Abstract
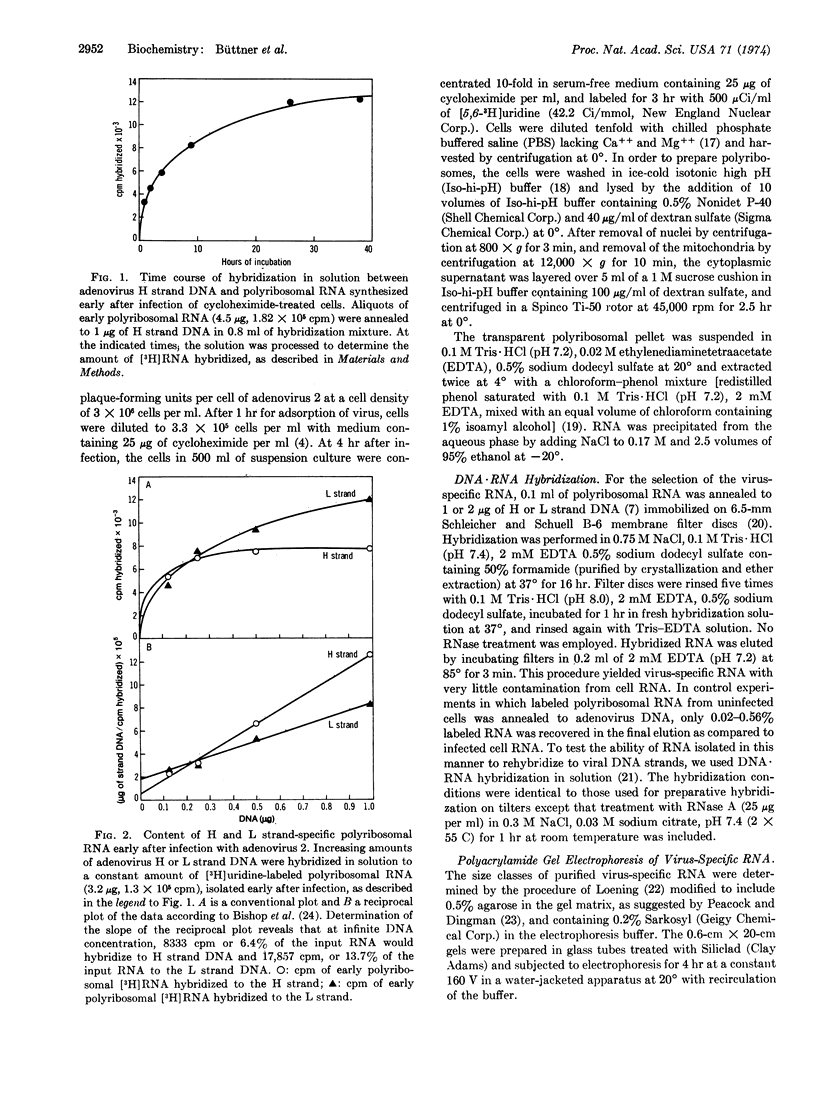
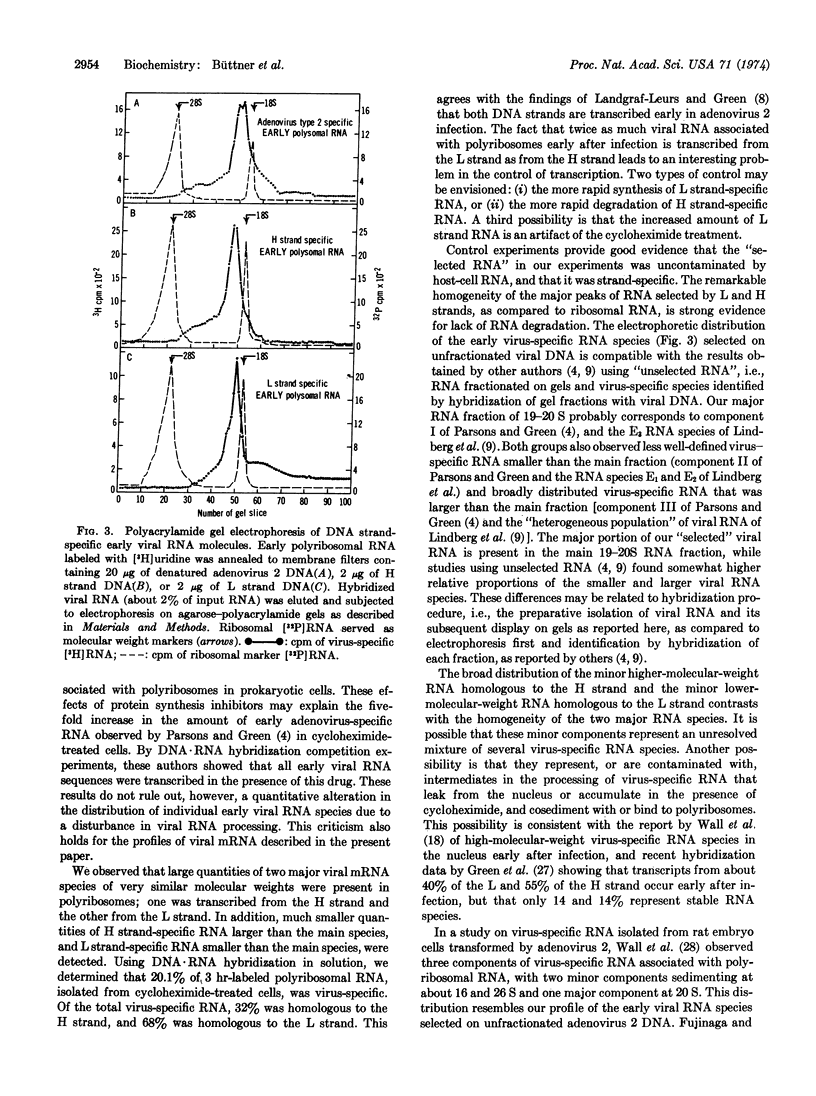
Hybridization to the separated light (L) and heavy (H) strands of adenovirus 2 DNA in 50% formamide at 37° was used to isolate undegraded virus-specific RNA molecules from the polyribosomes of cycloheximide-treated human KB cells early after infection with adenovirus 2. About 20% of polyribosomal RNA labeled with [3H]uridine from 4 to 7 hr after infection was virus-specific. Twice as much labeled RNA was homologous to the L strand as to the H strand. Polyacrylamide gel electrophoresis of RNA selected with unfractionated adenovirus DNA resolved a major component of virus-specific RNA in the 19-20 S region of the gel and smaller amounts of viral RNA in two heterogeneous fractions migrating at 15-18 S and 21-26 S. Selection with individual DNA strands showed that the 19-20 S main size class of early mRNA consists of two homogeneous RNA species with slightly different mobilities, the transcripts from the L and H strand having molecular weights of 7.4 × 105 and 7.7 × 105, respectively. The 15-18 S RNA hybridized with the L strand and the 21-26 S RNA with the H strand.
Keywords: DNA·RNA hybridization, gel electrophoresis, cycloheximide
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Robertson F. W., Burns J. A., Melli M. Methods for the analysis of deoxyribonucleic acid-ribonucleic acid hybridization data. Biochem J. 1969 Nov;115(3):361–370. doi: 10.1042/bj1150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Kung G., Bekhor I. A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry. 1967 Dec;6(12):3650–3653. doi: 10.1021/bi00864a005. