and thus had no exposure to the prior interruptive CDS), we required several months of data to satisfy the second power calculation. We therefore chose the time period of March 1, 2010 through Oct 14, 2010 (► Figure 4).
Fig. 4.
Data Collection Timeline
We excluded patients whose charts did not contain a properly completed nursing assessment form (► Figure 3). Institutional policy dictated that all patient charts should contain this form, but adherence to the policy was not universal. Patients who had elected to opt out from all research protocols were also excluded from all analyses. Finally, we also excluded ED orders to admit patients that had been placed by residents.
2.9 Data Analysis
We calculated the sensitivity and specificity of ED physicians’ assessment of whether patients needed contact isolation. Because a small number of physicians were involved in a large number of admissions, particularly among the new ED physicians who did not have exposure to the prior interruptive CDS, we conducted a sensitivity analysis that controlled for clustering of admissions within physicians. A ratio estimator for the variance due to clustering was used to adjust the 95% confidence intervals, and the Mantel-Haenszel test was then applied (SAS 9.2, Cary, NC).
3. Results
984 and 989 patient admissions by 35 and 37 ED physicians were recorded during the interruptive and non-interruptive CDS study periods, respectively. The studied alerts were activated for all patient admissions, which represents an institutional firing rate of 71 alerts daily. During these two study periods, the average alert exposure was 14 times per physician per week. Based on typical admission volumes, this alert would be expected to fire 4.5 times per eight hour ED physician shift. Seventy-nine patients were excluded from further analysis due to 70 cases that lacked a properly completed nursing assessment form, 8 patients who opted out of participating in any research, and 1 patient with an ED order for admission placed by a resident physician (► Figure 4). In identifying patients needing contact isolation, the physicians using interruptive CDS were 10% more sensitive than physicians using non-interruptive CDS (p = 0.04) (► Table 2). As a sensitivity analysis, we included only those 32 physicians that admitted patients during both time periods. Restricting our analysis to these physicians did not alter our point estimates, although it did widen our confidence intervals such that the p value changed to 0.06.
Table 2.
Comparing interruptive and non-interruptive CDS in terms of sensitivity and specificity.
| Reference Standard: Nursing assessment | Interruptive CDS (Oct 1 – Oct 14, 2009) | Non-interruptive CDS (Oct 1 – Oct 14, 2010) | Chi-square |
|---|---|---|---|
| Sensitivity [95% CI] | |||
| Unadjusted | 24% [17%, 32%] | 14% [9%, 21%] | 4.19 (p = 0.04)* |
| Clustering Adjustment | 24% [16%, 32%] | 14% [6%, 22%] | 4.17 (p = 0.04)† |
| Specificity [95% CI] | |||
| Unadjusted | 99% [97%, 99%] | 98% [97%, 99%] | 0.63 (p = 0.43)* |
| Clustering Adjustment | 99% [98%, 100%] | 98% [97%, 99%] | 0.63 (p = 0.43)† |
*Pearson chi-square test;
† Mantel-Haenszel test
To measure the effect of prior exposure to the interruptive CDS on the performance of physicians using the non-interruptive CDS, the 978 uses of the non-interruptive CDS were separated into 887 uses by 32 physicians with prior exposure and 91 uses by 5 physicians without prior exposure (► Figure 4). 639 more uses of the non-interruptive CDS by the 5 physicians without exposure to the prior interruptive CDS were identified during the expanded (March 1, 2010 – October 1, 2010) data collection period. Of these 639 uses, no cases met exclusion criteria. Thus, 730 (91 + 639 = 730) uses by the 5 physicians without prior exposure were compared with 887 uses by the 32 physicians with prior exposure to the interruptive CDS (► Figure 4). When using the non-interruptive CDS, the 5 physicians without prior exposure to the interruptive CDS were 7% less sensitive (p = 0.05) in identifying patients needing contact isolation (► Table 3).
Table 3.
