Abstract
We describe the first clinical case of cryptococcosis due C. gattii in a Cuban immunocompetent patient who had a traveling history two years before to Central America. Molecular characterization of the isolate showed it to be genotype AFLP5 of which MLST sequences clustered with clinical and environmental strains from Colombia. The patient died one year after the diagnosis despite a prolonged treatment with (liposomal) amphotericin B, fluconazole, voriconazole and gamma interferon.
Keywords: Cryptococcal meningitis, C. gattii, AFLP5, Imported mycoses, Treatment
1. Introduction
Cryptococcosis is a life-threatening mycosis that develops following the inhalation and dissemination of fungal conidia and/or desiccated yeast forms. The Cryptococcus neoformans/Cryptococcus gattii species complex are free-living saprophytic yeasts which can survive in diverse niches [1]. The complex includes two species with their varieties and serotypes: C. neoformans var. grubii (serotype A; genotype AFLP1), C. neoformans var. neoformans (serotype D; genotype AFLP2) and the intervarietal state C. neoformans serotype AD (genotype AFLP3), which mostly affect immunocompromised patients worldwide; the other species, C. gattii (serotypes B and C; genotypes AFLP4-7 and AFLP10) mainly affects individuals without apparent alterations of the immune system and used to have a more restricted distribution to tropical and subtropical climates, although recent findings indicates a drastic change in the adaptation of this organism to other environments [2–4]. Up to now, only C. neoformans var. grubii has been routinely isolated from environmental and human clinical samples in Cuba [5,6]. Here we report a fatal case of C. gattii meningitis in Cuba in an immunocompetent patient.
2. Case
A 56-year-old white Cuban male from Villa Clara, a city in the central part of the island, who had no significant medical history, was admitted on July 5th 2011 because of mild fever, myalgia, malaise and diffuse headache. He was a moderate smoker and used only sporadically alcohol. His most recent travel history was to Honduras (2003–2005) and Guatemala (2007–2009) where he worked as a general practitioner. Physical examination revealed a mild fever of 38 °C, signs of lung consolidation on the lower right side, and fine crackles with chest auscultation. A chest radiograph showed an inflammatory lesion on the lower right side consistent with pneumonia. No cultures or clinical chemical investigations were performed. The patient was treated empirically with penicillin but after seven days (July 12th, 2011) he was admitted to the local hospital because of worsening of his general condition and development of a severe headache. Hematologic and serologic tests, including HIV antibody screening, were negative. A lumbar puncture showed a high opening pressure (40 cm H2O) and cerebrospinal fluid (CSF) analysis revealed pleocytosis (215 cells/mm3 predominantly lymphocytes), reduced glucose level (2.6 mg/dL) and elevated protein level (480 mg/dL) (Table 1). Sputum and CSF was cultured and treatment was started with ceftriaxone (4 g/d). After isolation of Klebsiella pneumoniae from sputum cultures, amikacin (15 mg/kg/d during seven days) was added to the treatment. The patient did not improve satisfactorily and therefore a computerized tomography (CT) scan and magnetic resonance imaging (MRI) were performed which were unremarkable. To reduce suspected intracranial hypertension, the lumbar puncture was repeated a week later (July 20th, 2011). Opening pressure was still increased with signs of inflammation (Table 1). Direct microscopic examination of CSF with India ink showed the presence of a large number of rounded and encapsulated yeasts compatible with Cryptococcus. The treatment was changed to amphotericin B deoxycholate (0.7 mg/kg) intravenously and 400 mg (b.i.d.) of fluconazole orally. Flucytosine was not available in Cuba. After an initial improvement with antifungal treatment the patient experienced worsening pulmonary functions, intense headache, neurological impairment, decreased visual acuity and hearing loss on the right side. Therefore on August 23th, 2011, the patient was transferred to an infectious diseases reference center in Havana where the isolate was identified as Cryptococcus gattii. In vitro susceptibility testing showed low MICs of amphotericin B (0.125 mg/L), fluconazole (2 mg/L), flucytosine (0.5 mg/L), itraconazole (0.125 mg/L), posaconazole (0.125 mg/L), voriconazole (0.063 mg/L) and isavuconazole (0.016 mg/L).
Table 1.
