Abstract
Mucormycosis is an emergent and threatening invasive fungal invasion underdiagnosed by clinicians due to lack of awareness and aspecific clinical picture. The authors describe a clinical case of a diabetic and cirrhotic patient who developed rhino-orbital-cerebral and pulmonary mucormycosis, non-responsive to treatment. Typical gaps in the management of this deadly disease are addressed. There is a strong need for novel therapies and an expectation that sponsors will recognize the critical need for randomized clinical trials.
Keywords: Mucormycosis, Diabetes, High mortality, Delays, Hyperbaric medicine, Randomized clinical trials
1. Introduction
Over the past couple of decades, there has been an explosion in the number of published studies on this emergent invasive fungal infection [1]. However the situation in Portugal is still poorly understood (only two case reports published in the literature) [2,3]. This lack of awareness among Portuguese clinicians can lead to delays in diagnosis and initiation of treatment. Indeed, early diagnosis still remains an important bottleneck. A high index of suspicion in susceptible patient populations is the cornerstone in the management of this invasive fungal infection. The prognosis for mucormycosis in diabetic patients is better than for hematological malignancies patients, yet still there are significant gaps in the knowledge of how to manage these patients and as such more research is needed [4]. Current therapeutic limbo is associated with this fatal disease overwhelming by the delays in the diagnosis and treatment initiation [5]. Because of the relative rarity of mucormycosis, prospective, comparative studies of antifungal agents and strategies have not been conducted. Therefore, the management of mucormycosis is still based on the results of case series and case reports, animal model studies, and in vitro susceptibility data [6]. Hyperbaric medicine could be a promising supportive treatment but there is scarce evidence available on this [7,8].
The following case report describes a diabetic and cirrhotic patient who developed rhino-orbital-cerebral and pulmonary mucormycosis, non-responsive to treatment. The authors highlight the typical gaps in management that occur with respect to this deadly disease.
2. Case
A 60 year-old male, diagnosed with type 2 diabetes mellitus, class I obesity (BMI=33 kg/m2), chronic obstructive pulmonary disease (medicated with daily inhaled corticosteroid therapy) and alcoholic cirrhosis (Child-Pugh classification 8 points—B) presented several times to the emergency room of our hospital with right-sided headache which had been present for 1.5 years and was progressively worsening. Although being treated with insulin, his diabetes was poorly controlled [HgA1c 11.1 % (3.9–6.1)]. Due to his complaints, a cranial CT was performed (day 0), which showed a soft tissue density filling the paranasal sinuses, this was interpreted as an inflammatory process (Fig. 1). The patient was discharged with analgesic medication.
Fig. 1.
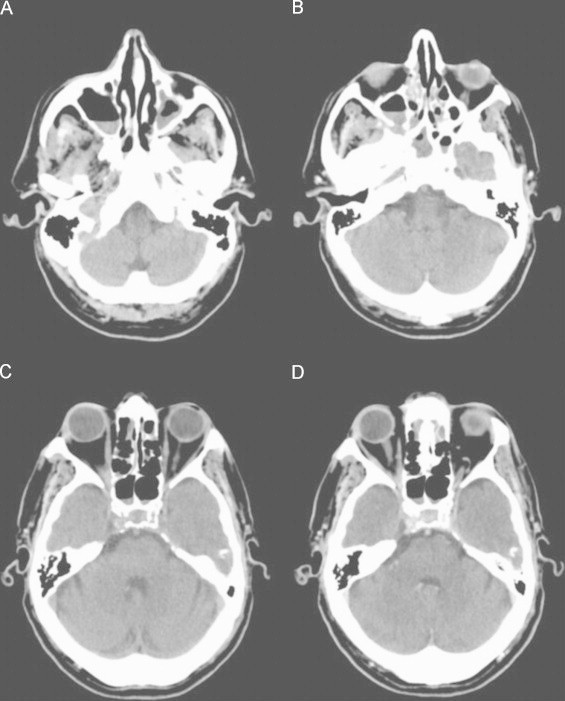
Cerebral CT scan, axial images. (A–C), Non-enhanced CT scan; (D) Contrast-enhanced CT scan. Partial soft tissue opacification of the maxillary, sphenoid and ethmoid sinuses.
