Abstract
Coccidioidomycosis is a fungal disease affecting humans and other mammals caused by the soil-dwelling fungi Coccidioides immitis and C. posadasii. Abortion due to Coccidioides spp. infection is rare in domestic animals and transplacental transmission is considered uncommon in women. This report describes a case of placental-fetal infection and abortion in an alpaca with disseminated C. posadasii infection. PCR amplification and DNA sequencing were used to confirm the etiology, C. posadasii, in fetal tissues.
Keywords: Coccidioides spp., Abortion, Alpaca, Camelids
1. Introduction
Coccidioidomycosis is a fungal disease affecting humans and other mammals caused by the soil-dwelling fungi Coccidioides immitis and C. posadasii. The infection is usually acquired in the Americas. The fungus is endemic in southwestern states of the United States, with the highest incidence in Arizona, California, Texas, and New Mexico [1]. It is generally accepted that the two species, C. immitis and C. posadasii, populate different geographic regions. C. immitis is more commonly found in central and southern California and Mexico, while C. posadasii has a broader region of endemicity, from central and southern Arizona to western Texas and southern New Mexico as well as areas of Central and South America [2].
Infection occurs normally via inhalation of arthroconidia, although transmission by direct inoculation may also occur. Horizontal (individual to individual) or vertical (mother to embryo/fetus) transmissions are considered very rare [1]. Coccidioidomycosis consists of a spectrum of disease ranging from a mild, self-limited, febrile illness to severe, life-threatening disease. While pregnancy is a risk factor for the development of severe and disseminated coccidioidomycosis, no cases of abortion due to coccidioidomycosis have been reported in humans [3]. South American camelids, such as llamas (Lama glama) and alpacas (Vicugna pacos), are very susceptible to coccidioidal infection and clinical cases usually present with severe respiratory and/or disseminated infection [4]. However, transplacental fetal infection and subsequent abortion has not been previously reported in South American camelids and is rare in other mammals [5,6]. This report describes a case of placental and fetal coccidioidomycosis and subsequent abortion in an alpaca with the disseminated form of the disease. PCR amplification and DNA sequencing were used to confirm the etiology, C. posadasii, in fetal tissues.
2. Case
An aborted 9-month gestation alpaca fetus and placenta were submitted for necropsy and diagnostic work-up to the California Animal and Health Safety Laboratory, San Bernardino branch. The abortion occurred while the herd was located in Somis, Ventura County, California. While the aborted dam never left California, other camelids on the premise had traveled briefly to shows in Arizona, New Mexico, Utah and Nevada. No other alpaca abortions were reported on the premise in recent past or in several weeks following this abortion. Prior cases of coccidioidomycosis occurring at the same farm included an adult alpaca, an 11-day old alpaca cria, and a guard dog. A full postmortem examination of the fetus, and gross examination of the placenta were performed and samples of heart, lungs, kidneys, liver, spleen, adrenal gland, gastrointestinal tract, brain, tongue, skin, skeletal muscle, eye and placenta were collected and fixed by immersion in 10% buffered formalin, pH 7.4, for approximately 24 h before preparing 4 µm thick histological sections. These were subsequently stained with hematoxylin and eosin, and a few select sections were additionally stained with GMS and PAS.
The fetus was in a mild to moderate state of post-mortem decomposition. Roughly spherical, slightly raised, white-grey and firm, 0.2–1 cm diameter nodules were detected (Fig. 1a and b) widely scattered throughout the lungs, spleen and intercostal skeletal muscles, less numerous on the diaphragm and skin, and rare in the liver and heart. The skin nodules were observed on the face, ventral abdomen, perianal region, and rear legs. The eyes showed marked external corneal opacity (edema) and a mid-sagittal section revealed anterior synechia and multiple white nodules expanding the iris, ciliary body and cornea. The placenta had many irregular, roughly round (~2–3 cm diameter) areas of hyperemia and hemorrhage covered by a fibrinous exudate on the chorionic and allantoic surfaces (Fig. 1c).
Fig. 1.
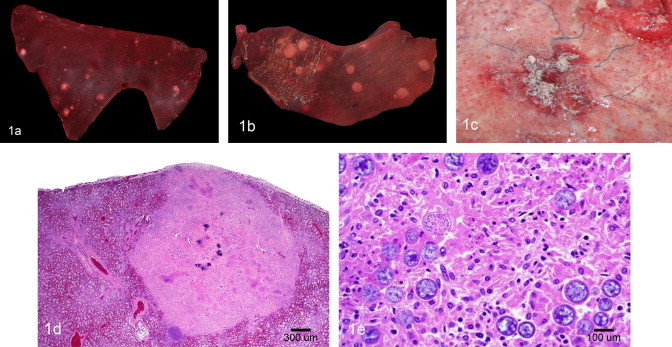
(a) Fetal lung depicting multifocal, variably sized, round, elevated, tan pyogranulomas. (b) Fetal spleen showing multifocal, variably sized, round, elevated, tan pyogranulomas. (c) Placenta showing a focal area of hyperemia and hemorrhage covered by a fibrinous exudate. (d) Microphotograph of the fetal lung showing a large, centrally mineralized pyogranuloma. Hematoxylin and eosin stain. (e) Higher magnification of Fig. 1d depicting numerous fungal spores compatible with Coccidioides spp. Hematoxylin and eosin stain.
