Abstract
We recently observed six cases of generalized dermatitis associated with Malassezia overgrowth in cats presented to the Veterinary College of Alfort, France. Elevated numbers of yeasts were observed in lesional skin by cytology and culture. Skin lesions occurred on the face, ventral neck, abdomen and ear canals and were characterized by some degree of alopecia, erythema and crusting. In most cases, pruritus was intense. The species M. pachydermatis was systematically isolated.
Keywords: Malassezia yeasts, Dermatitis, Cat, Predisposing factor, Treatment
1. Introduction
Malassezia yeasts belong to normal cutaneous or mucosal microbiota of many warm-blooded vertebrates [1]. These yeasts are now recognized as opportunistic pathogens that play a significant role in the development of different human and animal diseases such as otitis externa or seborrheic dermatitis. Malassezia yeasts have an affinity for lipids as substrates and the term “lipophilic yeasts” has frequently been used to characterize the genus. In fact, 13 out of the 14 currently described Malassezia species show an absolute requirement for long fatty acid chains [2]. These “lipid-dependent” yeasts are therefore seldom isolated in the laboratory unless specific nutrients are provided in the medium. The species M. pachydermatis is the only lipophilic yeast that may be isolated in regular media like Sabouraud dextrose agar.
In 1983, Dufait [3] was the first one to report Malassezia yeasts as a cause of generalized dermatitis in dogs. He described a series of 50 dogs with pruritic dermatitis from which the yeasts could be readily recovered by cytology or culture and which responded to antifungal therapy. Skin lesions consisted of erythema and hyperpigmentation that most often affected the ventral abdomen, although the face, feet and perineal regions were also commonly affected. A few years later, similar cases were described in dogs from Brazil and USA [4]. These reports forced the veterinary dermatology community to consider the potential role of M. pachydermatis as a cause of canine skin diseases [5]. Malassezia yeasts can also be isolated from the external ear canal and mucosae of healthy cats as well as cats with otitis externa and dermatitis. In healthy cats, reported percentages of isolation of Malassezia yeasts range from less than 10% to approximately 20% and up to 40% [1]. In parallel, the species M. pachydermatis has been identified in cats with otitis externa [5]. Lipid-dependent species, identified as M. furfur, M. globosa, M. sympodialis and M. slooffiae have also been reported from the skin or the external auditory canal of healthy cats or other felids [1], [6]. In 2004, a novel species M. nana was described from otic discharges of a cat in Japan [7].
2. Cases
All the cats were presented to the Dermatology consultations of the Veterinary College of Alfort, France. Elevated Malassezia populations were detected by cytological assessment of slides prepared using swabs or superficial scrapings. Contact plates filled with modified Dixon's medium were applied on lesional skin (Fig. 1). Plates were incubated at 32 °C for 5 days. Malassezia yeasts were identified by microscopic examination of the cells and by physiological tests [2]. For all the cases, positive subculture on Sabouraud dextrose agar confirmed that the colonies belonged to the non lipid-dependent species M. pachydermatis.
Fig. 1.

Numerous colonies of Malassezia after 5 days of incubation at 32 °C on a contact plate filled with the modified Dixon's medium.
Case 1 was a 4-year-old neutered male Persian cat presented to the veterinary college for a 1 month-old pruritus and a bilateral otitis externa associated with diarrhea. Alopecia, erythema, crusts and greasy adherent brownish scales were observed on the ventral part of the neck.
Case 2 was a 6-year-old neutered female short-haired cat presented with a 9 month-old pruritus associated with anorexia, diarrhea and weight loss. Dermatological signs included erythema, crusts, and alopecia on the face, the ventral neck and the abdomen (Fig. 2a).
Fig. 2.
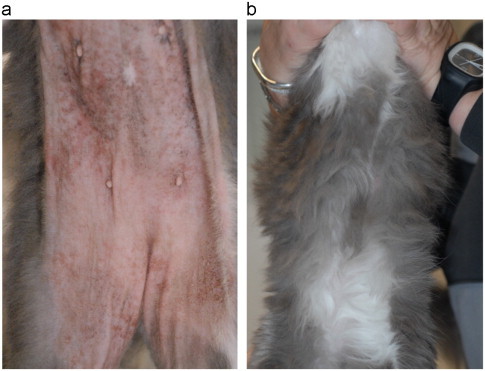
(a) Erythema, crusts, and alopecia on the abdomen of a 6-year-old neutered female short-haired cat (Case 2) with Malassezia overgrowth and (b) the same cat after the application of a 2% miconazole/2% chlorhexidine shampoo (Malaseb®, Dechra) at 3 days interval for 4 weeks.