About 2 months later (day +60), he presented again with complaints of progressive decreased right visual acuity, right proptosis and right hemifacial dysesthesia. On physical examination, right-sided proptosis, amaurosis and ophthalmoparesis, mainly in abduction, as well as slight reduction of right corneal reflex and right-sided V1 and V2 sensory deficit were observed. No eschars on the soft and hard palates were seen. He had a negative HIV serology.
A new cranial CT scan (day +60) was carried out and it continued to show opacification of the paranasal sinuses which was then associated with bone erosions, extension to the pterygopalatine fossa and intracranial extension, which led to the suspicion of a neoplastic lesion (Fig. 2). Magnetic resonance imaging (MRI) (day +62) confirmed the presence of a lesion centered on the apex of the right orbit, which filled the sphenoidal fissure and optic canal and extended to the pterygopalatine fossa and with intracranial extra-axial extension, in accordance with the hypothesis of a neoplastic lesion (Fig. 3).
Fig. 2.
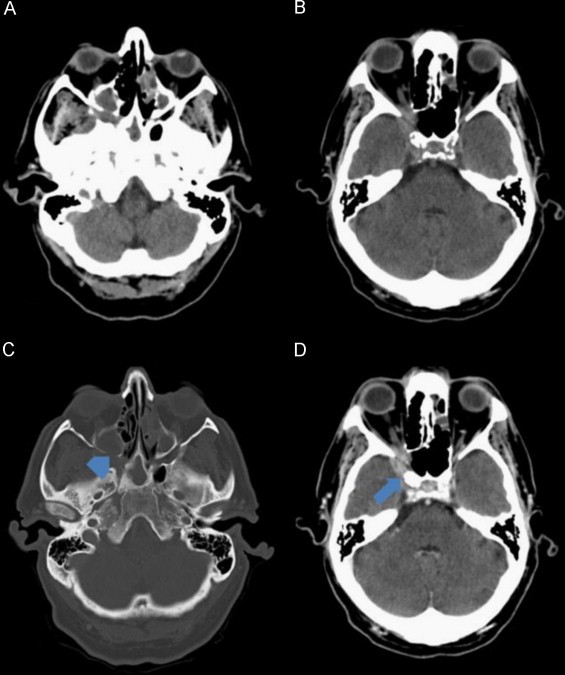
Cerebral CT scan, axial images. (A and B) Non-enhanced CT scan; (C) CT scan bone window; (D) Contrast-enhanced CT scan. Soft tissue opacification of the paranasal sinuses associated with focal areas of bone erosion of the posterior wall of the right maxillary sinus (arrowhead). Extension of the soft tissue lesion to the pterygopalatine fossa and intracranial extension to the temporal fossa (arrow) and to the anterior part of the cavernous sinus.
Fig. 3.
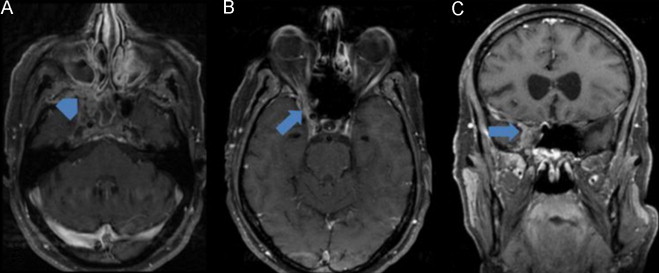
Cerebral MRI: (A, B) (axial T1 with fat saturation after gadolinium) and (C) (coronal T1 with fat saturation after gadolinium). Lesion centered on the apex of the right orbit, filling the sphenoidal fissure and optic canal, extending to the pterygopalatine fossa (arrowhead) and with mild intracranial extra-axial extension (arrow). Lesion is isointense in T1 and hypointense in T2 (not shown) with homogeneous enhancement after gadolinium. Associated inflammatory changes of the sinuses are also observed.