Comparing sensitivity and specificity between users of the non-interruptive CDS who did and did not have prior exposure to the interruptive CDS
| Reference Standard: Nursing assessment | Exposed to Interruptive CDS (Oct 1 – Oct 14, 2010) | Not Exposed to Interruptive CDS (Mar 15 – Oct 14, 2010) | Chi-square |
|---|---|---|---|
| Sensitivity [95% CI] | |||
| Unadjusted | 14% [9%, 21%] | 7% [3%, 13%] | 3.84 (p = 0.05)* |
| Clustering Adjustment | 14% [6%, 23%] | 7% [5%, 9%] | 3.83 (p = 0.05)† |
| Specificity [95% CI] | |||
| Unadjusted | 98% [97%, 99%] | 97% [96%, 98%] | 0.61 (p = 0.44)* |
| Clustering Adjustment | 98% [97%, 99%] | 97% [97%, 98%] | 0.61 (p = 0.44)† |
*Pearson chi-square test;
† Mantel-Haenszel test
After adjusting for clustering within physicians, several confidence intervals were slightly increased (► Table 2 and ► Table 3). Nonetheless, the aforementioned differences remained statistically significant. Specificity of physicians using the CDS tools was universally high.
4. Discussion
We retrospectively analyzed how a change from an interruptive to a non-interruptive user interface affected the performance of a clinician-CDS dyad. Furthermore, we analyzed whether clinicians’ prior exposure to an interruptive CDS improved these clinicians’ performance when subsequently using a similarly designed non-interruptive CDS.
Our first hypothesis was that the interruptive user interface would be more successful at helping clinicians to recognize patients needing contact isolation. Indeed, interruptive CDS helped physicians to be more sensitive in identifying these patients. Specificity was maintained at very high levels under both types of CDS, which suggests both that clinicians engaged appropriately with the CDS when it was used, and that physicians were not identifying any cases missed by our chosen reference standard. Although the absolute levels of sensitivity were low compared to most diagnostic tests, these measurements are only slightly lower than what has been reported elsewhere [24]. Our sensitivity was likely lower because identification was attempted in the ED. It may prove difficult to affect ED physicians’ behavior because ED they focus on emergent conditions and because they have not historically been responsible for initiating isolation precautions. Nonetheless, the aforementioned CDC guidelines have issued a Category 1B recommendation that ED leaders should “develop and implement systems for early detection and management of potentially infectious persons at initial points of encounter.”[17]
Our second hypothesis was that prior exposure to the interruptive CDS would improve clinician usage of a similarly designed non-interruptive CDS. This hypothesis was based on the supposition that clinicians with multiple exposures to the interruptive CDS would internalize the logic contained therein, such that they would retain this logic even when the CDS did not provide interruptive reinforcement to do so. Even though this was our hypothesis, we also considered the possibility that clinicians might begin to rely on this CDS, and thus overlook the need to initiate contact isolation if not prompted. Our results suggest that in this case, physicians learned from the initial interruptive CDS. Prior studies that have initiated a CDS tool and subsequently turned it off have shown that physicians quickly stopped following its logic [25, 26]. We suspect that our results are different because the transition we studied was slightly different: instead of just turning the interruptive CDS off completely, a similar but non-interruptive CDS replaced it. Finally, it is important to note that when we restricted our analysis to the 32 physicians who used both types of CDS, the p value increased slightly to 0.06. We believe this was because the sensitivity analysis was underpowered, as sample sizes were generated based on our primary endpoint. Nonetheless, it will be important for other researchers to further test these findings.
Our evaluation of these two hypotheses has several implications. It has been previously noted that there are several aspects of CDS that can be adjusted to achieve different results from the CDS-clinician dyad [27]. Just as laboratory tests undergo overall optimization and are then customized to the patient population of a given institution to provide the maximal area under the receiver operating curve, our analysis suggests that CDS could be similarly optimized. Although non-diagnostic CDS may not have sensitivity and specificity, we would nonetheless hold that the designer of a CDS tool inherently faces several tradeoffs that impact the effectiveness of the CDS tool. For example, in the case of an if-then alert triggered by a set of conditions, the designer faces a tradeoff between missing appropriate patients (similar to false negatives) and misidentifying inappropriate patients (similar to false positives). In the former case, an alert that is not interruptive enough runs the risk of not eliciting the desired action even when patients are appropriately identified. In the latter case, the interruptiveness of the CDS tool affects the extent to which clinicians are inappropriately burdened with unnecessary alerts. Even for CDS tools like the ones studied here, that are triggered broadly for all patients being admitted, the concepts of sensitivity and specificity can be applied to measure whether the clinician-CDS dyad achieved the results of a reference standard workflow. Just as some prior work has suggested that the content of CDS might be adjusted based on local conditions [28], we believe that the interruptiveness of a CDS tool could be adjusted in this manner. Other work has suggested measuring the appropriateness of an alert firing and the subsequent provider response [29]. This framework may be especially useful in adjusting the interruptiveness of an alert to optimize the level of clinician burden.
