Abstract
A 32-year-old HIV negative male presented with multiple pulmonary cavitation and skin abscesses up to 15 cm in diameter mimicking tuberculosis. Sporothrix brasiliensis was isolated and patient responded well to amphotericin B followed by itraconazole, except the skin lesions that had to be surgical drained to obtain cure.
Keywords: Sporothrix brasiliensis, Pulmonary sporotrichosis, Genotyping, Serology, Amphotericin B
1. Introduction
Sporotrichosis is an acute or subacute granulomatous mycosis caused by the cryptic species of the Sporothrix complex. In 80% of the cases the clinical presentation is the lymphocutaneous form. On the other hand, the widespread extracutaneous form is rare and usually found in immunosuppressed patients, especially HIV positive individuals [1–3].
The largest epidemic of zoonotic transmission of sporotrichosis was registered in the city of Rio de Janeiro, Brazil, since 1997, where it is now considered as an endemic disease [4]. This case reports aim to call attention for different and aggressive clinical presentations related to the zoonotic epidemics and also discusses differential diagnosis, etiology, transmission and treatment of these cases.
2. Case
On the 8th February 2012 (Day 0), a 32-year-old male presented at the hospital with cough, daily fever, asthenia, diarrhea, myalgia, weight loss and disseminated cutaneous lesions lasting for about 6 months. Lung murmur was reduced on auscultation and hepatomegaly was present. The patient had a history of alcohol and tobacco abuse for 18 years and contact with dogs, cats and pigs at the workplace. Figs. 1–3 show the skin lesions with different clinical aspects, as deep granulomatous ulcers with purulent discharge, nodules and abscesses of variable sizes reaching 15 cm in diameter, none of them had the typical lymphocutaneous clinical feature. Skin lesions were spread throughout the body except for the face and scalp. There was no mucosal, bone and other organs involvement. Laboratorial tests (Day 0) revealed normocytic and normochromic anemia (hemoglobin [Hb] 8 g/dL, hematocrit [Ht] 24%); leukocytosis 13,480/mm3; platelets 365,000/mm3; normal liver enzymes except slightly elevated GGT (80 U/L) and ALP (193 U/L); low albumin (2.6 g/dL) and preserved renal function. Serologies for HIV, hepatitis A, B and C, toxoplasmosis, cytomegalovirus and syphilis were negative. Chest radiography and computed tomography (CT) revealed numerous cavitary pulmonary nodules spread in both lungs (Fig. 4A and C, respectively). Tuberculosis, paracoccidiodomycosis and histoplasmosis were the strongest diagnostic hypothesis considered and for this reason he was transferred to an isolated room. Three sputum samples were BAAR negative. Serology was positive for sporotrichosis by an ELISA test using the SsCBF antigen and, an IgG serum titer of 102,400 (cut off<6400) was determined [5]. The skin biopsy of an abdominal lesion revealed an inflammatory infiltrate consisting of plasma cells, histiocytes and neutrophils. Isolated yeast cells and asteroid body could be seen in tissue sections stained by hematoxylin–eosin and Grocott. Sporothrix spp. was isolated from the sputum (isolate HUPE 117310), cutaneous secretion (isolate HUPE 117298) and biopsy of skin lesions. Molecular identification of the etiological agent species was ascertained by PCR amplification and sequencing of the partial calmodulin gene. Sequences were deposited in the GenBank under accession numbers KC329494 and KC329493, respectively. Both sequenced amplicons were aligned with the NCBI nucleotide nr databank using Blastn tool [6] revealing 100% of identity with previously deposited S. brasiliensis sequences [7–8]. In order to prove the genetic origin from the isolates HUPE 117310 and HUPE 117298, phylogenetic analysis were carried out in the MEGA software 5.0 [9] using Maximum Likelihood (ML) and Neighbor-Joining (NJ) methods. Sequences representing the pathogenics species of Sporothrix complex: S. schenckii clade I (AM117439 and AM117442) and clade II (AM399011 and JQ041906), S. brasiliensis (JQ041903 and AM116899), S. globosa (AM117430 and AM117434), S. mexicana (AM398393 and AM398392) and S. albicans (AM398382 and AM398396) were joined into nucleotide alignments. The nucleotide substitution model chosen was Kimura 2 parameter [10] and phylogenetic analysis was conducted in 1000 bootstraps replicates. As expected, the isolates HUPE 117310 and HUPE 117298 were placed in the S. brasiliensis clade supported by high boostraps values (ML-99 and NJ-97) (Fig. 5).