CSF findings during one year of C. gattii meningoencephalitis
| Date |
Results |
|||||
|---|---|---|---|---|---|---|
| OP (cm H2O) |
Clinical chemistry |
Microbiology |
||||
| Protein (mg/dL) | Glucose (mg/dL) | Cells (mm3) | India ink | Culture | ||
| 12/7/2011 | 40 | 480 | 2.6 | 70 | (+) | C. gattii |
| 20/7/2011 | 35 | 171 | 2.4 | 215 | (+) | C. gattii |
| 25/8/2011 | 32 | 40.2 | 3.4 | 17.5 | (+) | (−) |
| 5/9/2011 | 28 | 20 | 3.8 | 12 | (+) | (−) |
| 19/9/2011 | 25 | 30 | 3.8 | 10 | (+) | (−) |
| 3/10/2011 | 26 | 35 | 4 | 11 | (+) | (−) |
| 13/10/2011 | 38 | 28 | 4.18 | 13 | (+) | (−) |
| 23/10/2011 | 25 | 17.8 | 3.49 | 10 | (+) | (−) |
| 9/11/2011 | 35 | 17.8 | 3.49 | 10 | (+) | (−) |
| 5/12/2011 | 22 | 38 | 4.1 | 13.5 | (+) | (−) |
| 1/7/2012 | 50 | 45 | 2.5 | 15 | (+) | (−) |
So far the patient had received a cumulative dose of 1.500 mg amphotericin B deoxycholate and his kidney functions had become slightly disturbed (creatinine 130 mmol/L, ureum 10.6 mmol/L). Mayor findings at physical examination revealed a right VI cranial nerve palsy, blurred vision and diplopia in addition to a non-resolving pneumonia. Repeat CT scan with and without contrast did not show signs of cerebral edema or other abnormalities. Because of the poor clinical response and worsening kidney function amphotericin B deoxycholate was changed to liposomal amphotericin B (3 mg/kg) and fluconazole 800 mg/d was continued from August 29th to October 6th, 2011, but again without clinical improvement. Several lumbar punctures were necessary to control high intracranial pressure. All samples remained microscopically positive for cryptococci (Table 1). In this period the patient became blind and lost his hearing. After a total dose of 8 g liposomal amphotericin B, fluconazole was replaced by voriconazole (800 mg/d orally) and the patient was discharged on December 5th, 2011. During the last 2-month period the patient also received recombinant IFNγ (200 μg) subcutaneously thrice weekly. Because voriconazole was no longer available he was treated with fluconazole 400 mg from March 21st until July 1st 2012. The patient's blindness and loss of hearing remained permanent. He was re-admitted to hospital on July 1st, 2012, due to progressive deterioration, clinical signs of intracranial hypertension, seizures and papilledema. This improved slightly after CSF puncture but after seven days receiving amphotericin B deoxycholate (1 mg/kg/day) and fluconazole 800 mg/d he died on July 8th, 2012, one year after the start of the clinical presentation of the disease. An autopsy was not performed.
Identification of the isolated yeasts as C. gattii was performed by urease, inositol fermentation test and growth on canavanine-glycine bromothymol blue (CGB) agar to differentiate C. neoformans from C. gattii. Molecular identification as C. gattii genotype AFLP5 was done by using amplified fragment length polymorphism (AFLP) fingerprinting, as described previously [7,8]. The mating-type was determined using a conventional PCR using STE12α- and STE12a-specific primers [8] that revealed the presence of the mating-type α allele for both isolates. The two Cuban C. gattii AFLP5 isolates were sequenced for the seven nuclear loci CAP10, GPD1, IGS1, LAC1, MPD1, PLB1 and TEF1 to compare them to the data of other isolates with the genotype AFLP5, as published by Hagen et al. (2012) and Byrnes et al. (2011) [3,9]. Sequences were deposited in Genbank and can be accessed via the accession numbers KC424622–KC424635. Isolates were deposited in the CBS Fungal Biodiversity collection in Utrecht, the Netherlands as CBS 12755 (CUBA 250) and CBS 12754 (CUBA 350). A bootstrap Maximum Likelihood phylogenetic analysis was performed using the settings as being used for a previously published C. gattii MLST study [3] (Fig. 1).