It would be useful for researchers to develop a conceptual model that explicitly identifies the major tradeoffs inherent in the design of a CDS tool, and better still if this model could be used to assist in an explicit weighing of the various tradeoffs that are encountered. Extensive prior literature has documented the benefit of CDS [1], and this evidence could be used in such weighing. Much less work has been done in the quantitative assessment of the burden CDS places on the clinicians using it, and this knowledge would also be necessary to explicitly compare costs and benefits.
Our second hypothesis, more broadly stated, is as follows: temporary CDS with an interruptive user interface, followed by a similar CDS with a less burdensome non-interruptive interface, can achieve better results than clinicians using the non-interruptive CDS without prior exposure. Another way of stating this hypothesis is that clinicians learn from the content embedded within CDS. If further research continues to support this hypothesis, there would be several implications. Let us recognize that, currently, one of the major difficulties in implementing CDS is the burden it puts on clinician users [30]. The initial interruptive-subsequent passive implementation strategy could allow CDS designers to initiate clinicians’ attention on certain areas with an interruptive CDS, but then to maintain this focus over a longer period of time with a less burdensome non-interruptive CDS. A new interruptive CDS might be unveiled monthly, with all prior CDS maintained only by a non-interruptive user interface. To explore this area of inquiry, it would be important to gather more detailed measurements on how the interruptiveness of CDS interfaces relate to patient outcomes and clinician burdens, and to measure whether and when the effect of the initial interruptive CDS is eventually extinguished, even with non-interruptive CDS in place. Just as we have previously suggested that CDS interruptiveness might be customized to local conditions, we suggest here that it may be beneficial to customizing the interruptiveness of CDS across different time periods while the clinical setting is held constant.
5. Limitations
Our analysis has several limitations. First, our CDS tools differed slightly in their terminology. It is possible that the differing responses of clinicians could have been due to this variance, rather than the semi-hard stop present in the interruptive CDS. We recognized this difficulty in conducting our analysis, and we addressed it by separating terms into groups that would and would not have triggered contact isolation according to our epidemiologist-approved algorithm.
A second limitation is that the results of this CDS were not being used to isolate patients during the time period retrospectively analyzed. It is possible that some ED physicians were aware of this, and that their behavior towards this CDS was different than if the CDS had affected patient care. However, as we have noted in the methods section, we know that most ED physicians were unaware that this information was not being used to isolate patients once admitted. Furthermore, other types of operational initiatives have been successfully implemented in a stepwise manner at this institution, such that a patient identification phase may often precede an intervention phase. This was the intention of these CDS tools during the time period analyzed.
A third limitation is that other factors may have affected physician awareness of whether patients needed contact isolation. The ED physicians periodically receive oral and written reminders of the importance of isolating appropriate patients. In our institution, these educational activities were most prominent around the time of the H1N1 influenza outbreak. For this reason, we excluded the time period surrounding that outbreak from our initial analysis. Nonetheless, we would acknowledge that physicians who had worked more years in the Cedars-Sinai ED would have received more such educational outreach. We cannot exclude the possibility that these efforts, rather than exposure to an interruptive CDS, heightened their sensitivity in identifying patients requiring contact isolation. Furthermore, there may have been other relevant differences between physicians who initially contracted to work before and after the change to a non-interruptive CDS. We are not aware of any such differences that would be germane to this analysis. A related concern involves whether physicians using these CDS tools might be even more sensitive than our reference standard nursing assessment in identifying patients needing contact isolation. The universally high specificities we observed reassured us that this was not the case.
