Abstract
Hypersensitivity pneumonitis is a syndrome reported in humans, and occasionally animals, that results from the inhalation of very small antigenic particles (usually <5 µm) that are able to reach the alveolar space. This is the first report of hypersensitivity pneumonitis in a dog in Australia and the first associated with Geastrum triplex spores. Diagnosis was based on known antigen exposure, physical findings, radiographic signs of interstitial lung disease and molecular identification of Geastrum triplex in bronchoalveolar lavage fluid.
Keywords: Hypersensitivity pneumonitis, Canine, Geastrum triplex, Puffball, Lycoperdonosis
1. Introduction
Hypersensitivity pneumonitis is a syndrome reported in humans, and occasionally animals, that results from the inhalation of very small antigenic particles (usually <5 µm) that are able to reach the alveolar space. Such particles include fungal and bacterial antigens, animal proteins and chemicals. Acute, subacute and chronic forms of the disease have been reported but may be more reflective of a continuum of disease rather than distinct entities [1].
Lycoperdonosis is an acute form of hypersensitivity pneumonitis resulting from the inhalation of large volumes of fungal conidia from the family Agaricaceae, most commonly Lycoperdon spp. (“puffball” mushrooms). It has been reported in humans and dogs in North America and Europe [2–7]. These fungi have previously been arbitrarily classed together with the Geastraceae family (earthstars) as gastromycetes due to a common structure. The gastromycetes all contain fruiting bodies within which basidiospores are produced [8]. These spores are released following compression of the mushroom resulting in potential inhalation of very high numbers of spores. This is the first report of hypersensitivity pneumonitis associated with Geastrum triplex exposure and the first case of lycoperdonosis-like disease in a dog in Australia. We also report the use of molecular identification of the fungal species from a bronchoalveolar lavage (BAL) sample.
2. Case
A 3-month-old male entire Bull Mastiff was referred to the University Veterinary Teaching Hospital, Sydney with a 3-week history of increasing lethargy, dyspnoea and exercise intolerance. The puppy had lost body condition during this time despite a good appetite and appropriate diet. He had access to a large suburban garden and was often observed to be eating plants and bark. Prior to the development of clinical signs he had been found with large numbers of spores from a “puffball” mushroom on his muzzle.
Physical examination by the referring veterinarian 2 days prior to referral revealed tachypnoea with inspiratory dyspnoea and cyanosis. Thoracic radiographs showed a marked, diffuse interstitial lung pattern. Haematology and serum biochemistry were unremarkable. A tracheal wash was performed and the dog was commenced on amoxicillin-clavulanate (18 mg/kg per os (PO) every 12 h). A respiratory PCR panel on the wash fluid was negative for the respiratory pathogens H1N1 Influenza virus, Canine Distemper virus, Bordetella bronchiseptica, Canine Adenovirus 2, Canine Herpes Virus 1, Canine parainfluenza virus, Streptococcus equi and Mycoplasma cynos (IDEXX Reference Laboratories, West Sacramento, USA).
On physical examination at referral (Day 0) the dog weighed 10.4 kg and had a low body condition score (3/9). The respiratory rate was elevated (72 breaths per minute) with a marked inspiratory and expiratory dyspnoea and SpO2 of 65%. Arterial blood gas measurement whilst breathing room air showed a PaO2 of 43.5 mmHg, a PaCO2 of 45.4 mmHg, and a pH of 7.352. Oxygen supplementation by mask resulted in an increase in the SpO2. There was no evidence of pulmonary hypertension on echocardiography.
Bronchoscopy revealed moderate rounding of the carina with no tracheal or bronchial mucosal erythema or oedema. There was a small volume of clear mucoid exudate within the lower airways. Bronchoalveolar lavage (BAL) from the left tertiary bronchus demonstrated neutrophilic inflammation (50% neutrophils, 25% predominantly small lymphoid cells and 25% macrophages). Moderate numbers of spherical, 4 μm diameter, refractile grey-green structures were presented intra- and extra-cellularly. The outer surface of these structures was covered in regular short projections (Fig. 1). Additionally, low numbers of 5–6 μm diameter ovoid, non-capsulated, thin-walled structures were identified. Bacterial and fungal cultures of the BAL were negative as was a real-time PCR to detect Pneumocystis DNA (Molecular Microbiology Laboratory, Sydney South West Pathology Service, NSW, Australia).
