Abstract
Diaporthe phaseolorum is a frequent fungal parasite of plants, rarely involved in human diseases. We describe a case of cutaneous infection caused by this fungus diagnosed by morphology and molecular biology, on the hands and on a foot of a renal transplanted Brazilian farmer. The infection was resolved with oral itraconazole.
Keywords: Diaporthe, Phomopsis, Brazil, Granulomatous
1. Introduction
Solid organ transplant patients are frequently infected by fungi, in nearly 5% of cases within the first six months after transplantation and principally by Candida, Aspergillus and Cryptococcus [1]. We describe here a case of a patient infected after a renal transplant with the fungus Diaporthe, a common plant parasite [2] that can occasionally infect humans [3].
2. Case
A 43-year-old farmer man with end-stage renal failure, caused by focal segmental glomerulosclerosis, underwent kidney transplantation from a cadaveric donor. The patient developed renal vein thrombosis in the transplanted kidney that had to be removed. The patient underwent kidney transplantation for the second time, again from a cadaveric donor and received antithymocyte globulin, tacrolimus and prednisone as immunosuppressive regimen. He developed diabetes mellitus that was adequately controlled by metformin.
Two years later, the patient related cutaneous indurated and eritematous lesions on the both right extremities for two months, without history of previous trauma. Part of these lesions (one from the right arm and another from of the right leg) were surgically removed to histologic examination, which revealed foci of granulomatous lesions with central microabscesses (chronic necrotising granulomatous lesions) in the deep dermis (Fig. 1). Grocott–Gomori silver staining showed the presence of septate hyphae, occasionally branched at 45° angles, and yeast-like cells with a thick wall sometimes in chains (Fig. 2). Portions of the biopsied specimens were cultured on Sabouraud dextrose agar tubes (SDA; Difco Laboratories, Detroit, Mich.) with chloramphenicol (0.05 mg/mL) and incubated at 25° and 37 °C for 7 days. Cultures of both samples yielded identical colonies of the same filamentous dematiaceous fungus, which was sent to the Faculty of Medicine of the Rovira i Virgili University for identification.
Fig.1.
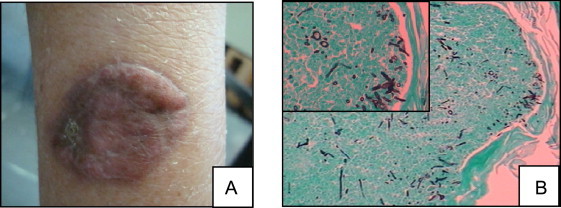
Macroscopic aspect of the lesion in the right arm (A). Grocott–Gomori silver staining revealed the presence of septate hyphae, and yeast-like cells with thick wall, 40× magnification (B).
Fig. 2.
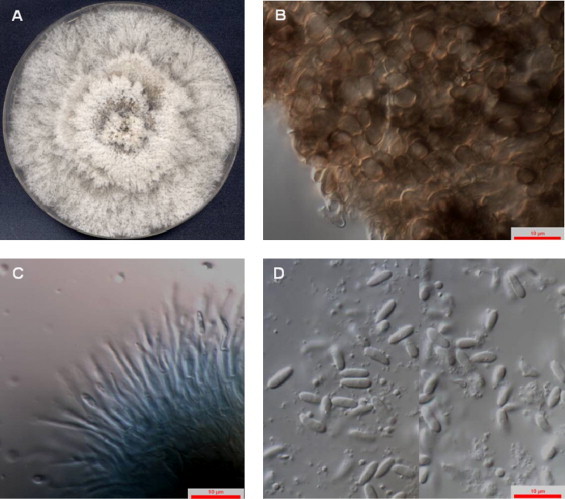
Diaporthe phaseolorum (FMR 12294). (A) Colony on PDA after 7 days at 25 °C, (B) pycnidial outer wall of textura globulosa, (C) conidiophores and (D) conidia.
Treatment was initiated after diagnose with oral itraconazole at 100 mg twice a day. After one month, the skin lesions remission was noticed and surgically excised, also continued with itraconazole. At a 5-month follow-up visit, while still receiving itraconazole and continuing with the immunosuppressive regime, there was no recurrence of skin lesions.
