Abstract
A case of chromoblastomycosis of the hand caused by Rhinocladiella aquaspersa is described. The case was acquired locally in tropical Venezuela and was successfully treated with oral itraconazole.
Keywords: Chromoblastomycosis, Rhinocladiella aquaspersa, Itraconazole
1. Introduction
Chromoblastomycosis is a chronic granulomatous fungal infection that causes hyperproliferation of cutaneous and subcutaneous tissues and is histologically characterized by the presence of muriform cells. Infection originates after the etiologic agent gains entrance via percutaneous trauma [1]. Main agents responsible for its etiology are Fonsecaea pedrosoi, Fonsecaea monophora, Cladophialophora carrionii, Phialophora verrucosa, and Rhinocladiella aquaspersa. The disease is found in tropical and subtropical areas [2–4], with the vicarious agents [5] of Fonsecaea and Phialophora occurring under hot and humid conditions [6], and Cladophialophora being restricted to arid climates [7–10]. R. aquaspersa is an uncommon cause of chromoblastomycosis, with most cases having been reported from the American continent [11–14]. The present paper presents a further case from Venezuela and summarizes the climatic conditions of the areas where R. aquaspersa is endemic.
2. Case report
A 63-year-old male construction worker, native and resident of a rural area in Siquisique (Caserío La Unión), Lara State, Venezuela, presented with an asymptomatic, localized lesion affecting the dorsal surface of the left hand, involving the knuckles and proximal interphalangeal joints of the third and fourth fingers. The dermatosis produced retraction of the fifth finger. Examination of the skin disclosed a scaly, crusted, dull-red plaque, friable with hemorrhagic dots. The lesion was almost flat and eroded. According to the patient, the lesion had developed over an 18-month period and trauma or inoculation was not recalled. There were no lymphatic commitments. He has never been outside Siquisique city. The patient denied other problems but was in poor health and nutritional conditions. To recover the etiologic agent, the area was soaked with alcohol and scrapings from the lesions were collected into a Petri dish using a sterile scalpel blade. Direct KOH examination of the crusts of the lesions revealed numerous thick-walled, globose or ovoidal, dark-brown muriform cells approximately 6−8 µm in diameter, either singly or arranged in groups.
Microscopic examination of a biopsy specimen from the lesion stained with periodic acid Schiff showed pseudoepitheliomatous hyperplasia and a mixed cell granulomatous infiltrate consisted of lymphocytes, histiocytes, giant cells, and neutrophils. Muriform cells were observed in the granulomatous tissue. These results confirmed the clinical diagnosis of chromoblastomycosis (Fig. 1). There were no budding cells, hyphal elements, swollen cells, pseudohyphal elements, or moniliform hyphae.
Fig. 1.
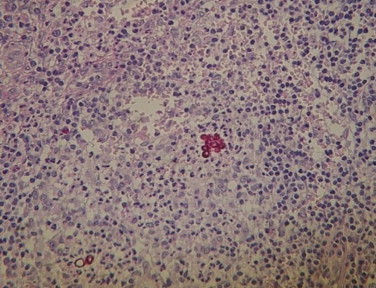
Histopathology examination showing cluster of muriform cells stained with periodic acid Schiff.
Cultures of skin scrapings were performed on Sabouraud's glucose agar (SGA) with chloramphenicol (0.5 mg/ml). After incubation at 30 °C a slow-growing dark mold was recovered. Microscopically scarce conidia were observed which did not allow identification of the fungus. The isolate was submitted to the Departamento de Microbiología, Facultad de Medicina, Universidad Autónoma de Nuevo León, Monterrey, Mexico, for morphological and molecular identification. The patient was treated with oral itraconazole (100 mg/day).The lesions improved after 2 months, with significant healing, desquamations having disappeared, and culture became negative. No side effects were noted by the patient during the course of treatment. Unfortunately, the patient could not be contacted for further follow-up.