Fig. 1.
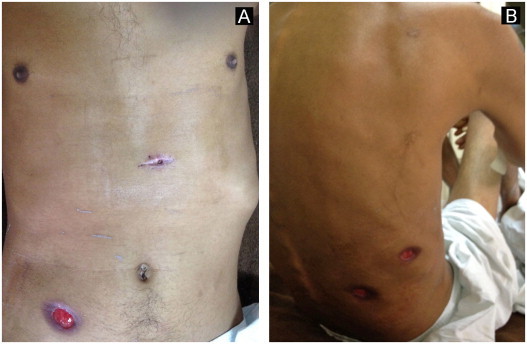
Cutaneous ulcers on the chest, abdomen and back of a patient with pulmonary sporotrichosis (A and B). Cutaneous biopsy scar is visible in Fig. 1A.
Fig. 2.
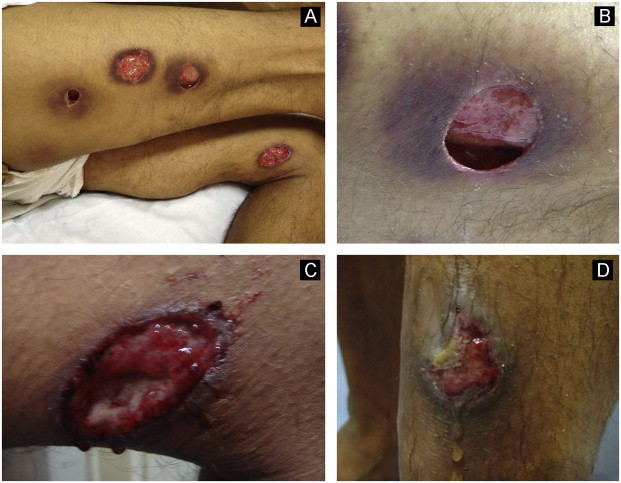
Numerous deep ulcerated nodules on the legs and thighs in the same patient (A, B, C and D).
Fig. 3.
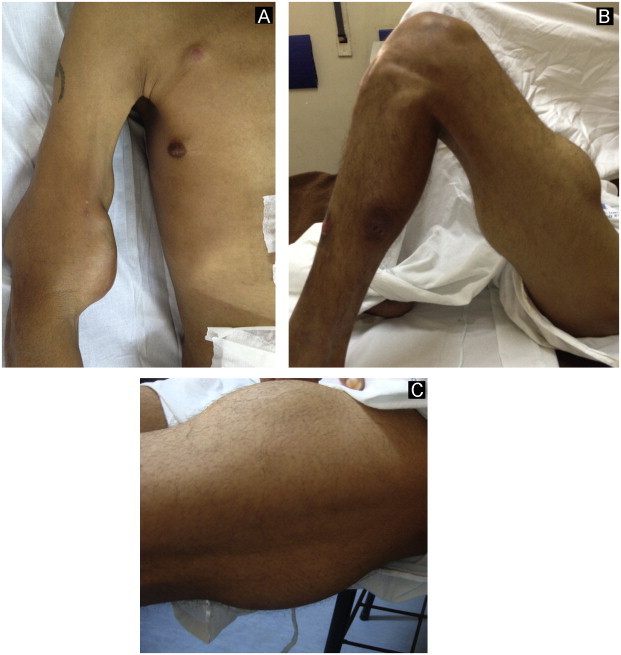
Large cutaneous abscesses on the arms (A) and thighs (B and C) due to S. brasiliensis infection.
Fig. 4.