Fig. 1.
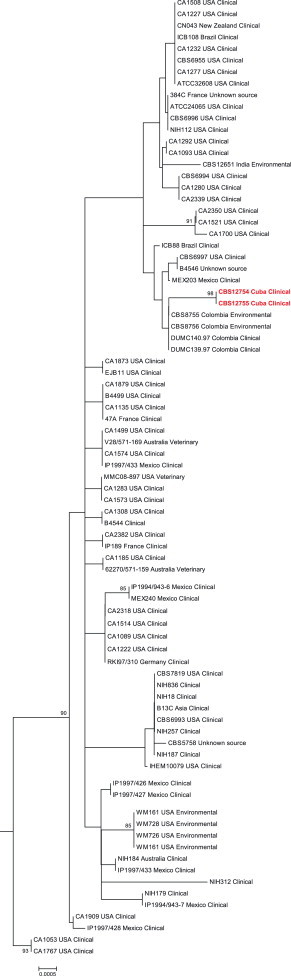
A 1000× bootstrap Maximum Likelihood phylogenetic analysis of the two Cuban strains (CBS 12755 and CBS 12754), compared to C. gattii AFLP5 strains from previously published MLST studies [3,9]. Both Cuban strains (marked in red) clustered together with clinical and environmental strains from Colombia. Numbers next to branches represent bootstrap values≥75. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
C. gattii differs from the more commonly isolated C. neoformans in clinical aspects, ecological niche and genetic makeup. Unlike C. neoformans, it most often affects immunocompetent patients exposed to an environmental source causing pulmonary and cerebral infections. Furthermore C. gattii is known to be more resistant to antifungal agents. In the Caribbean, cryptococcosis appears to be a relatively rare disease with an estimated 7800 new patients each year [10]. In Cuba, the disease was first reported in the early 1950s [11]. Since then sporadic cases were associated with alcoholism, organ transplantations and immunological disorders. In 1986, with the beginning of the AIDS epidemic in Cuba, the amount of individuals suffering from cryptococcosis had increased over the years with an average of eight new cases each year [12]. This might still be an underrepresentation since a retrospective autopsy study of HIV/AIDS patients performed during a 10-year period showed that disseminated or central nervous system cryptococcosis was a serious and common disorder in 29% of the cases [13]. The clinical presentation of cryptococcal infection varies from asymptomatic pulmonary colonization to severe pneumonia with respiratory failure and life-threatening meningitis [14,15]. Mostly infections due to C. gattii genotypes AFLP4 and AFLP6 usually occurs in patients without detectable predisposing factors while genotypes AFLP5, AFLP7 and AFLP10 appear to be associated with an impaired immune system (mainly AIDS, but also any other immunosuppressive condition including solid organ transplantation) which is similar to C. neoformans [3,16]. Interestingly none of these underlying diseases, previously associated with C. gattii AFLP5 infection, could be demonstrated in our patient.
About 65%–77% of cases with C. gattii infections presents more often with lung involvement [1]. Initial symptoms of our patient fitted with a community-acquired pneumonia and sputum cultures only demonstrated Klebsiella. It might be hypothesized that the development of a community-acquired pneumonia could generate appropriated conditions favoring rapid multiplication and dissemination of C. gattii from its dormant state. Cryptococcal infection involves any part of the central nervous system and leads to a wide spectrum of neurological symptoms such as headache, altered consciousness, seizures, and cranial nerve palsies. Seaton et al. [17] reviewed the ophthalmic findings in 82 immunocompetent patients in whom C. gattii was involved. A high rate of visual loss was observed in 52.6% of survivors similar what had occurred in our patient.