The patient underwent incisional biopsy through maxillary sinusotomy by endoscopic sinus surgery (day +66) which revealed multiple broad non-septated, ribbon-like, right-angle hyphae consistent with Mucormycosis (Grocott technique and hematoxylin-eosin) (Fig. 4). Tissue culture was negative for fungi. Liposomal amphotericin B (L-AmB) was started (day +70) at a dose of 5 mg/kg/day, intravenous, in combination with intravenous caspofungin 50 mg/ day. Despite having completed 55 days of treatment clinical worsening was proven by control MRI (day +125) (Fig. 5). Surgical debridement (day +130) was performed with anterior ethmoidectomy and bilateral maxillary sinusotomy. He then presented with acute renal failure (day +131) either associated with amphotericin and contrast products used in neuroimaging studies, requiring hemodialysis. As a result, therapy was changed to posaconazole 800 mg/ day (day +131) (8.7 mg/kg/day) divided into 4 doses orally and with ambulatory monitoring. A chest CT scan (day +130) (Fig. 6) revealed endobronchial spread of infection (multiple nodules and endobronchial lesions). Hyperbaric oxygen was proposed, but while waiting for this, his clinical situation deteriorated and the patient died (8 months after starting posaconazole) (day +371).
Fig. 4.
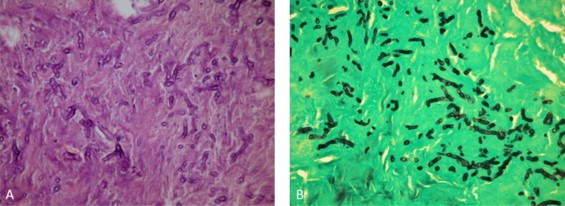
(A) Hematoxylin and eosin stain, 400× and (B) Grocott's methenamine silver stain, 400×. Multiple broad non-septated, ribbon-like, right-angle hyphae.
Fig. 5.
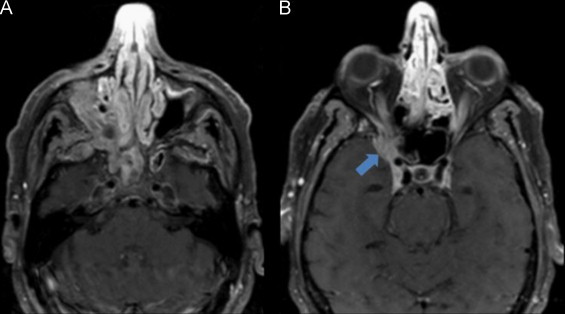
(A and B) (Axial T1 with fat saturation after gadolinium) Cerebral MRI at day 20 of Liposomal amphotericin B and caspofungin treatment, showing imagiological worsening with increased intracranial infiltration of the fungal lesion (arrow).
Fig. 6.
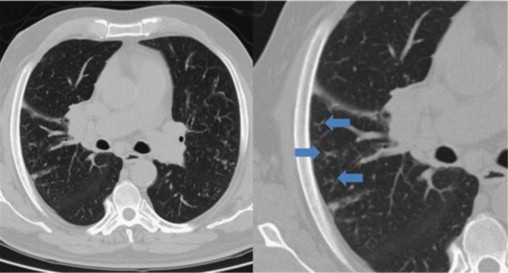
Thorax CT scan: muliple peripherical centrilobular nodules with “tree-in-bud” images (arrows), showing endobronchial spread of infection.
3. Discussion
No clinical history is completely specific for the diagnosis of invasive mucormycosis [9]. Any diabetic patient with a headache and visual changes or recurrent sinusitis is a candidate for prompt evaluation using diagnostic imaging and nasal endoscopy to rule out mucormycosis [10].