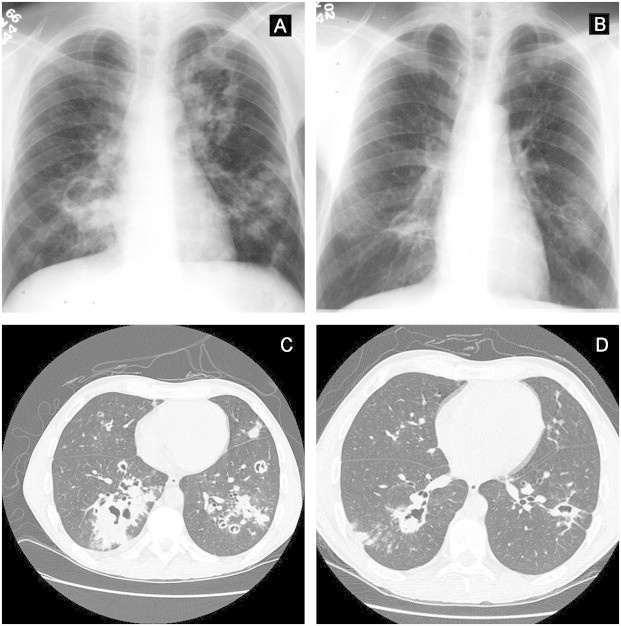
Pretreatment x-ray (A) showing numerous cavitary nodules spread in both lungs due to S. brasiliensis infection; 3 months post-treatment image (B); Pretreatment CT of the same patient (C) and 4 months post-treatment CT (D).
Fig. 5.
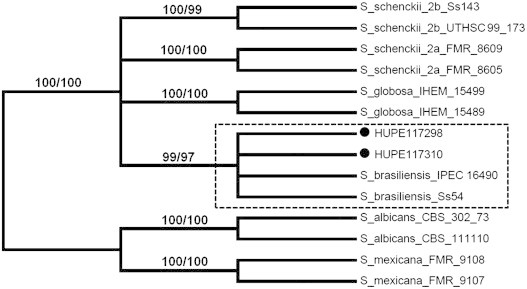
Phylogenetic tree generated by Maximum Likelihood and Neighbour-Joining methods revealing the placement of the isolates HUPE 117310 and HUPE 117298 (●) in the S. brasiliensis clade (dashed clade). The percentage of replicate trees in which isolates clustered together in the bootstrap test (1000 replicates) are shown in the branches in both methods used.
Treatment was started on 02/15/2012 with intravenous amphotericin B deoxycholate 42 mg/day for 14 days perceiving a cumulative dose of 588 mg. On 02/29 deoxycholate was replaced for a liposomal formulation (Ambisome®), 300 mg/day, reaching a total dose of 12,300 mg after 42 days of treatment. After one month of amphotericin B the patient had a marked improvement in his general health condition (weight gain and no fever or cough). Ulcerated skin lesions responded well to systemic treatment (Fig. 6) although skin abscesses remained unchanged. Therefore, he was submitted to successfully surgical drainage of skin abscesses (Figs. 7–9). Intravenous medication was replaced by 400 mg/day of oral itraconazole on 04/10/2012. Chest radiography (3 months post-treatment) and CT images (4 months post-treatment) showed marked improvement after 4 months of treatment (Fig. 4B and D, respectively). The patient remains under oral therapy which must be maintained for at least 12 months.
Fig. 6.
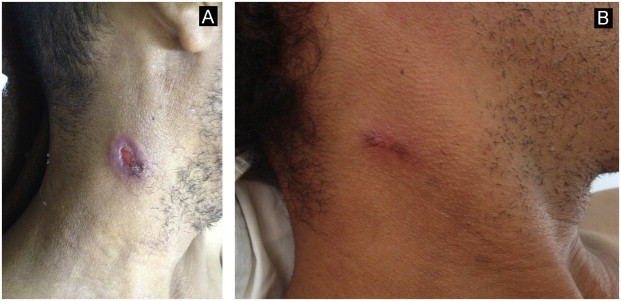
Lymph node fistula on the neck mimicking scrofuloderma: images before (A) and after systemic therapy (B).
Fig. 7.
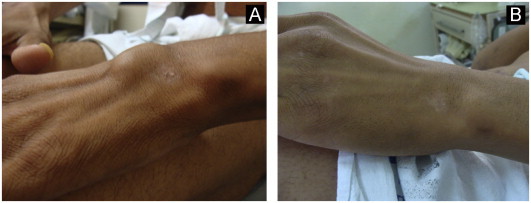
Surgical treatment of an abscess on the left wrist: pretreatment (A) and post-treatment (B) images.
Fig. 8.