2.1. Mycology
The mold was subcultured on SGA and on potato dextrose agar (PDA) on culture plates (20 mm diam.) incubated at 30 °C. After 2 weeks velvety colonies developed which were dark olive with an olivaceous black reverses. Slide cultures were performed on PDA blocks. After 2 weeks slides were stained with lactophenol cotton blue and evaluated in a Nikon Eclipse 50i microscope fitted with a Nikon digital sight DS-2Mv camera. Microscopically brown, septate hyphae were observed. Conidiophores were erect, 70−90 µm long, unbranched, thick-walled and darkly pigmented, bearing conidia at their distal portions. Scars were noticed on the conidiophores at the sites where conidia had been implanted. Conidia were single-celled, smooth, ellipsoidal to clavate, 4.0−6.6 µm long, light brown. The mold was tentatively identified by morphology as a Rhinocladiella species.
Carbohydrate assimilation was tested using API 20C AUX galleries (bioMérieux, Mexico) [15]. Hydrolysis of urea was tested using Christensen´s urea agar. Inoculated tubes were incubated at 30 °C for 10 days. Cycloheximide tolerance was investigated on commercial Mycosel agar. Plates were incubated at 30 °C for 10 days. Proteolytic activity was evaluated in gelatin agar slants after 20 days at 30 °C [16]. Thermotolerance was explored with strains incubated on PDA and Mycosel agar for 2 weeks at 30, 37, and 40 °C [17]. Salt tolerance was investigated in 2% malt extract agar with 5%, 10%, 17%, and 20% sodium chloride [18]. Plates were incubated at 30 °C for 20 days.
The isolate assimilated d-glucose, glycerol, 2-k-d-gluconate, L-arabinose, d-xylose, adonitol, d-galactose, inositol, d-sorbitol, methyl-d-glucopiranoside, N-acetyl-glucosamine, d-cellobiose, d-maltose, d-saccharose, d-trehalose, d-melezitose, d-raffinose. In contrast, d-lactose and xylitol were not assimilated. Urea hydrolysis was positive, growth on Mycosel agar was not inhibited by cycloheximide, and liquefaction of gelatin was absent. The isolate grew at 30 °C, had limited growth at 37 °C, and failed to grow at 40 °C. Growth in malt extract agar with all sodium chloride concentrations tested was negative.
For molecular identification, approximately 1 cm2 mycelium from 2-week-old cultures was transferred to 2 ml Eppendorf tubes containing 200 µl of enzymatic lyses buffer [20 mM Tris–HCl (pH 8), 2 mM EDTA, 1.2% Triton X-100, 20 mg/ml lysozyme], which were then incubated at 37 °C for 2 h with agitation. Afterwards, 390 µl TE 1× with 1% SDS and 4 µl (10 mg/ml) proteinase K were added and cells were incubated for 1 h at 55 °C. After, 100 µl 5 M NaCl and 80 µl of 10% CTAB/4.1% NaCl were added and the mixture was vortexed and incubated for 10 min at 65 °C. DNA was extracted twice with 1 vol of phenol–chloroform–isoamyl alcohol (25:24:1), precipitated with absolute ethanol, washed with 70% ethanol, air dried, and resuspended in 200 µl of Tris–EDTA buffer [19]. Ribosomal DNA ITS domains were amplified using primers ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS-5 (5′GGAAGTAAAAGTCGTAACAAGG-3′) [20]. Amplifications were performed in a final volume of 25 µl containing 1× PCR buffer, 2 mM MgCl2, 0.2 mM of each dNTP, 200 nM of each primer, 1 U of AmpliTaq polymerase (Bioline, Randolph, MA, U.S.A.) and 100 ng DNA. The thermocycling conditions were: 94 °C for 4 min, followed by 30 cycles of 94 °C for 60 s, 55 °C for 90 s, and 72 °C for 90 s, with final extension at 72 °C for 5 min. The final products were electrophoresed in 1.5% agarose gel and stained with ethidium bromide. Hyper Ladder I (Bioline) was used as a molecular weight marker for size determinations. The pattern of amplified bands was photographed and analyzed with the UVP Biolmaging System, EpiChemi III Darkroom. PCR products were purified using the commercial Wizard PCR Preps DNA purification system (Promega, Madison, WI, U.S.A.). Sequencing was performed in both directions at the Instituto de Biotecnología, Universidad Nacional Autónoma de México. DNA sequence fragments were compared to NCBI GenBank sequence entries using the BLAST algorithm and to a research database on black fungi at CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. The sequence was deposited in GenBank with accession number GU177853 as species of order Chaetothyriales. Later, the isolate was identified at CBS as R. aquaspersa. The culture was deposited in the CBS reference collection as CBS 132913.
