Abstract
Kerion celsi is rarely associated with Microsporum audouinii infection. We report the case of a 3-year-old girl with a kerion celsi caused by M. audouinii and successfully treated with oral terbinafine. Fungi identification was made by macro and microscopical colony morphology analyses and molecular (genotypic) studies.
Keywords: Kerion, Child, Microsporum
1. Introduction
Tinea capitis may have a range of clinical manifestations, from mild descamative to highly inflamed lesions such as kerion celsi. Kerion of the scalp usually occurs in individuals infected with zoophilic dermatophytes, but occasionally a geophilic organism is isolated and rarely it can be caused by anthropophilic fungal infections [1].
We report an unusual case of a 3-year-old girl with a kerion celsi caused by an anthropophilic dermatophyte, Microsporum audouinii, successfully treated with oral terbinafine.
2. Case
A 3-year-old Caucasian healthy girl, with an unremarkable past medical history, presented since day −60 a history of scaly pruriginous plaques on the scalp unsuccessfully treated with ciclopiroxolamin and zinc pyrithione shampoo and lately with a topical preparation containing betamethasone valerate, urea and salicylic acid. At day −5 she was admitted to the pediatric emergency room with fever and extensive inflamed scalp lesions. Laboratory analyses showed leukocytosis (leukocyte count of 27,000 cells/lL) and serum C-reactive protein level of 6.56 mg/dL. The remaining blood tests, urinalysis and chest radiography revealed no abnormalities, and immunodeficiency states were excluded. She was started empirically on oral flucloxacilin and fluconazole but as there was no clinical improvement she was referred to our Pediatric Dermatology outpatient clinic where she was examined at day 0. Physical examination demonstrated a large infiltrated 8-cm×10-cm red plaque with pustules on skin surface, exudative, with overlying crust, surrounded by adjacent edematous, erythematous infra-centimetrical plaques, located in the right temporo-parietal area as well as small, bilateral, mobile, nontender, and clearly demarcated palpable cervical lymphadenopathy. The hairs were matted with mucopurulent exudate so hair loss was difficult to identify but they were easily pulled out of the hair follicle and Wood's lamp examination revealed green fluorescence. Samples were promptly collected at day 0 for mycological studies (direct microscopic examination with potassium hydroxide solution and subsequent culture on Sabouraud Dextrose Agar Medium). The direct microscopic examination at day 0 revealed microsporum-type endo-ectothrix parasitism on some hairs. The diagnosis of a kerion was made, and anti-fungal treatment was changed at day 0 to a 10-week course of oral terbinafin at 4.5 mg/kg per day (62.5 mg/day) Fig. 1 and Fig. 2. We opted to maintain flucloxacilin till day +5 and add a short course of oral prednisolone at 1 mg/kg per day (12.5 mg/day) from day 0 till day +8. Treatment was very well tolerated and without systemic adverse effects. Recommendations about the use of non-pharmacological anti-infective measures such as not sharing combs, towels and other hair products, were made. All the cohabitants of the child were also examined and they started sertaconazole 2% shampoo to reduce the risk for transmission from asymptomatic carriers, although we did not identify any infected individual among them.
Fig. 1.
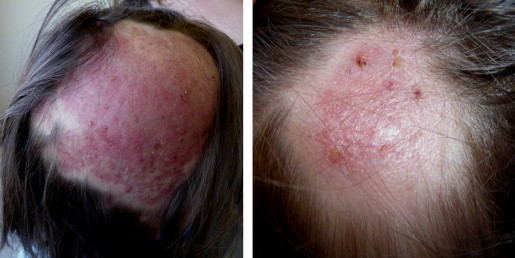
Clinical course (day +30).
Fig. 2.
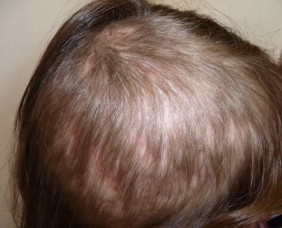
Clinical course (day +110).
Bacterial cultures of the drainage taken at day 0 were negative. At day +5 of incubation a culture of dermatophytes grew on Sabouraud Dextrose Agar, identified as M. audouinii by conventional and molecular methods Fig. 3 and Fig. 4. At follow-up visit on day +15 the appearance of her scalp was improved, however large areas of alopecia were observed. We added sertaconazole 2% shampoo and petrolatum for removing the residual crusts. The patient was clinically and mycologically cured at day +70 and alopecia completely resolved in two months Fig. 1 and Fig. 2.
Fig. 3.
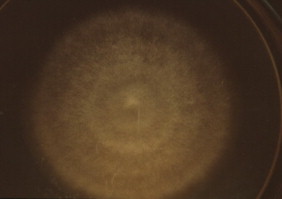
Fungal colony (Sabouraud Dextrose Agar Medium, 26 °C, day +15).
Fig. 4.
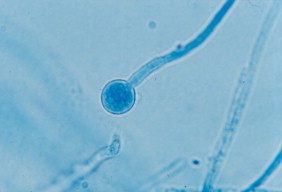
Slide-culture examination.