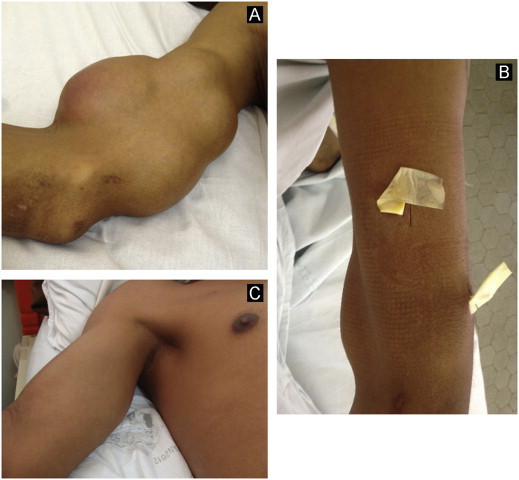
Surgical approach of intercommunicating skin abscesses on the right arm: images before surgery (A); with the drain (B) and post-treatment (C).
Fig. 9.
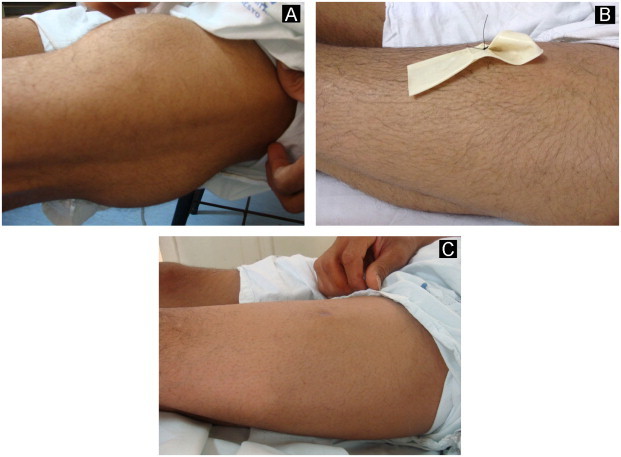
Surgical treatment of a large cutaneous abscess on the left thigh: images before surgery (A); with the drain (B) and post-treatment (C).
3. Discussion
On the extracutaneous presentation of sporotrichosis the bones and joints are the preferred affected sites [11]; dissemination to skin or other organs is considered rare and generally affecting immunosuppressed individuals, especially HIV positive patients [12–14]. Lung damage by Sporothrix spp. is uncommon and thought to occur by inhalation of conidia instead of local trauma or dissemination from a skin lesion. Reports of pulmonary sporotrichosis have been published sporadically, nor always with cutaneous involvement [15–17]. Many times the source of infection is unknown. Pulmonary tuberculosis is endemic in Brazil and is the first hypothesis for the diagnosis of a patient manifesting with pulmonary cavitation, fever, cough and weight loss. Considering the symptoms presented by this patient, paracoccidiodomycosis and histoplasmosis were also included as differential diagnosis. Since the outbreak of sporotrichosis became first epidemic and then endemic through the last 15 years new clinical forms of this disease have been diagnosed [18,19]; lung involvement at these endemic areas could now be included as a differential diagnosis of pulmonary diseases, especially those showing nodular, cavitary or fibronodular radiological images. Lung injury probably had spread the fungus to skin by hematological dissemination, due to the extensive pulmonary parenchymal involvement and lack of cutaneous trauma history or skin lesions suggesting lymphatic spread of his disease. In these cases surgical approach as suggested by literature is unfeasible. Alcohol and tobacco abuse, thought to cause immunosupression, and infection by S. brasiliensis, known to be the prevalent species in this epidemic, may both predispose to the destructive clinical form presented in this case. Strain and species virulence are being studied by different groups [7,8,18]. According to the guideline of the American Society of Infectious Diseases [20], the initial treatment of these cases should be performed with intravenous amphotericin B followed by oral itraconazole for at least 12 months. This therapeutic protocol was done in this case and resulted in good clinical response although the patient will be followed for a long period under treatment. Dermatologists should be aware of cutaneous abscesses since medication has a poor penetration in these closed lesions, and consider surgical approaches in cases where skin lesions seem to be unresponsive to systemic drug treatment.
Conflict of interest
There are none.