2.2. Antifungal susceptibility
Amphotericin B (AMB) (Bristol-Myers Squibb, Princeton, NJ, U.S.A.), itraconazole (ITZ) (Janssen Pharmaceutica, Beerse, Belgium), voriconazole (VRZ) (Pfizer, Inc., New York, NY, U.S.A.), posaconazole (PSZ) (Schering-Plough, Kenilworth,NJ, U.S.A.), and terbinafine (TBF) (Novartis, Mexico) were obtained in reagent-grade powder form from their respective manufacturers. Isolates were evaluated by using Clinical and Laboratory Standard Institute broth macrodilution approved standard reference method M38-A2 [21]. After adequate sporulation occurred on PDA, the mycelium was overlaid with sterile distilled water, and suspensions were made by softly scraping the colonies with wooden applicators. Heavy fragments were allowed to settle, and the upper, homogeneous supernatant was transferred to sterile tubes. Inocula suspension of 106 CFU/ml were prepared by hemacytometer counts and then diluted in RPMI 1640 medium with glutamine and morpholinopropanesulphonic acid buffer at a concentration of 0.165 M (Angus, Niagara Falls, NY, U.S.A.) to obtain a final organism concentration of 0.4−5×104 CFU/ml. The inoculum size for all tests was verified by plating 10 µl onto PDA, incubating the plates at 35 °C, and counting the number of colonies. Serial dilution of the drugs was made following the CLSI guidelines in order to obtain final concentrations of the drugs ranging from 0.015 to 8 mg/L for all antifungal compounds. Candida krusei ATCC 6258 and Paecilomyces variotii ATCC MYA3630 were used for quality control.
Results of susceptibility testing were: AMB=2 µg/ml, TBF=0.06 µg/ml, ITZ=0.125 µg/ml, VRZ=1 µg/ml, and PSZ=0.25 µg/ml, respectively.
3. Discussion
R. aquaspersa is a rare agent of human chromoblastomycosis. An overview of clinical cases is presented in Table 1; several reports provided only very scant information. The majority of historical cases have not been verified by sequence data, but the morphology of R. aquaspersa is unmistakable. Most cases report the presence of muriform cells in tissue, but in Case 3 these were absent. The clinical appearance of the disease is highly diverse [23]. The main agents of the disease are classified in Cladophialophora and Fonsecaea and are restricted to arid and tropical climates, respectively [5,9], but environmental data of cases of R. aquaspersa do not show any consistency (Table 1). Caserío La Unión of the present case is located in the northernmost part of the state, between 600 and 700 m above mean sea level. This area presents temperatures between 23 and 28 °C, its annual precipitation varies between 950 and 1400 mm, and its annual evaporation rates range from 1400 to 2400 mm. The predominance of semi-deciduous vegetation is associated to the aforementioned climatic conditions. The global distribution of R. aquaspersa is (sub)tropical. The species has thus far not been recovered from the environment, but Badali et al. [14] mentioned a case acquired by from a cactus thorn suggesting a natural habitat on living or dead plants. Marques et al. [13] reported a case secondary to an insect bite.
Table 1.