For identification, the fungus (FMR 12194) was subcultured on oatmeal agar (OA; 30 g filtered oat flakes, 20 g agar, 1 L distilled water) and potato dextrose agar (PDA; Difco Laboratories, Detroit, Mich.) and incubated at 25 °C±2 °C for up to two months. Growth rates were determined on PDA plates at 25°, 37°, 40° and 42 °C for 14 days in darkness. In the following description colour colony codes (in parenthesis) refer to Kornerup and Wanscher [4]. The microscopic features were determined by making wet mounts with lactic acid, which were then examined under a light microscope.
The fungus growing on OA and PDA reached a diameter of 50–70 mm in 5 days and covered the whole agar surface after 7 days at 25 °C; at 37 °C, colonies reached 21–30 mm diameter after 21 days, and did not grow at 40 °C. On PDA, the fungus produced a yellowish orange (4/A/7–8) diffusible pigment, orange (5/B/7) on the colony reverse, which was absent in OA. The colony morphology was very similar on the two culture media tested. At the beginning they were white with cottony to lanose or feathery aerial mycelium, with a slightly fringed margin; reverse brown (5/E/4) to dark brown (6/F/4) at the centre. After 2–3 weeks immersed or semi-immersed, dark pigmented stromata, 350–800 μm diameter, appeared scattered on the agar, some ripened to give pycnidial conidiomata. Pycnidia usually single, globose to subglobose, unilocular, ostiolate, dark brown or black, often with 1–2 long necks; wall composed of an outer layer of cells of textura globulosa to angularis, dark brown, thick-walled, 7–13 μm diameter, and a thick inner layer of cells of textura globulosa, colourless; necks more or less cylindrical, 250–460 μm long, coated with short dark brown hyphae. Conidiophores lining the cavity of the conidiomata, generally composed of a hyaline, cylindrical basal cell, 5–7×2.5–3 μm, usually bearing 1 or 2 terminal phialidic conidiogenous cells. Conidiogenous cells hyaline, cylindrical, tapering towards the apex, 8–13×1.5–2 μm, with barely visible collarettes. Conidia unicellular, hyaline, ellipsoidal to cylindrical, rounded at both ends, 5–8×1.8–2 μm, biguttulate, sometimes bearing a basal conidiogenous scar, arranged in slimy masses. Teleomorph were not observed (Fig. 2). Based on these features, the fungus was tentatively identified as Diaporthe sp.
To confirm its identity, the internal transcribed spacer (ITS) region of rDNA was amplified and sequenced as previously described [5]. BLAST sequence identity searches [6] were carried out to compare the sequence obtained (554 bp, GenBank accession ♯ HF586483) from the case isolate with those of other fungi deposited in the GenBank database. The BLAST query with the ITS regions sequenced from the isolate showed a 99% similarity with several sequences of Diaporthe phaseolorum (accession ♯ AF 001020.2, AY 577815.1, JN 541222.1 and JF 896458.1; 100% query coverage), and two other sequences of Diaporthe sp. (FJ799938.1) and Phomopsis sp. (DQ480356.1).
3. Discussion
The members of the genus Diaporthe are important plant pathogens, endophytes or saprobes on plant debris that are most commonly seen as their Phomopsis anamorphs. These anamorphs are usually characterised by the production of two types of conidia (α-conidia, short and more or less ellipsoidal or cylindrical; β-conidia, long and filiform), into pycnidial conidiomata [7].
Traditionally, the identification of the species belonging to the Diaporthe/Phomopsis complex relies on morphology, culture characteristics and host association. However, several studies have demonstrated that such features are not always suitable for that purpose mainly due to their inter- and intra-species variability [8,9]. The identification of these fungi is currently mainly based on the sequence analyses of the ITS region, although other genomic regions have also been used for taxonomic reevaluation of Diaporthe/Phomopsis species [9–11]. In the present case, the morphological features of the clinical isolate are very similar to that of Diaporthe longicolla (=Phomopsis longicolla) mainly by the absence of β-conidia and the sexual state [10]. However, the ITS sequence analysis confirmed its identification as D. phaseolorum. It is worth mentioning that Santos et al. [10] described D. phaseolorum as a homothallic species most strains of which, in addition to having sexual structures, produce the anamorph in culture with the two types of conidia, features that were not observed in any of the culture conditions tested in our study. Diaporthe longicolla, the species that shows morphological similarity with our isolate, was recently described as the etiological agent of a cutaneous infection. In that case its identification was based only on ITS sequences since the fungus did not sporulate in culture [12].