Acknowledgments
This work was supported by Faperj and Health Ministry of Brazil, grant E-26/110.761/2010. LMLB is a research fellow of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Lopes-Bezerra LM, Schubach AO, Costa RO. Sporothrix schenckii and sporotrichosis. Anais da Academia Brasileira de Ciências. 2006;78(2):293–308. doi: 10.1590/s0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 2.da Rosa AC, Scrofenerker ML, Vettorato R, Gervini RL, Vettorato G, Weber A. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. Journal of the American Academy of Dermatology. 2005;52(3 Pt 1):451–459. doi: 10.1016/j.jaad.2004.11.046. Review. [DOI] [PubMed] [Google Scholar]
- 3.Barros MB, Schubach Ade O, do Valle AC, Gutierrez Galhardo MC, Conceição-Silva F, Schubach TM. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clinical Infectious Diseases. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 4.Barros MBL, Schubach AO, Schubach TMP, Wanke B, Lambert-Passos SR. An epidemic of sporotrichosis in Rio de Janeiro, Brazil: epidemiological aspects of a series of cases. Epidemiology and Infection. 2008;136:1192–1196. doi: 10.1017/S0950268807009727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardes-Engemann AR, Orofino RC, Miguens BP. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Medical Mycology. 2005;43:487–493. doi: 10.1080/13693780400019909. [DOI] [PubMed] [Google Scholar]
- 6.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 7.Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. Journal of Clinical Microbiology. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues AM, de Hoog GS, de Camargo ZP. Emergence of pathogenicity in the Sporothrix schenckii complex. Medical Mycology. 2012 doi: 10.3109/13693786.2012.719648. [DOI] [PubMed] [Google Scholar]
- 9.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 11.Costa RO, de Mesquita KC, Damasco PS, Bernardes-Engemann AR, Dias CM, Silva IC. Infectious arthritis as the single manifestation of sporotrichosis: serology from serum sample and synovial fluid as an aid to diagnosis. Revista Iberoamericana de Micología. 2008;25:54–56. doi: 10.1016/s1130-1406(08)70014-7. [DOI] [PubMed] [Google Scholar]
- 12.Bunce PE, Yang L, Chun S, Zhang SX, Trinkaus MA, Matukas LM. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Medical Mycology. 2012;50(2):197–201. doi: 10.3109/13693786.2011.584074. [DOI] [PubMed] [Google Scholar]
- 13.Callens SF, Kitetele F, Lukun P, Lelo P, Van Rie A, Behets F. Pulmonary Sporothrix schenckii infection in a HIV positive child. Journal of Tropical Pediatrics. 2006;52(2):144–146. doi: 10.1093/tropej/fmi101. [DOI] [PubMed] [Google Scholar]
- 14.Barros MBL, Scubach AO, Galhardo MCG, TMPB Scubach, Reis RS, Conceição MJ. Sporotrichosis with widespread cutaneous lesions: report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. International Journal of Dermatology. 2003;42:677–681. doi: 10.1046/j.1365-4362.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 15.England DM, Hochholzer L. Sporothrix infection of the lung without cutaneous disease. Primary pulmonary sporotrichosis. Archives of Pathology & Laboratory Medicine. 1987;111(3):298–300. [PubMed] [Google Scholar]
- 16.Padhye AA, Kaufman L, Durry E, Banerjee CK, Jindal SK, Talwar P. Fatal pulmonary sporotrichosis caused by Sporothrix schenckii var. luriei in India. Journal of Clinical Microbiology. 1992;30(9):2492–2494. doi: 10.1128/jcm.30.9.2492-2494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhai M, Rawat V, Verma P, Jha PK, Shree D, Goyal R, Umesh Primary pulmonary sporotrichosis in a sub-Himalayan patient. J Lab Physicians. 2012;4(1):48–49. doi: 10.4103/0974-2727.98674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez-Galhardo MC, Barros MB, Schubach AO, Cuzzi T, Schubach TM, Lazéra MS. Erythema multiforme associated with sporotrichosis. Journal of the European Academy of Dermatology and Venereology. 2005;19(4):507–509. doi: 10.1111/j.1468-3083.2005.01148.x. [DOI] [PubMed] [Google Scholar]
- 19.Orofino-Costa R, Bóia MN, Magalhães GA, Damasco PS, Bernardes-Engemann AR, Benvenuto F. Arthritis as a hypersensitivity reaction in a case of sporotrichosis transmitted by a sick cat: clinical and serological follow up of 13 months. Mycoses. 2010;53(1):81–83. doi: 10.1111/j.1439-0507.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Clinical practice guidelines for the management of sporotrichosis: 2007 Update by the infectious diseases society of America. IDSA guidelines for management of sporotrichosis. Clinical Infectious Diseases. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]