Published cases of Rhinocladiella aquaspersa.
| Case | Strain | Patient, age | Site, duration | Geography | Therapy | Reference |
|---|---|---|---|---|---|---|
| 1 | CBS 313.73 | No data | Mexico | [24] | ||
| 2 | CBS 122635 | Male 56 | Hand, 15 yr | Alpuyeca, Morelos, Mexico, 1000 m alt, arid | Terbinafin 6 mo (500 mg/d), cured | [14] |
| 3 | FMR 7699 | Female 62 | Foot, cactus puncture, 20 yr | Solanea, Paraiba, Brazil, 600 m alt, tropical dry | Electrocauterization, itraconazole (200 mg/d), cured | [14] |
| 4 | Male 60 | Ear, 5 yr | Medellin, Colombia, 1400 m alt, temperate dry | Itraconazole 7 mo (200 mg/d), cured | [11] | |
| 5 | Female 52 | Abdomen | South Korea | Itraconazole 4 mo (200 mg/d), surgery; cured | [25] | |
| 6 | Male 52 | Arm, leg, forehead, 2 y, insect bite | Maranhão, Brazil, 300 m alt, tropical | Ketoconazole; failure | [13] | |
| 7 | Male 5 | Upper limb, 2 yr | Santa Cruz de Bucaral, Falcon state, Venezuela, 800 m, arid | Itraconazole; failure | [12] | |
| 8 | No data | Minas Gerais, Brazil, 300 m alt, tropical | [26] | |||
| 9 | No data | Costa Rica, tropical | [12] | |||
| 10 | CBS 132913 | Male 63 | Hand, 18 mo | Siquisique, Lara State, Venezuela, 600 m alt, tropical | Itraconazole 2 mo (100 mg/day); cured | Present case |
The species is susceptible to commonly used azoles, and in our case treatment of the infection with itraconazole led to healing within two months.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
Please acknowledge anyone who contributed towards the study by making substantial contributions to conception, design, acquisition of data, or analysis and interpretation of data, or who was involved in drafting the manuscript or revising it critically for important intellectual content, but who does not meet the criteria for authorship.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Rubin H.A., Bruce S., Rosen T., McBride M.E. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. Journal of the American Academy of Dermatology. 1991;25:951–954. doi: 10.1016/0190-9622(91)70292-a. [DOI] [PubMed] [Google Scholar]
- 2.Esterre P., Andriantsimahavandy A., Ramarcel E.R., Pecarrere J.L. Forty years of chromoblastomycosis in Madagascar: a review. American Journal of Tropical Medicine and Hygiene. 1996;55:45–47. doi: 10.4269/ajtmh.1996.55.45. [DOI] [PubMed] [Google Scholar]
- 3.Silva J.P., de Souza W., Rozental S. Chromoblastomycosis: a retrospective study of 325 cases on amazonic region (Brazil) Mycopathologia. 1998;143:171–175. doi: 10.1023/a:1006957415346. [DOI] [PubMed] [Google Scholar]
- 4.Bonifaz A., Carrasco-Gerard E., Saul A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1–7. doi: 10.1046/j.1439-0507.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 5.Najafzadeh M.J., Sun J., Gerrits van den Ende A.H.G., Vicente V.A., Menken S.B.J., de Hoog G.S. Dispersal and evolution of pathogenicity in Fonsecaea, agent of human chromoblastomycosis. Emerging Infectious Diseases. 2010;17:464–469. doi: 10.3201/1703.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badali H., Fernández González M., Mousavi B., Illnait-Zaragozi M.T., González Rodríguez J.C., de Hoog G.S. Chromoblastomycosis due to Fonsecaea pedrosoi and F. monophora in Cuba. Mycopathologia. 2013;175:439–444. doi: 10.1007/s11046-013-9634-3. [DOI] [PubMed] [Google Scholar]
- 7.Yegres F., Richard-Yegres N. Cladophialophora carrionii: aportes al conocimiento de la endemia en Venezuela durante el siglo XX. Revista de la Sociedad Venezolana de Microbiología. 2002;2:153–157. [Google Scholar]
- 8.Pérez-Blanco M., Hernandez Valles R., Garcia-Humbria L., Yegres F. Chromoblastomycosis in children and adolescents in the endemic area of the Falcón State, Venezuela. Medical Mycology. 2006;44:467–471. doi: 10.1080/13693780500543238. [DOI] [PubMed] [Google Scholar]
- 9.Deng S, Gerrits van den Ende AHG, Yang L, Badali H, Najafzadeh MJ, Li RY, et al. Global distribution pattern of Cladophialophora carrionii, agent of human chromoblastomycosis. Emerging Infectious Diseases, submitted for publication.