Fourth, we recognize that the CDS provided a relatively generic recommendation. Although this low degree of customization may not be what first comes to mind when CDS is discussed, the tools discussed still meet formal criteria to be classified as CDS. Furthermore, it is quite similar to other simple CDS tools that have been shown to impact patient care. Just as many effective outpatient CDS tools are triggered by a patient’s age and remind clinicians that preventive care may be indicated, these tools are triggered by a decision to admit and remind clinicians that contact isolation may be indicated. Most importantly, even if these tools were not considered to be CDS, our findings would still apply to CDS with more complex, customized recommendations.
A related concern might be that the CDS provided recommendations unsupported by evidence, or at least that clinicians believed them to be unsupported by evidence. Either situation could explain low adherence to these recommendations, regardless of user interface. Although we would acknowledge that the cited guideline is quite general and that the CDS recommendations were dependent on a local implementation, we believe the CDS recommendations to be evidence-based and consistent with the cited guideline. Furthermore, even if physicians disagreed with the recommendations of the CDS, we still found a difference between the extent to which they were willing to act on these recommendations based on the type of CDS user interface, and based on their prior exposure to the CDS.
6. Conclusion
We found that an interruptive CDS interface was more effective in affecting clinician responses than a non-interruptive interface. As CDS adoption efforts are intensified at the community medical centers and clinics that serve the vast majority of US patients, and are frequently staffed by non-employee physicians, it will be more important than ever to weigh the benefits of interruptive CDS interfaces with their associated clinician burden. Consideration should be made towards customizing CDS interfaces to fit local factors, including both patient and physician characteristics. We also advocate further testing of an initial interruptive and then subsequent non-interruptive CDS implementation strategy, which could potentially be a helpful tool to optimize the long term balance between patient outcomes and clinician burden.
Clinical Relevance Statement
We found that an interruptive CDS interface was more effective in altering clinician behavior than a non-interruptive interface. As CDS adoption efforts are intensified across the US, it will be more important than ever to weigh the benefits of interruptive CDS interfaces and their associated clinician burden. Consideration should be made towards customizing CDS interfaces to fit local factors, including both patient and physician characteristics.
Conflicts of Interest Statement
The authors declare that they have no conflicts of interest in this research.
Human Subjects Information
The Cedars-Sinai institutional review board approved our study.
List of Abbreviations used
CDC: Centers for Disease Control
CDS: Clinical Decision Support
ED: Emergency Department
EMR: Electronic Medical Record
HAI: Healthcare Associated Infection
MDRO: Multi-drug Resistant Organism
MRSA: Methicillin-resistant Staphylococcus aureus
SNF: Skilled Nursing Facility (SNF)
VRE: Vancomycin-resistant Enterococcus
Table 1.
Responses indicating a need for contact isolation, and how these differed by assessment tool.
| CDS-enabled ED Physician Assessment | Reference Standard | |
|---|---|---|
| Interruptive CDS (Figure 1) | Non-interruptive CDS (Figure 2) | Epidemiologist-designed, structured, objective assessment (Figure 3) |
| 1 – Contact: Open Wounds | 1 – Contact – Open Wound/Abscess | 1 – Draining Wound |
| 1 – Draining Wound | ||
| 2 – Contact Precautions | ||
| 2 – Isolation Contact | ||
| 2 – Mod Contact Isolation | ||
| 3 – Contact: SNF | 3 – Contact – SNF | 3 – Admit/Transfer from: Skilled Nursing Facility / Acute Care Hospital |
| 4 – Known MDRO | 4 – Contact – Known MDRO | 4 – History of MRSA/MDRO |
| 4 – Known MRSA | ||
| 4 – Known VRE | ||
| 5 – Ventilator Dependent | 5 – Ventilator Dependent | 5 – Ventilator Dependent |
Acknowledgement