Although our patient had diabetes mellitus, which is a documented risk factor for mucormycosis, he did not present with ketoacidosis. It is thus possible that this patient´s impaired immunity is related to liver cirrhosis, rather than diabetes. However, fungal invasive infections rarely occur in patients with liver cirrhosis [11]. Indeed, only 10 mucormycosis-infected cirrhotic patients are reported in the English-language medical literature, the majority with diabetes mellitus as comorbidity. As baseline glucose levels were not provided in most of these cases, it is difficult to conclude that liver cirrhosis is a risk factor for mucormycosis [11].
Optimal clinical care and clinical investigation of patients with mucormycosis are limited by absence of controlled trials, and absence of well-defined predictors of mortality or clinical response. A recent study described some clinical factors associated with 90-day mortality: active malignancy or neutropenia at enrollment, higher baseline serum concentrations of iron and ferritin were associated with increased mortality. However, age, diabetes mellitus, transplant status, or antifungal therapy were not associated with mortality [12].
An interesting point in the epidemiology of mucormycosis is that despite the explosion of the diabetes epidemic in the last 2 decades, the incidence of mucormycosis has been shown to be in constant decline in the diabetic population in some studies during this time [13] but not all [14]. Statins are nowadays frequently prescribed for diabetic patients and with some evidence of anti-Zygomycetes effects [15] and so the association may be more than a coincidence. These drugs have fungicidal activity against Glomeromycota (former Zygomycota) and act synergistically with other antifungal agents, such as voriconazole [16].
The diagnosis of mucormycosis is challenging and treatment should start as early as possible in order to decrease mortality [17].
Tissue culture was negative for fungi. Nevertheless, it is known that culture often yields no growth and histopathological identification of an organism structurally identical to Mucorales frequently is the only evidence of infection [9].
Tissue-based diagnosis remains the gold standard [1,18]. Nonetheless, treatment initiation should not wait for fixed histopathological staining results and should therefore be based on frozen tissue samples from biopsy [19]. Demonstration of hyphae in clinical samples by direct microscopy is important because it is rapid and highly suggestive of disease. Specimens can be observed after treatment with potassium hydroxide, staining with an optical brightener (calcofluor white), or with Grocott's methenamine silver stain. When hyphae are fragmented a definitive diagnosis of mucormycosis can be difficult by direct examination and culture is required to confirm the diagnosis [20].
Other bottleneck in mucormycosis´ management is delayed initiation of treatment. Chamilos et al [17] have shown a twofold increase in mortality with delayed initiation of therapy (starting by more than 6 days after diagnosis). This approach is multimodal, including antifungal agents, surgical debridement, and correction of the underlying condition predisposing the patient to the disease. However multiple controversies still remain [1]. Because both surgical and medical interventions are simultaneously or sequentially performed, it is difficult to ascertain the relative efficacy of drug therapy alone.
Control of underlying conditions is critical in mucormycosis [10]. Rapid correction of metabolic abnormalities is mandatory in uncontrolled diabetes. Corticosteroids should be discontinued, if feasible, and other immunosuppressive drugs should be tapered as much as possible [21].
Among the more recent therapeutic developments in mucormycosis treatment are the lipid formulations of amphotericin B (LAmB), which are now the drugs of first choice; the new triazole posaconazole, with promising efficacy as salvage treatment; the iron chelators deferasirox and deferiprone; the echinocandins in combination with LAmB and recombinant growth factors such as granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) [6].
Generally, polyene should be used as backbone therapy either in monotherapy or in combination-therapy [5]. Although AmB deoxycholate was the cornerstone of mucormycosis therapy for decades, LAmB are less nephrotoxic and can be safely administered for higher doses for a longer period of time than AmB [22]. Selection of which dose of lipid polyene for a combination-therapy study is also difficult based on available data. Nonetheless, a reasonable dose for LAmB would be 5 mg/kg/day, up to a maximum of 10 mg/kg/day for CNS infections. Combined complete and partial response rates are 32–100%, and overall mortality ranges from 5% to 61% [16].