3. Discussion
Nowadays, we are facing changes in dermatophytoses' causative agents in Europe, mainly because of immigration [2–4]. In Portugal, anthropophilic species are increasing. Currently, M. audouinii and Trichophyton soudanense are the most frequently isolated species in patients with tinea capitis in Lisbon Metropolitan Area [3]. Tinea capitis caused by anthropophilic dermatophytes may exist in a quite suppressed state for weeks and if a high degree of hypersensitivity develops it may suddenly become inflammatory and develop into kerion [1]. The possibility of a kerion celsi caused by an anthropophilic infection, although unusual, should be considered [5].
The strain isolated in our patient's lesions was characterized by microscopic examination and culture characteristics, showing morphological and physiological features compatible with M. audouinii Fig. 3 and Fig. 4. The disparities that can be seen between different manifestations of the same underlying infection raise the question whether all strains of M. audouinii have similar virulence. Even drug resistance is possibly related to particular genotypes. The dermatophytes polymorphisms and strains variations are significant and may disclose particular different virulence factors, possibly because of different keratinolytic proteinases implicated, an important subject to further explore in future research studies [6–8]. We confirmed the species using molecular genetic methods. Direct DNA sequencing (Macrogen® Inc, Korea) showed a high filogenetic homology in the internal transcribed spacer region of ribosomal RNA gene (ITS1, 5.8 S, ITS2 of rDNA) for M. audouinii (GenBank: JX101949.1), confirming the diagnosis [9].
The main treatment of kerion celsi requires prompt systemic therapy, because topical agents do not penetrate in the hair follicle [10,11]. Griseofulvin is the treatment of choice for cases caused by Microsporum species, and it has a long-term safety profile [12]. Nevertheless, as it was not easily available for prescription in our country, we opted to give terbinafine, a broad-spectrum agent that have been shown to be comparable in efficacy and safety with griseofulvin, with good results in our patient and no adverse effects [10,11]. Invariably, the excessive degree of inflammation is detrimental and may lead to permanent scarring hair loss. Although in the past the use of corticosteroids have been thought to minimize the risk of permanent alopecia, current evidence does not suggest any reduction in clearance time compared with only systemic anti-fungal agents [11].
Our case report draws attention to certain particular aspects of this diagnosis. The help of a careful anamnesis and a complete physical examination are crucial to identify a fungal infection as a clinical diagnostic hypothesis. It is essential to promptly obtain scrapings of scale and pluck loose hairs for establishing the diagnosis, which may be otherwise misdiagnosed. The identification of the species and strain of the dermatophyte causing the infection is important to quickly start appropriate systemic treatment, reducing the inflammation, minimizing damage and the possibility of permanent scarring hair loss.
Conflict of interest
There are none.
References
- 1.Hay R.J., Ashbee H.R. Mycology. In: Burns T., Breathnach S., Cox N., Griffiths C., editors. Rook's textbook of dermatology. 8th edition. Wiley-Blackwell; Oxford: 2010. pp. 36.1–36.93. [Google Scholar]
- 2.Seebacher C., Bouchara J.F., Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–352. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 3.Roque H.D., Vieira R., Rato S., Luz-Martins M. Specific primers for rapid detection of Microsporum audouinii by PCR in clinical samples. Journal of Clinical Microbiology. 2006;44:4336–4341. doi: 10.1128/JCM.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilaberte Y., Rezusta A., Coscojuela C. Tinea capitis in a newborn infected by Microsporum audouinii in Spain. Journal of the European Academy of Dermatology and Venereology. 2003;17:239–240. doi: 10.1046/j.1468-3083.2003.00577_10.x. [DOI] [PubMed] [Google Scholar]
- 5.Ikutomi M., Nishikawa T., Nakayama H. A case of kerion celsi due to Microsporum audouinii. Nihon Ishinkin Gakkai Zasshi. 1980;21:184–187. [Google Scholar]
- 6.Giddey K., Favre B., Quadroni M., Monod M. Closely related dermatophyte species produce different patterns of secreted proteins. FEMS Microbiology Letters. 2007;267:95–101. doi: 10.1111/j.1574-6968.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 7.Monod M., Capoccia S., Lechenne B., Zaugg C., Holdom M., Jousson O. Secreted proteases from pathogenic fungi. International Journal of Medical Microbiology. 2002;292:405–419. doi: 10.1078/1438-4221-00223. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R., de Hoog S., Presber W., Gräser Y. A virulent genotype of Microsporum canis is responsible for the majority of human infections. Journal of Medical Microbiology. 2007;56:1377–1385. doi: 10.1099/jmm.0.47136-0. [DOI] [PubMed] [Google Scholar]
- 9.Li H.C., Bouchara J.P., Hsu M.M.L., Barton R., Su S., Chang T.C. Identification of dermatophytes by sequence analysis of the rRNA gene internal transcribed spacer regions. Journal of Medical Microbiology. 2008;57:592–600. doi: 10.1099/jmm.0.47607-0. [DOI] [PubMed] [Google Scholar]
- 10.Kakourou T., Uksal U. European society for pediatric dermatology. Guidelines for the management of tinea capitis in children. Pediatric Dermatology. 2010;27:226–228. doi: 10.1111/j.1525-1470.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 11.Higgins E.M., Fuller L.C., Smith C.H. Guidelines for the management of tinea capitis. British Journal of Dermatology. 2000;143:53–58. doi: 10.1046/j.1365-2133.2000.03530.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A.K., Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatric Dermatology. 2013;30:1–6. doi: 10.1111/j.1525-1470.2012.01866.x. [DOI] [PubMed] [Google Scholar]