Fig. 1.
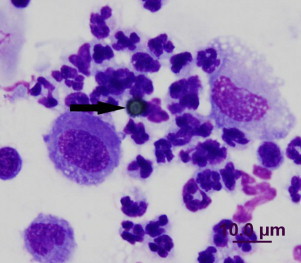
Inflammation and fungal basidiospore (arrow) in BAL sample. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
The dog was initially stabilised with intranasal oxygen supplementation, intravenous crystalloids and dexamethasone (0.29 mg/kg intravenously). Terbutaline (0.12 mg/kg PO every 12 h), trimethoprim–sulfonamide (18 mg/kg PO every 8 h), prednisolone (0.5 mg/kg PO every 12 h) and itraconazole (5 mg/kg PO every 12 h) were prescribed pending the results of microbiological studies. Upon discharge from hospital, 7 days after admission, the dog had gained weight (10.9 kg) and was able to maintain a SpO2 of 94% with a normal respiratory rate (28 breaths per minute) and mildly increased respiratory effort.
At reassessment 7 days after discharge (Day 14) there was marked clinical improvement with increased respiratory effort only evident during periods of excitement. Haematology revealed a leukocytosis (30.6×109/L; reference interval: 4.5–17.0×109/L) secondary to a neutrophilia (25.4×109/L; reference interval: 3.5–12.0×109/L) and monocytosis (1.8×109/L; reference interval: 0.0–1.1×109/L). A 2-week course of doxycycline (7.6 mg/kg PO every 12 h) was initiated and trimethoprim–sulfonamide was discontinued at this time. Prednisolone and terbutaline therapy were discontinued after 1 month of therapy (Day 28) at which time thoracic radiographs showed a mild interstitial pulmonary pattern.
The dog represented with acute onset tachypnoea and lethargy 59 days after initial presentation. Just prior to the development of clinical signs he had been observed to be playing with a “puffball” mushroom in the garden. On physical examination the respiratory rate was 80 breaths per minute with a SpO2 of 93% and body temperature of 38.8 °C. Thoracic radiographs were unchanged from 1 month prior and an ultrasound-guided fine needle lung biopsy was performed. Pulmonary parenchymal cytology revealed 67% neutrophils, 30% vacuolated macrophages and 3% small lymphocytes with negative bacterial and fungal cultures. Prednisolone therapy (0.9 mg/kg PO every 12 h) was reinstituted and amoxicillin-clavulanate (20 mg/kg PO every 12 h) was administered for 14 days. There was a rapid improvement in the clinical signs.
A sample of the “puffball” fungus was obtained and microscopic examination of its spores revealed a number of 4 μm diameter, refractile, greyish-green spherical structures identical to those found in the original BAL. A conventional panfungal PCR targeting the rDNA gene cluster including the ITS1, 5.8S gene and ITS2 regions using primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) was performed on DNA extracted from the BAL and the fungus spores, as described previously [9]. This indicated the BAL and spore sequences had a 99.23% sequence homology with GenBank sequences of Geastrum triplex (collared earthstar) (GenBank accession no. JN845162.1) (Fig. 2) [10,11].
Fig. 2.

Geastrum triplex. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
The prednisolone therapy was weaned and discontinued over 1 month. At reassessment 132 days after initial presentation the dog had a normal respiratory rate and effort. Thoracic radiographs revealed a mild interstitial lung pattern.
3. Discussion
This is the first report of a hypersensitivity pneumonitis in a dog following exposure to a Geastrum spp. and of the antemortem use of molecular identification in its diagnosis. The four previously reported cases of canine fungal-associated hypersensitivity pneumonitis have been classified as suffering from lycoperdonosis with only two surviving to discharge [5–7].