This research was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number KL2TR000122 (Dr. Pevnick) and Grant UL1TR000124 (Dr. Bell). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Garg AX, Adhikari NKJ, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes. JAMA 2005; 293(10): 1223–1238 doi: 10.1001/jama.293.10.1223 [DOI] [PubMed] [Google Scholar]
- 2.Moxey A, Robertson J, Newby D, Hains I, Williamson M, Pearson SA. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc 2010; 17(1): 25–33 Epub 2010/01/13. doi: 10.1197/jamia.M3170. PubMed PMID: 20064798; PubMed Central PMCID: PMC2995634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald CJ. Use of a computer to detect and respond to clinical events: its effect on clinician behavior. Annals of Internal Medicine 1976; 84(2): 162 PubMed PMID: 6964557 [DOI] [PubMed] [Google Scholar]
- 4.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. Journal of the American Medical Informatics Association 2006; 13(2): 138–47 doi: 10.1197/jamia.M1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians’ decisions to override computerized drug alerts in primary care. Archives of internal medicine 2003; 163(21): 2625–2631 Epub 2003/11/26. doi: 10.1001/archinte.163.21.2625. PubMed PMID: 14638563. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. New England Journal of Medicine 2010; 363(6): 501–504 doi: doi:10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 7.Langberg M. Challenges to implementing CPOE: a case study of a work in progress at Cedars-Sinai. Mod Physician 2003;7:21–22 [Google Scholar]
- 8.Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc 2005; 12(4): 4384–47 Epub 2005/04/02. doi: 10.1197/jamia.M1777. PubMed PMID: 15802482; PubMed Central PMCID: PMC1174889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strom BL, Schinnar R, Aberra F, Bilker W, Hennessy S, Leonard CE, Pifer E. Unintended effects of a computerized physician order entry nearly hard-stop alert to prevent a drug interaction: a randomized controlled trial. Archives of internal medicine. 2010; 170(17): 1578–1583 Epub 2010/09/30. doi: 10.1001/archinternmed.2010.324. PubMed PMID: 20876410. [DOI] [PubMed] [Google Scholar]
- 10.Strom BL, Schinnar R, Bilker W, Hennessy S, Leonard CE, Pifer E. Randomized clinical trial of a customized electronic alert requiring an affirmative response compared to a control group receiving a commercial passive CPOE alert: NSAID–warfarin co-prescribing as a test case. Journal of the American Medical Informatics Association 2010; 17(4): 411–415 doi: 10.1136/jamia.2009.000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamblyn R, Huang A, Taylor L, Kawasumi Y, Bartlett G, Grad R, Jacques A, Dawes M, Abrahamowicz M, Perreault R, Winslade N, Poissant L, Pinsonneault A. A randomized trial of the effectiveness of on-demand versus computer-triggered drug decision support in primary care. J Am Med Inform Assoc 2008; 15(4): 430–438 Epub 2008/04/26. doi: 10.1197/jamia.M2606. PubMed PMID: 18436904; PubMed Central PMCID: PMCPMC2442270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matheny ME, Sequist TD, Seger AC, Fiskio JM, Sperling M, Bugbee D, Bates DW, Gandhi TK. A randomized trial of electronic clinical reminders to improve medication laboratory monitoring. J Am Med Inform Assoc 2008; 15(4): 424–429 Epub 2008/04/26. doi: 10.1197/jamia.M2602. PubMed PMID: 18436905; PubMed Central PMCID: PMCPMC2442256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rollman Bl, Hanusa BH, Gilbert T, Lowe HJ, Kapoor WN, Schulberg HC. The electronic medical record: A randomized trial of its impact on primary care physicians and initial management of major depression. Archives of Internal Medicine 2001; 161(2): 189–197 [DOI] [PubMed] [Google Scholar]
- 14.Phansalkar S, van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, Middleton B, Bates DW. Drug–drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. Journal of the American Medical Informatics Association 2013; 20(3): 489–493 doi: 10.1136/amiajnl-2012-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobach D, Sanders GD, Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux R, Samsa G, Hasselblad V, Williams JW, Wing L, Musty M, Kendrick AS. Enabling health care decisionmaking through clinical decision support and knowledge management. Evidence Report No. 203. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I.) AHRQ Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality; April 2012 [PMC free article] [PubMed] [Google Scholar]