- 10.González G.M., Carolina R.O., Bocanegra-García V., González J.G., Garza-González E. Molecular diversity of Cladophialophora carrionii in patients with chromoblastomycosis in Venezuela. Medical Mycology. 2012;51:170–177. doi: 10.3109/13693786.2012.695457. [DOI] [PubMed] [Google Scholar]
- 11.Arango M., Jaramillo C., Cortés A., Restrepo A. Auricular chromoblastomycosis caused by Rhinocladiella aquaspersa. Medical Mycology. 1998;36:43–45. [PubMed] [Google Scholar]
- 12.Pérez-Blanco M., Fernández-Zeppenfeldt G., Hernández R., Yegres F, Borelli D. Chromomycosis by Rhinocladiella aquaspersa: the first case in Venezuela. Revista iberoamericana de Micología: Órgano de la Asociación Española de Especialistas en Micología. 1998;15:51–54. [PubMed] [Google Scholar]
- 13.Marques SG, Pedrozo Silva CM, Resende MA, Andreata LS, Costa JML. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Medical Mycology. 2004;42:261–265. doi: 10.1080/13693780310001597700. [DOI] [PubMed] [Google Scholar]
- 14.Badali H., Bonifaz A., Barrón-Tapia T., Vázquez-González D., Estrada-Aguilar L., Cavalcante Oliveira N.M. Rhinocladiella aquaspersa, proven agent of verrucous skin infection and a novel type of chromoblastomycosis. Medical Mycology. 2010;48:696–703. doi: 10.3109/13693780903471073. [DOI] [PubMed] [Google Scholar]
- 15.Espinel-Ingroff A., McGinnis M.R., Pincus D.H., Goldson P.R., Kerkering T.M. Evaluation of the API 20C yeast identification system for the differentiation of some dematiaceous fungi. Journal of Clinical Microbiology. 1989;27:2565–2569. doi: 10.1128/jcm.27.11.2565-2569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinel-Ingroff A., Goldson P.R., McGinnis M.R., Kerkering T.M. Evaluation of proteolytic activity to differentiate some dematiaceous fungi. Journal of Clinical Microbiology. 1988;26:301–307. doi: 10.1128/jcm.26.2.301-307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Hoog G.S., Attili-Angelis D., Vicente V.A., Gerrits van den Ende A.H.G., Queiroz-Telles F. Molecular ecology and pathogenic potential of Fonsecaea species. Medical Mycology. 2004;42:405–416. doi: 10.1080/13693780410001661464. [DOI] [PubMed] [Google Scholar]
- 18.Zalar P., de Hoog G.S., Schroers H.J., Crous P.W., Groenewald J.Z., Gunde Cimerman N. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with description of seven new species from hypersaline environments. Studies in Mycology. 2007;58:157–183. doi: 10.3114/sim.2007.58.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ausubel F.M. Preparation of genomic DNA from bacteria. In: Ausubel F.M., Brent R., Kingston R.E., More D.D., Seidman J.G., Smith J.A., Struhl K., editors. Short protocols in molecular biology. 4th ed. John Wiley and Sons Inc.; New York, NY: 1999. [Google Scholar]
- 20.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute.Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard, 2nd ed. NCCLS document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- 23.Queiroz-Telles F., Wagner de Santos D. Challenges in the therapy of chromoblastomycosis. Mycopathologia. 2013;175:477–488. doi: 10.1007/s11046-013-9648-x. [DOI] [PubMed] [Google Scholar]
- 24.Borelli D. Acrotheca aquaspersa nova species agente de cromomicosis. Acta Científica Venezolana. 1972;23:193–196. [PubMed] [Google Scholar]
- 25.Jun J.B., Park J.Y., Kim D.W., Suh S.B. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Korean Journal of Medical Mycology. 2004;9:117–122. [Google Scholar]
- 26.Silva Lacaz C. da. Micología Médica. São Paulo: Sarvier; 1984.