The diagnosis of hypersensitivity pneumonitis in humans is complicated by the lack of unique features to distinguish it from other forms of interstitial lung disease and relies on a high level of clinical suspicion, known antigen exposure and a combination of clinical findings [12]. This diagnostic dilemma may be exacerbated in veterinary patients due to the apparent rarity of the condition and unknown or unrecognised antigen exposure. The diagnosis of hypersensitivity pneumonitis due to inhalation of Geastrum triplex spores in this case was based on clinical signs (progressive dyspnoea and cyanosis), radiographic findings, known antigen exposure (with recurrence of signs on re-exposure) and isolation from bronchoalveolar fluid, exclusion of other potential causes of interstitial lung disease and response to therapy. At least four of the previously defined six significant predictors of hypersensitivity pneumonitis were identified in this case in addition to other findings [13].
The antemortem diagnosis of lycoperdonosis in dogs has previously been based on the patient's history and clinical signs with variable emphasis placed on the identification of fungal spores in cytological samples [5–7]. The spores identified in this case appear morphologically similar to those previously reported. Post-mortem confirmation of the presence of Lycoperdon DNA has been described previously in dogs following DNA extraction and PCR of paraffin-embedded lung tissue [6]. However, this is the first report of a similar technique being used as a diagnostic tool antemortem using a BAL sample. The use of panfungal PCR also allowed the exclusion of concurrent fungal pneumonia in the disease process. Molecular identification of infectious agents is being utilised increasingly in veterinary medicine due to the high sensitivity and relative speed of such methods [14]. The use of such methods in the exclusion of other causes of disease is advantageous in cases such as these. Although PCR can result in false-positive results secondary to sample contamination, the concurrent historical and physical findings in this case support the diagnosis. Furthermore, the DNA extraction and PCR of BAL was processed before receiving the ‘puffball’ garden sample, thus eliminating contamination of the BAL sample from this source.
The thoracic radiographs in this case revealed a marked interstitial lung pattern. The pulmonary changes reported in lycoperdonosis are similar to those seen in Pneumocystis pneumonia [15] with this condition considered an important differential diagnosis in humans [4] and dogs [7]. The age of the dog in this case also supported this differential diagnosis. Pneumocystis infection was ruled out in this case based on PCR of BAL fluid. This method has been demonstrated to have a greater sensitivity than cytology and immunocytochemistry in affected children [16].
Both the bronchoalveolar lavage and lung fine needle biopsy cytology showed a predominantly neutrophilic inflammation. This is consistent with BAL findings in the previous reports of canine lycoperdonosis, but differs to disease in humans in which a lymphocytic cellular pattern is more typical [17]. This difference is likely reflective of the acute course of disease seen in these dogs and is supported by similar findings in the acute form in people [12].
As only relatively small numbers of individuals are affected despite many of the antigens being ubiquitous in the environment, it has been proposed that a genetic susceptibility or prior environmental exposure increases the risk of reaction [12]. The dog in this case was treated with corticosteroids in addition to antibiotics, an antifungal and a bronchodilator. Immunosuppressive therapy with corticosteroids has been reported in the majority of human cases [3,4] and all of the cases of canine lycoperdonosis [5–7]. Similarly, treatment with tapering doses of prednisolone for 3–6 months can result in disease remission in adult patients with subacute hypersensitivity pneumonitis [12].
The paucity of reports of hypersensitivity pneumonitis in dogs may be associated with the rarity of the condition or a lack of recognition. It should be considered as a differential diagnosis in cases with interstitial lung disease in which there is the possibility of antigen exposure or in which another underlying cause has not been identified.
Conflict of interest
There are none.