Posaconazole has activity against the Mucorales. It is an oral agent and can be used as a combination salvage therapy with LAmB in patients with refractory mucormycosis or intolerant to LAmB or as an oral step-down therapy in patients who have responded to treatment with AmB, but not as primary therapy [4,19,21]. The future of this combination strategy might be at risk due to concerns about the reliability of achieving adequate in vivo levels of oral posaconazole without a parenteral formulation. To date, poor activity of this agent was observed in preclinical efficacy studies and no activity in mouse models against Rhizopus oryzae (the most common agent) [5].
A recent review of 96 cases of mucormycosis cases published from 2003 to 2011 [23] showed that diagnosis was based on histology alone in 2 (2.1%) and microbiological evidence in 67 (69.8%), while no data on the diagnostic approach was reported in 27 (28.1%) patients. The most frequent pathogens were Rhizopus spp. (31.2%), followed by Mucor spp. (14.6%). The site of infection was predominantly rhino-orbital (38.5%), of which 43% also had CNS involvement, followed by disseminated disease (22.1%). A complete response was achieved in 62 (64.6%), partial response in 7 (7.3%) patients, and stable disease in 1 (1%). Overall mortality was 24% (lacking data for three patients). In published case reports on posaconazole treatment for mucormycosis, the drug was frequently and successfully used in combination or as a second line therapy.
Despite the use of posaconazole, our patient died. Whether this was a case of posaconazole-resistant mucormycosis remains unclear, but probably it was not the case of this patient as he was not under prophylaxis with posaconazole. In fact, researchers have already reported cases of breakthrough mucormycosis to posaconazole in patients receiving this agent as prophylaxis [24]. Another reason could be under-dosing of posaconazole, as we didn´t perform therapeutic drug monitoring [25].
Echinocandins are regarded as inactive against Glomeromycota [5]. However, studies have shown that Rhyzopus oryzae does express the target enzyme for echinocandins, so these agents might have a therapeutic role against mucormycosis when combined with polyenes [5]. Low-dose, but not high-dose caspofungin improved survival in mice with diabetes, ketoacidosis and Rhyzopus oryzae infection. A small, retrospective clinical study in which combination AmB complex or LAmB plus caspofungin therapy was associated showed significantly improved survival for patients with rhino-orbito-cerebral mucormycosis compared with polyene monotherapy demonstrated by multivariate analysis, that only combination therapy was significantly associated with superior outcomes (OR 10.9 for success vs monotherapy; p=0.02) [22]. Further randomized clinical trials are needed.
A maximal aggressive treatment was already studied in mice models and demonstrated that triple combination therapy enhanced survival and reduced the tissue fungal burden of mice infected with mucormycosis compared with placebo, monotherapy, or dual drug therapy [26]. This was supported with a case report of an adolescent with osteosarcoma and disseminated Lichtheimia corymbifera infection receiving successful triple combination therapy with high doses of LAmB, posaconazole, and caspofungin [27]. First, dual therapy has to be shown to be superior to monotherapy, and only then a triple-therapy study can be possibly conducted.
The Deferasirox-AmBisome Therapy for mucormycosis (DEFEAT Mucor) study was the first randomized clinical trial conducted on patients with mucormycosis, and demonstrated that adjunctive deferasirox therapy did not improve outcomes of the disease [28].
This patient should have been proposed for hyperbaric oxygen earlier. Despite scarce and old evidence on utility of hyperbaric oxygen, the increased oxygen pressure achieved with this technique may improve neutrophil activity and the putative oxidative killing effects of polyene antifungals [29]. John et al [30] concluded, by reviewing 28 cases, that hyperbaric oxygen is a promising treatment modality, showing up to 94% survival among diabetic patients, but does not make any firm conclusions given that most of the evidence is from case reports/series. Besides absence of vigorous clinical evidence supporting the benefits, hyperbaric oxygen is expensive and often logistically cumbersome.
Multiple immune-augmentation strategies have been proposed for mucormycosis, such as G-CSF, GM-CSF, or interferon-ɣ alone (IFN- ɣ) or in combination with granulocyte transfusions but again, their efficacy has not yet been evaluated through adequately powered randomized controlled trials[21].