In both cats, cytological examination of ear canals revealed the presence of a large number of Malassezia yeasts. Contact plates on the face, the neck and the abdomen yielded many (more than 50) colonies of Malassezia. In case 2, complete blood cell count (CBC) indicated leukocytosis with marked eosinophilia (24 eosinophils/μL). In cats, hypereosinophilic syndrome can be associated with allergic diseases, parasitic diseases or some neoplastic syndroms. Bone marrow aspiration showed no abnormalities.
Because cutaneous adverse food reaction was suspected in both cats, a homemade diet with a novel source of protein (horse meat) associated with boiled potatoes and colza oil was prescribed. To control Malassezia overgrowth a 2% miconazole/2% chlorhexidine shampoo (Malaseb®, Dechra) was prescribed at 3 days interval for 4 weeks. One month later, a significant clinical improvement was noticed (Fig. 2b). At that time, cytology and cultures were negative. In case 2, the re-introduction of the original food lead to a clinical relapse.
Case 3 was a 13-year-old neutered female short-haired cat presented with a 3 month-old pruritus associated with anorexia, depression and vomiting. Dermatological signs included erythema, crusts, and alopecia on the face, the ventral and dorsal parts of the neck, the forelimbs and the tail.
Case 4 was a 7-year-old neutered male short-haired cat presented with a 1-year-old pruritus and otitis externa associated with episodic diarrhea. Dermatological signs included erythema, crusts, and alopecia on the face, the ventral part of the neck, and a brown, greasy material in the nail folds (Fig. 3).
Fig. 3.

Brown and greasy material observed in the nail folds of a cat (Case 4) with Malassezia overgrowth.
Case 5 was a 3-year-old neutered male Chartreux cat presented with a 2-month-old pruritus associated with fever, conjunctivitis and cough. Dermatological signs included erythema, crusts, and alopecia on the ears, the abdomen and the inner face of thighs. Cytological examination of both ear canals and nail folds (for Case 4) revealed the presence of Malassezia yeasts. Contact plates on the face and the neck yielded many (more than 50) colonies of Malassezia. The presence of anti-feline coronavirus (FCoV) antibodies (>1:800) confirmed that two cats (Cases 3 and 4) were infected by the virus responsible for Feline Infectious Peritonitis (FIP). Serum RT-PCR was also positive for the FIP virus. In Case 5, the lesions appeared 6 days after the cat was vaccinated. Feline herpes virus (FelHV-1) was identified in conjunctival swabs by PCR.
In Cases 3 and 4, pads containing 0.5% climbazole and 3% chlorhexidine (Douxo® Pyo pads, Sogeval) were applied every day on the lesions (for 4 weeks). In Case 4, ear drops containing miconazole (20 mg/mL) (Surolan® Elanco) were also applied twice a day (for 2 weeks). A dermatological improvement was noticed after antifungal therapy, but cats conditions finally declined, probably due to kidney failure, and the animals died.
In Case 5, no antifungal treatment was applied. The cat condition rapidly declined and the animal was sacrificed.
Case 6 was a 6-year-old male short-haired cat presented with a 10-month-old middle pruritus associated with polyphagia and weight loss. Dermatological signs included erythema, crusts, and alopecia on the face and the rest of the body. Footpads were fissured. Cytological examination did not reveal the presence of Malassezia yeasts but contact plates on the face yielded 30 colonies of Malassezia. Serum biochemistry tests showed alkaline phosphatase activity (372 IU/L) and alanine aminotransferase (458 IU/L) to be above the upper limit of the reference ranges (20–90 IU/L and 6–83 IU/L, respectively). Cutaneous biopsy revealed the presence of many Malassezia yeasts on the epidermis (Fig. 4) associated with hyperkeratosis and irregular epidermal hyperplasia. Liver biopsy showed chronic cirrhosis. This case was compatible with a superficial necrolytic dermatitis associated with hepatopathy. After 6 weeks, the cat condition declined and the animal was sacrificed.
Fig. 4.
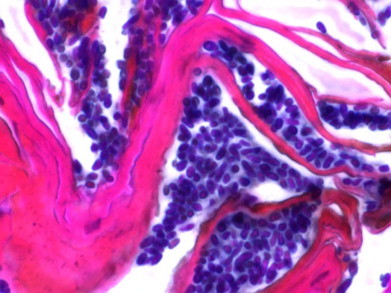
Large number of Malassezia yeasts detected on the epidermis of a cat (Case 6). The sample was stained with Periodic Acid–Schiff. Bar=10 μm (Pathology Department, Ecole Nationale Vétérinaire d'Alfort).