Environmental exposure is known to be the dominant risk factor for C. gattii infection, but so far all pathogenic clinical and environmental Cryptococcus isolates from Cuba have been identified as C. neoformans var. grubii [5]. However a single case of a fatal veterinary C. gattii genotype AFLP4 infection was described recently in a cheetah imported from South Africa. Molecular epidemiologic studies strongly suggested that the animal was not infected in Cuba, but most likely in Africa [18]. It has been demonstrated before that C. gattii infections can be imported subclinically and reactivate after being dormant for many years [3]. There are three main arguments that support the hypothesis that our patient had a dormant infection when he returned to Cuba from Central America. First, there is no evidence suggesting environmental presence of this species despite multiple attempts to recover C. gattii from plants, trees and cacti in Cuba [6], which is in strong contrast to for example India where C. gattii can be isolated from up to 50% of investigated trees [16]. Secondly, it has been ample demonstrated that the time interval between exposure to C. gattii and the development of the disease is highly variable; at one extreme, acute infection have been reported while reactivation of long silent subclinical infections is also possible depending of the immunological status of the patient [3,19]. We have no indication that a hereditary immune disorder might have been involved but there is in vitro evidence that clinical C. gattii isolates induced a more pronounced inflammatory response compared to other Cryptococcus species and environmental C. gattii [20]. This might explain the severe immunopathology seen in human (and animal) C. gattii infections. And finally, molecular biological characterization showed that the strain belonged to C. gattii genotype AFLP5. Although this genotype was originally reported from India and the USA, current literature show that it has spread throughout Latin-American countries (Argentina, Brazil, Colombia, Guatemala Mexico, and Venezuela), the west coast of the USA., as well as over Asia and Australia [3,16]. Comparison with a large C. gattii genotype AFLP5 MLST dataset [3,9] yielded a high similarity degree to strains from clinical and environmental sources in Colombia. The patient's travel history to Central America suggests acquisition during this time period.
The neurological picture in conjunction with the presence of yeasts in the CSF let to suspect cryptococcal infection in our patient initiating treatment according to management guidelines [21] and availability of drugs. For meningoencephalitis and disseminated disease due to C. gattii, recommended induction, consolidation, and suppressive treatment are basically the same as for C. neoformans. Because C. gattii infection is associated with more neurological complications and delayed response to therapy, as demonstrated in this case, a longer course of therapy, increasing dose and/or recombinant IFNγ as salvage therapy for patients unresponsive to multiple antifungal drugs has been suggested [21]. The latter salvage therapy was successful in patients with C. neoformans infections [22]. For persistent and relapse isolates it is highly recommended to look for changes in the MIC from the original isolate (≥3-dilution difference suggests development of direct drug resistance) [13,21]. Unfortunately, although the direct examination of CSF stayed positive during the whole disease course, cultures remained negative. Our case is a quick alert to suspect C. gattii AFLP5 infection also in HIV negative patients, to consider the growing possibility of imported infections from endemic regions and the need for more effective alternatives for treating this mycosis.
Conflict of interest
JFM received grants from Astellas, Merck, Pfizer, and Schering-Plough. He has been a consultant to Basilea and Merck and received speaker fees from Merck and Gilead. All other authors: no potential conflicts of interest.
References
- 1.Kwon-Chung K.J., Boekhout T., Wickes B.L., Fell J.W. Systematics of the genus Cryptococcus and its type species C. neoformans. In: Heitman J., editor. Cryptococcus: from human pathogen to model yeast. ASM Press; Washington, DC: 2011. pp. 3–15. [Google Scholar]