This state of confusion and unacceptable position of not knowing the best management of mucormycosis enhanced by the lack of evidence of randomized clinical trials can explain the undertreatment or the overtoxicity and cost associated with this clinical case.
Delays in the diagnosis and initiation of treatment were the most important causes of the fatal outcome for this patient. Hyperbaric oxygen may have a role earlier in diabetic patients with mucormycosis. Clinicians' awareness (starting with general practitioners) and prompt, aggressive and combination treatment can be helpful in diminishing the higher mortality associated to mucormycosis. Improving training and education of physicians (risk factors and clinical manifestations), diagnostic imaging skills as well as spread of the mycological diagnostic capacity through several technicians will probably strengthen institutional expertise in the diagnosis of mucormycosis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kontoyiannis D.P., Lewis R.E., Lotholary O., Spellberg B., Petrikkos G., Roillides E. Future directions in mucormycosis research. Clinical Infectious Diseases. 2012;54(S1):S79–S85. doi: 10.1093/cid/cir886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marques DS, Pinho Vaz C, Branca R, Campilho F, Lamelas C, Afonso LP et al. Rhizomucor and scedosporium infection post hematopoietic stem-cell transplant. Case Report Med 2011;830769. doi:10.1155/2011/830769. Epub 2011 April 5. [DOI] [PMC free article] [PubMed]
- 3.Oliveira V., Costa A. Cerebral hematoma caused by mucormycosis. Reviews in Neurological Diseases. 2001;33(10):951–953. [PubMed] [Google Scholar]
- 4.Rammaert B., Lanternier F., Poirée S., Kania R., Lortholary O. Diabetes and mucormycosis: a complex interplay. Diabetes and Metabolism. 2012;38(3):193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B., Ibrahim A., Roilides E., Lewis R.E., Lortholary O., Petrikkos G. Combination therapy for mucormycosis: Why, what, and how? Clinical Infectious Diseases. 2012;54(S1):S73–S78. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2012; 97:xxx doi:10.3324/haematol.2012.065110. [DOI] [PMC free article] [PubMed]
- 7.García-Covarrubias L., Barratt D.M., Bartlett R., van Meter K. Treatment of mucormycosis with adjunctive hyperbaric oxygen: five cases treated at the same institution and review of the literature. Revista de investigación clínica; organo del Hospital de Enfermedades de la Nutrición. 2004;56(1):51–55. [PubMed] [Google Scholar]
- 8.Kajs-Wyllie M. Hyperbaric oxygen therapy for rhinocerebral fungal infection. Journal of Neuroscience Nursing. 1995;27(3):174–181. doi: 10.1097/01376517-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Walsh T.J., Gamaletsou M.N., McGinnis M.R., Hayden R.T., Kontoyiannis D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomicosis) Clinical Infectious Diseases. 2012;54(S1):S55–S60. doi: 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- 10.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clinical Infectious Diseases. 2012;54(S1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 11.Lin S.Y., Lu P.L., Tsai K.B., Lin C.Y., Lin W.R., Chen T.C. A mucormycosis case in a cirrhotic patient successfully treated with posaconazole and review of published literature. Mycopathologia. 2012;174:499–504. doi: 10.1007/s11046-012-9561-8. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B., Kontoyiannis D.P., Fredricks D., Morris M.I., Perfect J.R., Chin-Hong P.V. Risk factors for mortality in patients with mucormycosis. Medical Mycology. 2012;50(6):611–618. doi: 10.3109/13693786.2012.669502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clinical Infectious Diseases. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 14.Bitar D., Van Cauteren D., Lanternier F., Dannaoui E., Che D., Dromer F. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerging Infectious Diseases. 2009;15:1395–1440. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamilos G., Lewis R.E., Kontoyiannis D.P. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrobial Agents and Chemotherapy. 2006;50:96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsin-Yun Sun, Nina Singh. Mucormycosis: its contemporary face and management strategies. Lancet Infectious Diseases. 2011;11:301–311. doi: 10.1016/S1473-3099(10)70316-9. [DOI] [PubMed] [Google Scholar]