Acknowledgements
The authors thank Dr. Melinda Salter from Kirrawee Veterinary Hospital for initial case investigation and management.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Selman M., Buendia-Roldan I. Immunopathology, diagnosis, and management of hypersensitivity pneumonitis. Seminars in Respiratory and Critical Care Medicine. 2012;33:543–554. doi: 10.1055/s-0032-1325163. [DOI] [PubMed] [Google Scholar]
- 2.Strand R., Neuhauser E., Sornberger C., Lycoperdonosis N. England Journal of Medicine. 1967;277:89–91. doi: 10.1056/NEJM196707132770209. [DOI] [PubMed] [Google Scholar]
- 3.Henriksen N. Lycoperdonosis. Acta Paediatrica Scandinavica. 1976;65:643–645. doi: 10.1111/j.1651-2227.1976.tb04945.x. [DOI] [PubMed] [Google Scholar]
- 4.Munson E.L., Ponko D.M., Fink J.G. Lycoperdonosis: report of two cases and discussion of the disease. Clinical Microbiology Newsletter. 1997;19:17–24. [Google Scholar]
- 5.Rubensohn M. Inhalation pneumonitis in a dog from spores of puffball mushrooms. Canadian Veterinary Journal. 2009;50:93. [PMC free article] [PubMed] [Google Scholar]
- 6.Alenghat T., Pillitteri C., Bernis D., Kellet-Gregory L., Jackson KV, Kania SA. Lycoperdonosis in two dogs. Journal of Veterinary Diagnostic Investigation. 2010;22:1002–1005. doi: 10.1177/104063871002200629. [DOI] [PubMed] [Google Scholar]
- 7.Buckeridge D., Torrance A., Daly M. Puffall mushroom toxicosis (lycoperdonosis) in a two-year-old dachshund. Veterinary Record. 2011;168:304–305. doi: 10.1136/vr.c6353. [DOI] [PubMed] [Google Scholar]
- 8.Robert P., Evans S. The University of Chichago Press, 2011; London: 2011. The book of fungi: a life-size guide to six hundred species from around the world; pp. 506–547. [Google Scholar]
- 9.Lau A., Chen S., Sorrell T., Carter D., Malik R., Martin P. Development and clinical application of a Panfungal PCR assay to detect and identify fungal DNA in tissue specimens. Journal of Clinical Microbiology. 2007;45:380–385. doi: 10.1128/JCM.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S.C., Halliday C.L., Meyer W. A review of nucleic acid-based diagnostic tests for systemic mycoses with an emphasis on polymerase chain reaction-based assays. Medical Mycology. 2002;40:333–357. doi: 10.1080/mmy.40.4.333.357. [DOI] [PubMed] [Google Scholar]
- 11.Kasuya T.H.K., Uno K., Kakishima M. Phylogenetic placement of Geastrum melanocephalum and polyphyly of Geastrum triplex. Mycoscience. 2012;53:411–426. [Google Scholar]
- 12.Selman M., Pardo A., King T. Hypersensitivity pneumonitis: insight in diagnosis and pathobiology. American Journal of Respiratory and Critical Care Medicine. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 13.Lacasse Y., Selman M., Costabel U., Dalphin J-C., Ando M., Morrell F. Clinical diagnosis of hypersensitivity penumonitis. American Journal of Respiratory and Critical Care Medicine. 2003;168:952–958. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- 14.Dial S. Fungal diagnostics: current techniques and future trends. Veterinary Clinics of North America – Small Animal Practice. 2007;37:373–392. doi: 10.1016/j.cvsm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kerberger R., Lobetti R. Radiographic aspects of Pneumocystis carninii pneumonia in the miniature dachshund. Veterinary Radiology & Ultrasound. 1998;39:313–317. doi: 10.1111/j.1740-8261.1998.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 16.Samuel C., Whitelaw A., Corocoran C., Morrow B., Hsiao NY., Zampoli M. Improved detection of Pneumocystis jirovencii in upper and lower respiratory tract specimens from children with suspected pneumocystis pneumonia using real-time PCR: a prospective study. BMC Infectious Diseases. 2011;11:320–334. doi: 10.1186/1471-2334-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer K., Raghu G., Baughman R., Brown KK., Costabel U., du Bois RM. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. American Journal of Respiratory and Critical Care Medicine. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]