- 16.Horsky J, Schiff G, Johnston D, Mercincavage L, Bell DS, Middleton B. Interface design principles for usable decision support: A targeted review of best practices for clinical prescribing interventions. Journal of Biomedical Informatics 2012; 45(6): 1202–1216 [DOI] [PubMed] [Google Scholar]
- 17.Siegel JD, Rhinehart E, Jackson M, Chiarello Lthe Healthcare Infection Control Practices Advisory Committee, 2007Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afif W, Huor P, Brassard P, Loo VG. Compliance with methicillin-resistant Staphylococcus aureus precautions in a teaching hospital. American Journal of Infection Control 2002; 30(7): 430–433 doi: 10.1067/mic.2002.125174. [DOI] [PubMed] [Google Scholar]
- 19.Clock SA, Cohen B, Behta M, Ross B, Larson EL. Contact precautions for multidrug-resistant organisms: Current recommendations and actual practice. American Journal of Infection Control 2010; 38(2): 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamlin BW, Overhage JM, Tierney W, Dexter P, McDonald CJ.Clinical decision support within the Regenstrief medical record system. In: Berner ES: Springer New York; 2007: 190–314 [Google Scholar]
- 21.Kho AN, Dexter PR, Warvel JS, Belsito AW, Commiskey M, Wilson SJ, Hui SL, McDonald CJ. An effective computerized reminder for contact isolation of patients colonized or infected with resistant organisms. International journal of medical informatics 2008; 77(3): 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen CB, Galewsky A. EmSTAT™, A comprehensive point-of-care clinical information system for emergency medicine. Proc Annu Symp Comput Appl Med Care 1990: 944–945 [Google Scholar]
- 23.Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America 2003; 24(5): 362–386 Epub 2003/06/06. doi: 10.1086/502213. PubMed PMID: 12785411. [DOI] [PubMed] [Google Scholar]
- 24.Pittet D, Safran E, Harbarth S, Borst F, Copin P, Rohner P, Scherrer JR, Auckenthaler R. Automatic alerts for methicillin-resistant Staphylococcus aureus surveillance and control: role of a hospital information system. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America 1996; 17(8): 496–502 Epub 1996/08/01. PubMed PMID: 8875292 [PubMed] [Google Scholar]
- 25.Schriger DL, Baraff LJ, Buller K, Shendrikar MA, Nagda S, Lin EJ, Mikulich VJ, Cretin S. Implementation of clinical guidelines via a computer charting system. Journal of the American Medical Informatics Association 2000; 7(2): 186–195 doi: 10.1136/jamia.2000.0070186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schriger DL, Baraff LJ, Rogers WH, Cretin S. Implementation of clinical guidelines using a computer charting system. JAMA: The Journal of the American Medical Association 1997; 278(19): 1585–1590 doi: 10.1001/jama.1997.03550190049043 [PubMed] [Google Scholar]
- 27.Berner ES, Lande TJ. Overview of clinical decision support systems, clinical decision support systems. In: Berner ES: Springer New York; 2007: 3–22 [Google Scholar]
- 28.Ray HN, Boxwala AA, Anantraman V, Ohno-Machado L. Providing context-sensitive decision-support based on WHO guidelines. Proceedings of the AMIA Symposium 2002: 637–641 PubMed PMID: 12463901 [PMC free article] [PubMed] [Google Scholar]
- 29.McCoy AB, Waitman LR, Lewis JB, Wright JA, Choma DP, Miller RA, Peterson JF. A framework for evaluating the appropriateness of clinical decision support alerts and responses. Journal of the American Medical Informatics Association 2012; 19(3): 346–352 doi: 10.1136/amiajnl-2011-000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B. Ten commandments for effective clinical decision support: Making the practice of evidence-based medicine a reality. Journal of the American Medical Informatics Association 2003; 10(6): 523–530 doi: 10.1197/jamia.M1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sim I, Berlin A. A framework for classifying decision support systems. AMIA Annu Symp Proc. 2003: 599–603 Epub 2004/01/20. PubMed PMID: 14728243; PubMed Central PMCID: PMC1480261. [PMC free article] [PubMed] [Google Scholar]
- 32.Shea S, DuMouchel W, Bahamonde L. A Meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. Journal of the American Medical Informatics Association 1996;3:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]