3. Discussion
In cats, generalized Malassezia dermatitis remains extremely rare. A causal relationship between the overgrowth of Malassezia organisms and the development of seborrheic dermatitis was first proposed during the 1994 American College of Veterinary Dermatology meeting. The two cats had generalized exfoliative and greasy erythroderma, which responded to antifungal therapy. However, relapses were frequent and required repeated therapy. No predisposing disorders were identified and the histopathological changes were nonspecific. In cats, Malassezia overgrowth has since been associated with retroviral infections [8], [9], paraneoplastic syndromes [10], [11], thymoma [11], [12], and diabetes mellitus [13], [14]. In a retrospective study, Maudlin et al. evaluated the presence and importance of Malassezia yeasts in feline skin biopsy specimens submitted for histopathological examination between January 1999 and November 2000 [11]. Fifteen (2.7%) submissions contained Malassezia yeasts in the stratum corneum of the epidermis or follicular infundibulum. Eleven of the 15 cats presented with an acute onset of multifocal to generalized skin lesions. All 11 cats were sacrificed or died within 2 months of the onset of clinical signs. Based on these findings and the descriptions of the present study, Malassezia overgrowth should be considered as a marker of life-threatening, underlying diseases in cats.
Malassezia yeasts have also been associated with feline acne [15], [16] and idiopathic facial dermatitis [17]. Atopic dermatitis has been described as a common predisposing factor for Malassezia dermatitis in dogs, whereas this association has been reported less frequently in cats [14], [18]. In a series of 18 allergic cats with Malassezia spp. overgrowth [18], atopic dermatitis was diagnosed in 16 animals. All the cats were otherwise healthy and those tested (16 out of 18) were free from retroviral infections. The beneficial effects of azole antifungal therapy alone in five out of seven of these cats led the authors to conclude that Malassezia yeasts can exacerbate the clinical signs of allergy in cats as well as in dogs. Two out of six cases of the present report were associated with cutaneous food adverse reaction, and topical antifungal therapy was useful. In atopic animals, cutaneous lesions related to Malassezia overgrowth commonly occur on the face, ventral neck, abdomen and ear canals. The factors involved in the transition, from commensalism to parasitism, by Malassezia yeasts in cats are not fully understood.
The criteria required for the diagnosis of Malassezia dermatitis have not been definitely established in pet carnivores. However, it may be proposed that such a diagnosis is appropriate when a cat with elevated numbers of Malassezia yeasts on lesional skin shows good clinical and mycological responses to antifungal therapy. Cytological examination is considered as the most useful technique for assessment of Malassezia density on the skin surface in animals. Several cytological criteria have been proposed to diagnose canine Malassezia dermatitis, including the observation of more than two yeasts per high power field in skin specimens. However, no report has been published that provided details of yeast density on the surface of normal feline skin and the use of threshold values is not recommended in cats. Contact-plate cultural techniques provide a rapid and convenient method for isolation of Malassezia yeasts from the skin surface. Because of the presence of lipid-dependent yeasts on the skin of cats, the use of lipid-supplemented media, especially the modified Dixon's medium, is required. However, in the present study, all the cases were attributed to M. pachydermatis and we may imagine that the use of regular media (not supplemented with lipids) would have been appropriate for the isolation of Malassezia yeasts. In cats, histopathological examination of skin biopsy specimens typically shows hyperkeratosis and irregular epidermal hyperplasia. As in dogs, Malassezia yeasts are not always visible in the epidermal stratum corneum, even in cases where large numbers are seen cytologically; this probably reflects disruption of this layer during processing.
A recent systematic review evaluated the efficacy of antifungal treatments for Malassezia dermatitis in dogs [19]. This review allowed the recommendation, with good evidence, of the use of only one topical treatment: a shampoo containing 2% miconazole and 2% chlorhexidine, twice a week for 3 weeks. A similar review has not been made for cats, but we observe that the 2% miconazole/2% chlorhexidine shampoo had a beneficial effect in cats with adverse food reaction.
Conflict of interest
None declared.
Acknowledgments
The authors would like to thank Nathalie Cordonnier for examination of histological sections. This study was performed with the financial support of the Dechra company.