The present study is the second case of human infection by D. phaseolorum [3] and the first in Brazil. Another three cases of invasive infection were attributed to Diaporthe spp. They were a cutaneous infection, keratitis and osteomyelitis that generally involved farmers and gardeners receiving immunosuppressive therapy [12–14]. In this study, our patient developed the skin lesions two years after transplantation. García-Reyne et al. [12] also described a patient who showed the lesions one year after the renal transplantation. There needs to be greater attention paid to transplant patients concerning the occurrence of fungal infections, especially those who are immunosuppressed and who have other diseases such as diabetes mellitus [15].
In the clinical cases caused by saprobic fungi, as in the present case, the diagnosis is not easy, the combination of culture, histopathology and patient's clinical history have being fundamental for a definitive diagnosis [16]. Here, the isolation of the same fungus, from two different lesions, on two different extremities was helpful to avoid considering a possible contamination. However, how this microorganism could infect two different sites is intriguing.
In the previous cases of human infections by Diaporthe diverse therapies have been used. The cutaneous infection by D. longicolla in a renal transplant recipient patient was treated unsuccessfully with itraconazole and terbinafine, while surgical resection and voriconazole resolved the infection [12]. The case of keratitis was successfully treated with a combination of keratoplasty and topical voriconazole/amphotericin B therapy. The previous cutaneous infections were also successfully treated with itraconazole [3,13,14].
Conflict of interest
There are none.
References
- 1.Badiee P., Alborzi A. Invasive fungal infections in renal transplant recipients. Experimental and Clinical Transplantation. 2011;9:355–362. [PubMed] [Google Scholar]
- 2.Thompson S.M., Tan Y.P., Young A.J., Neate S.M., Aitken E.A.B., Shivas R.G. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia. 2011;27:80–89. doi: 10.3767/003158511X617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iriart X., Binois R., Fior A., Blanchet D., Berry A., Cassaing S. Eumycetoma caused by Diaporthe phaseolorum (Phomopsis phaseoli): a case report and a mini-review of Diaporthe/Phomopsis spp. invasive infections in humans. Clinical Microbiology and Infection. 2011;17:1492–1494. doi: 10.1111/j.1469-0691.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 4.Kornerup A., Wanscher J.H. Methuen Publishing Ltda; London: 1978. Methuen Handbook of Colour; p. 1978. [Google Scholar]
- 5.Gilgado F., Cano J., Gené J., Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. Journal of Clinical Microbiology. 2005;43:4930–4942. doi: 10.1128/JCM.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 7.Sutton BC The Coelomycetes. Kew, U.K: Commonwealth Mycological Institute; 1980.
- 8.Van der Aa H.A., Noordeloos M.E., Gruyter J. Species concepts in some larger genera of the Coelomycetes. Studies in Mycology. 1990;32:3–19. [Google Scholar]
- 9.Santos J.M., Phillips A.J.L. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009;34:111–125. [Google Scholar]
- 10.Santos J.M., Vrandecic K., Cosic J., Duvnjak T., Phillips A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia. 2011;27:9–19. doi: 10.3767/003158511X603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udayanga D., Liu X., Crous P.W., McKenzie E.H.C., Chukeatirote E., Hyde K.D. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis) Fungal Divers. 2012:2012. [Google Scholar]
- 12.García-Reyne A., López-Medrano F., Morales J., Esteban C., Martin I., Eraña I. Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: first report of human infection by this fungus. Transplant Infectious Disease. 2011;13:204–207. doi: 10.1111/j.1399-3062.2010.00570.x. [DOI] [PubMed] [Google Scholar]
- 13.Sutton D., Timm W., Morgan-Jones G., Rinaldi M.G. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis Saccardo 1905: Criteria for identification, case history, and therapy. Journal of Clinical Microbiology. 1999;37:807–811. doi: 10.1128/jcm.37.3.807-811.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandell K.J., Colby K.A. Penetrating keratoplasty for invasive fungal keratitis resulting from a thorn injury involving Phomopsis species. Cornea. 2009;28:1167–1169. doi: 10.1097/ICO.0b013e31819839e6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X.D., Hu X.P., Yin H., Wang W., Zhang X., Ma L.L. Aspergillus pneumonia in renal transplant recipients. Chinese Medical Journal (English) 2008;121:791. 479. [PubMed] [Google Scholar]
- 16.Sutton D.A. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Revista Iberoamericana de Micología. 1999;16:171–179. [PubMed] [Google Scholar]