- 17.Chamilos G., Lewis R.E., Kontoyiannis D.P. Delaying amphotericin B–based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clinical Infectious Diseases. 2008;47(4):503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 18.Hammond S.P., Bialek R., Milner D.A., Petschnigg E.M., Baden L.R., Marty F.M. Molecular methods to improve diagnosis and identification of mucormycosis. Journal of Clinical Microbiology. 2011:2151–2153. doi: 10.1128/JCM.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Burik J.A., Hare R.S., Solomon H.F., Corrado M.L., Kontoyiannis D.P. Posaconazole is effective as salvage therapy in zygomicosis: a retrospective summary of 91 cases. Clinical Infectious Diseases. 2006;42:61–65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 20.Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clinical Microbiology and Infection. 2009;15(5):60–65. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- 21.Gamaletsou M.N., Sipsas N.V., Roilides E., Walsh T.J. Rhino-orbital-cerebral mucormycosis. Current Infectious Disease Reports. 2012;14:423–434. doi: 10.1007/s11908-012-0272-6. [DOI] [PubMed] [Google Scholar]
- 22.Reed C., Bryant R., Ibrahim A.S., Edwards J., Jr, Filler S.G., Goldberg R. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clinical Infectious Diseases. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vehreschild JJ, Birtel A, Vehreschild MJ, Liss B, Farowski F, Kochanek M, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Critical Reviews in Microbiology . Posted online on August 24, 2012. (doi:10.3109/1040841X.2012.711741). [DOI] [PubMed]
- 24.Lekakis L.J., Lawson A., Prante J., Ribes J., Davis G.J., Monohan G. Fatal rhizopus pneumonia in allogeneic stem cell transplant patients despite posaconazole prophylaxis: two cases and review of the literature. Biology of Blood and Marrow Transplantation. 2009;15:991–995. doi: 10.1016/j.bbmt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Egelund E.F., Egelund T.A., Ng J.S., Wassil S.K., Peloquin C.A. Posaconazole pharmacokinetics in a 2-year-old boy with rhino-cerebral-orbital zygomycosis. Pharmacotherapy. 2013;33(1):1–8. doi: 10.1002/phar.1172. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim A.S., Gebremariam T., Luo G., Fu Y., French S.W., Edwards J.E., Jr Combination therapy of murine mucormycosis or aspergillosis with iron chelation, polyenes, and echinocandins. Antimicrobial Agents and Chemotherapy. 2011;55:1768–1770. doi: 10.1128/AAC.01577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux B.G., Méchinaud F., Gay-Andrieu F., Lortholary O., Dannaoui E., Hoinard D. Successful triple combination therapy of disseminated Absidia corymbifera infection in an adolescent with osteosarcoma. Journal of Pediatric Hematology Oncology. 2010;32:131–133. doi: 10.1097/MPH.0b013e3181ca0dcf. [DOI] [PubMed] [Google Scholar]
- 28.Spellberg B., Ibrahim A.S., Chin-Hong P.V., Kontoyiannis D.P., Morris M.I., Perfect J.R. The deferasirox-ambisome therapy for mucormycosi (DEFEAT Mucor) Study: a randomized, double-blinded, placebo-controlled trial. Journal of Antimicrobial Chemotherapy. 2012;67(3):715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colin G. Kaide, Sorabh Khandelwal. Hyperbaric oxygen: applications in infectious diseases. Emergency Medicine Clinics of North America. 2008;26:571–595. doi: 10.1016/j.emc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.John B.V., Chamilos G., Kontoyiannis D.P. Hyperbaric oxygen as an adjunctive treatment for zygomicosis. Clinical Microbiology and Infection·. 2005;11(7):515–517. doi: 10.1111/j.1469-0691.2005.01170.x. [DOI] [PubMed] [Google Scholar]