References
- 1.Sugita T., Boekhout T., Guillot J. In: Malassezia and the skin. 1st ed. Velegraki A., Mayzer P., Guého E., Boekhout T., editors. Springer; Berlin: 2010. Epidemiology; pp. 65–119. [Google Scholar]
- 2.Guého E. In: Malassezia and the skin. 1st ed. Velegraki A., Mayzer P., Guého E., Boekhout T., editors. Springer; Berlin: 2010. Biodiversity, phylogeny and ultrastructure; pp. 17–63. [Google Scholar]
- 3.Dufait R. Pityrosporum canis as the cause of canine chronic dermatitis. Veterinary Medicine and Small Animal Clinician. 1983;78:1055–1057. [Google Scholar]
- 4.Mason K.V., Evans A.G. Dermatitis associated with Malassezia pachydermatis in 11 dogs. Journal of the American Animal Hospital Association. 1991;27:13–20. [Google Scholar]
- 5.Bond R., Guillot J., Cabanes J. In: Malassezia and the skin. 1st ed. Velegraki A., Mayzer P., Guého E., Boekhout T., editors. Springer; Berlin: 2010. Malassezia yeasts in animal diseases; pp. 271–299. [Google Scholar]
- 6.Crosaz O., Legras A., Hubert B. Malassezia yeasts in cats: from normal cutaneous carriage to pathogenic overgrowth. Mycoses. 2012;55(Suppl. 4):91. [Google Scholar]
- 7.Hirai A., Kano R., Makimura K. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. International Journal of Systematic and Evolutionary Microbiology. 2004;54:623–627. doi: 10.1099/ijs.0.02776-0. [DOI] [PubMed] [Google Scholar]
- 8.Sierra P., Guillot J., Jacob H. Fungal flora on cutaneous and mucosal surfaces of cats infected with feline immunodeficiency virus or feline leukemia virus. American Journal of Veterinary Research. 2000;61:158–161. doi: 10.2460/ajvr.2000.61.158. [DOI] [PubMed] [Google Scholar]
- 9.Reche A., Jr, Daniel A.G., Lazaro Strauss T.C. Cutaneous mycoflora and CD4:CD8 ratio of cats infected with feline immunodeficiency virus. Journal of Feline Medicine and Surgery. 2010;12:355–358. doi: 10.1016/j.jfms.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey D.R. A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis. Journal of Small Animal Practice. 1998;39:394–396. doi: 10.1111/j.1748-5827.1998.tb03739.x. [DOI] [PubMed] [Google Scholar]
- 11.Maudlin E.A., Morris D.O., Goldschmidt M.H. Retrospective study: the presence of Malassezia in feline skin biopsies. A clinicopathological study. Veterinary Dermatology. 2002;13:7–14. doi: 10.1046/j.0959-4493.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 12.Forster-Van Hijfte M.A., Curtis C.F., White R.N. Resolution of exfoliative dermatitis and Malassezia pachydermatis overgrowth in a cat after surgical thymoma resection. Journal of Small Animal Practice. 1997;38:451–454. doi: 10.1111/j.1748-5827.1997.tb03439.x. [DOI] [PubMed] [Google Scholar]
- 13.Perrins N., Gaudiano F., Bond R. Carriage of Malassezia spp. yeasts in cats with diabetes mellitus, hyperthyroidism and neoplasia. Medical Mycology. 2007;45:541–546. doi: 10.1080/13693780701435333. [DOI] [PubMed] [Google Scholar]
- 14.Bensignor E. Malassezia dermatitis in cats: 15 cases treated with itraconazole. Veterinary Record. 2010;167:1011–1012. doi: 10.1136/vr.c3854. [DOI] [PubMed] [Google Scholar]
- 15.White S.D., Bourdeau P., Blumstein P. Feline acne and results of treatment with mupirocin in an open clinical trial: 25 cases (1994–96) Veterinary Dermatology. 1997;8:157–164. doi: 10.1046/j.1365-3164.1997.d01-16.x. [DOI] [PubMed] [Google Scholar]
- 16.Jazic E., Coyner K.S., Loeffler D.G. An evaluation of the clinical, cytological, infectious and histopathological features of feline acne. Veterinary Dermatology. 2006;17:134–140. doi: 10.1111/j.1365-3164.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 17.Bond R., Curtis C.F., Ferguson E.A. An idiopathic facial dermatitis of Persian cats. Veterinary Dermatology. 2000;11:35–41. doi: 10.1046/j.1365-3164.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 18.Ordeix L., Galeotti F., Scarempella F. Malassezia spp. overgrowth in allergic cats. Veterinary Dermatology. 2007;18:316–323. doi: 10.1111/j.1365-3164.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- 19.Nègre A., Bensignor E., Guillot J. Evidence-based veterinary dermatology: a systematic review of interventions for Malassezia dermatitis in dogs. Veterinary Dermatology. 2009;20:1–12. doi: 10.1111/j.1365-3164.2008.00721.x. [DOI] [PubMed] [Google Scholar]