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Mak S., Green M. A method for determining the fraction of the viral genome transcribed during infection and its application to adenovirus-infected cells. Proc Natl Acad Sci U S A. 1968 Jul;60(3):959–966. doi: 10.1073/pnas.60.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Apirion D., Schlessinger D. Polyribosome metabolism in Escherichia coli treated with chloramphenicol, neomycin, spectinomycin or tetracycline. J Mol Biol. 1969 Oct 28;45(2):205–220. doi: 10.1016/0022-2836(69)90100-4. [DOI] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Isolation of phi X174 specific messenger ribonucleic acids in vivo and identification of their 5' terminal nucleotides. J Virol. 1972 Feb;9(2):207–215. doi: 10.1128/jvi.9.2.207-215.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. Adenovirus DNA. 3. Separation of the complementary strands of adenovirus types 2, 7 and 12 DNA molecules. J Mol Biol. 1971 Aug 28;60(1):185–202. doi: 10.1016/0022-2836(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. DNA strand selection during the transcription of the adenovirus 2 genome in infected and transformed cells. Biochim Biophys Acta. 1973 Jul 27;312(4):667–673. doi: 10.1016/0005-2787(73)90070-1. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Congruent transcriptional controls and heterology of base sequences in coliphages lambda and phi-80. Virology. 1969 Nov;39(3):373–388. doi: 10.1016/0042-6822(69)90085-3. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Covalently linked cell and SV40-specific sequences in an RNA from productively infected cells. Virology. 1972 Nov;50(2):558–566. doi: 10.1016/0042-6822(72)90407-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]