- 2.Chowdhary A., Randhawa H.S., Boekhout T., Hagen F., Klaassen C.H., Meis J.F. Temperate climate niche for Cryptococcus gattii in Northern Europe. Emerging Infectious Diseases. 2012;8:172–174. doi: 10.3201/eid1801.111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagen F., Colom M.F., Swinne D., Tintelnot K., Iatta R., Montagna M.T. Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerging Infectious Diseases. 2012;18:1618–1624. doi: 10.3201/eid1810.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd S.E., Hagen F., Tscharke R.L., Huynh M., Bartlett K.H., Fyfe M. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proceedings of the National Academy of Sciences USA. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illnait-Zaragozi M.T., Martínez-Machín G.F., Fernández-Andreu C.M., Boekhout T., Meis J.F., Klaassen C.H. Microsatellite typing of clinical and environmental Cryptococcus neoformans var. grubii isolates from Cuba shows multiple genetic lineages. PLoS ONE. 2010;5:e9124. doi: 10.1371/journal.pone.0009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illnait-Zaragozí M.T., Martínez-Machín G.F., Fernández-Andreu C.M., Perurena-Lancha M.R., Theelen B., Boekhout T. Environmental isolation and characterization of Cryptococcus species from living trees in Havana City, Cuba. Mycoses. 2012;55:138–144. doi: 10.1111/j.1439-0507.2012.02168.x. [DOI] [PubMed] [Google Scholar]
- 7.Boekhout T., Theelen B., Diaz M., Fell J.W., Hop W.C., Dromer F. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology. 2001;147:891–907. doi: 10.1099/00221287-147-4-891. [DOI] [PubMed] [Google Scholar]
- 8.Hagen F., Illnait-Zaragozi M.T., Bartlett K.H., Swinne D., Geertsen E., Klaassen C.H. In vitro antifungal susceptibilities and AFLP genotyping of a worldwide collection of 350 clinical, veterinary and environmental Cryptococcus gattii isolates. Antimicrobial Agents and Chemotherapy. 2010;54:5139–5145. doi: 10.1128/AAC.00746-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrnes E.J., Li W., Ren P., Lewit Y., Voelz K., Fraser J.A. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathogens. 2011;7:e1002205. doi: 10.1371/journal.ppat.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 11.Curbelo A. Septicemia y meningitis a Cryptococcus neoformans. Archivos del Hospital Universitario (Cuba) 1951;9:324–330. [PubMed] [Google Scholar]
- 12.Illnait M.T., Valdés I.C., Martínez G.F., Fernández C.M. Criptococcosis. Una alerta necesaria. Revista Cubana de Medicina Tropical. 2001;53:224–225. [PubMed] [Google Scholar]
- 13.Illnait-Zaragozí M.T., Martínez G.F., Fernández C.M., Hagen F., Boekhout T., Klaassen C.H. Microsatellite typing and susceptibilities of serial Cryptococcus neoformans isolates from Cuban patients with recurrent cryptococcal meningitis. BMC Infectious Diseases. 2010;10:289–296. doi: 10.1186/1471-2334-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman J.D., Vollmer M.E., Luks A.M. Cryptococcosis in the immunocompetent patient. Respiratory Care. 2010;55:1499–1503. [PubMed] [Google Scholar]
- 15.Kim Y.S., Lee I.H., Kim H.S., Jin S.S., Lee J.H., Sung-Kyoung K. Pulmonary cryptococcosis mimicking primary lung cancer with multiple lung metastases. Tuberculosis and Respiratory Diseases. 2012;73:182–186. doi: 10.4046/trd.2012.73.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhary A., Prakash A., Randhawa1 H.S., Hagen F., Klaassen C.H., Meis J.F. First environmental isolation of Cryptococcus gattii, genotype AFLP5, from India and a global review. Mycoses. 2013:56. doi: 10.1111/myc.12039. [DOI] [PubMed] [Google Scholar]
- 17.Seaton R.A., Verma N., Naraqi S., Wembri J.P., Warrell D.A. Visual loss in immunocompetent patients with Cryptococcus neoformans var. gattii meningitis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:44–49. doi: 10.1016/s0035-9203(97)90391-6. [DOI] [PubMed] [Google Scholar]
- 18.Illnait-Zaragozí M.T., Hagen F., Fernández C.M., Martínez G.F., Polo J.L., Boekhout T. Reactivation of a Cryptococcus gattii infection in a cheetah (Acinonyx jubatus) held in the National Zoo, Havana, Cuba. Mycoses. 2011;54:e889–e892. doi: 10.1111/j.1439-0507.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagen F., van Assen S., Luijckx G.J., Boekhout T., Kampinga G.A. Activated dormant Cryptococcus gattii infection in a Dutch tourist who visited Vancouver Island (Canada): a molecular epidemiological approach. Medical Mycology: 2010;48:528–531. doi: 10.3109/13693780903300319. [DOI] [PubMed] [Google Scholar]
- 20.Schoffelen T., Illnait-Zaragozí M.T., Joosten L.A.B., Netea M.G., Boekhout T., Meis JF. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS ONE. 2013;8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea M.G., Brouwer A.E., Hoogendoorn E.H., Van der Meer J.W., Koolen M., Verweij P.E. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: defective cytokine production and reversal by recombinant interferon- gamma therapy. Clinical Infectious Diseases. 2004;39:e83–e87. doi: 10.1086/425121. [DOI] [PubMed] [Google Scholar]