Histologically, the lesions of the fetal and placental tissues consisted of multifocal pyogranulomas composed of a core of degenerate and viable neutrophils admixed with cellular debris (Fig. 1d). These were surrounded by numerous epithelioid macrophages and a few lymphocytes, plasma cells and multinucleate giant cells, often admixed with hemorrhage, fibrin, edema and cellular debris. There were large numbers of round, 60–100 μm fungal spherules (sporangia) with a 4–5 μm thick refractile and hyaline double wall present in most tissues examined. These were either within or around the pyogranulomas and occasionally within the cytoplasm of surrounding multinucleate giant cells (Fig. 1e). The sporangia contained flocculent basophilic to amphophilic material and, occasionally, multiple 5–7 μm endospores. The spherules stained positive with GMS and PAS. The pyogranulomatous inflammation with intralesional spherules in the eyes was centered in the iris and extended onto the ciliary body and cornea. In the brain, pyogranulomas with spherules were observed in the leptomeninges and cerebral parenchyma. Additional laboratory tests of the fetal lung included immunohistochemistry for equine herpesvirus type-1, bovine herpesvirus type-1, and bovine viral diarrhea virus. The results were all negative.
A serum sample obtained from the dam at the time of the abortion was tested at the coccidioidomycosis serology laboratory at the University of California, Davis. The serum was positive for coccidioidal IgG antibodies by qualitative immunodiffusion (in-house assay) and the titer determined by quantitative immunodiffusion (in-house assay) was 1:256, which is interpreted as an indicator of disseminated coccidioidomycosis. The dam was euthanized. A postmortem examination and histopathology revealed disseminated coccidioidomycosis severely affecting the lungs (Fig. 2) and serosal membranes of the thoracic cavity, and mildly involving other organs such as the liver, kidneys, and uterine neck and horns. Fungal spherules similar to those described for the fetus were scant and were only seen within a few of the pyogranulomas in the lungs and liver, but were not seen within the pyogranulomas of the kidneys or uterus. The dam was seropositive for bluetongue virus by a cELISA (VMRD Inc.) and seronegative for Brucella abortus by diagnostic card test (National Veterinary Services Laboratories), bovine viral diarrhea virus type-1 and 2 by serum viral neutralization (CAHFS in-house assay), equine herpesvirus type-1 by serum viral neutralization (CAHFS in-house assay), Toxoplasma by latex agglutination (Eiken Chemical Co., Ltd.), and Leptospira canicola, L. grippotyphosa, L. hardjo, and L. pomona by the microscopic agglutination test (National Veterinary Services Laboratories).
Fig. 2.
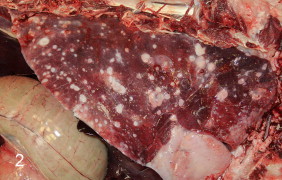
Dam's lung showing widespread, multifocal, granulomatous pneumonia caused by Coccidioides spp.
At the time of necropsy, additional samples of lung from the dam and fetus, and placenta were fixed in formalin for approximately 12 h and then kept in ethanol. DNA isolation and real-time PCR testing for Coccidioides was performed by the UC Davis Real-time PCR Research and Diagnostics Core Facility (UC Davis). DNA was isolated from a pea size piece of each fixed tissue that was previously soaked in PBS for 90 min to remove excess formalin. Tissues were mechanically ground, placed into QIAGEN Binding Reagent with proteinase K (Germantown, MD), and incubated at 56 °C for 15 min. DNA was isolated using the QIAGEN QIAxtractor. This DNA was used as the template for real-time PCR and nested endpoint PCR.
The Coccidioides real-time PCR assay (RT-Coccy; UC Davis Real-time PCR Research and Diagnostic Core Facility, UC Davis) uses primers and probe that target the ribosomal DNA internal transcribed spacer (ITS) region and indicated that Coccidioides DNA was present in both the fetal lung and placenta samples (CT value 36.74 and 38.24 respectively). Coccidioides DNA was not detected in the dam's lung tissue (CT value 40). The 18S ribosomal gene, used as the quality control gene, was positive for all samples.
Ribosomal DNA was amplified for sequence analysis using endpoint nested PCR as previously described by Baptista-Rosas with minor modifications [7]. Amplification was performed using 5 units of AmpliTaq Gold DNA polymerase (Life Technologies, Grand Island, NY) and 100 μl reactions. The sequence of primer NSA3-2013 (5′-AAACCCTGTCGTCGTGGGGATA-3′) was changed from that published [8] to reflect the Coccidioides consensus sequence rather than the subkingdom Dikarya. The cycling conditions for the first amplification step (Nest 1) were 1 cycle of 94° for 10 min; 35 cycles of 94°for 30 s, 55° for 40 s, and 72° for 40 s; and 1 cycle of 72° for 10 min. The cycling conditions for the second amplification step (Nest 2) were 1 cycle of 94° for 10 min; 35 cycles of 94°for 30 s, 60° for 40 s, and 72° for 40 s; and 1 cycle of 72° for 10 min. The cycling conditions of the “Coccidioides spp. specific ITS2 region” (Nest 3) were as published with the exception that the samples were heated to 94° and the template (product of the Nest 2 amplification) was diluted 1/10 [7].
PCR products were purified using the QiaQuick PCR purification kit (QIAGEN Sciences, Valencia, CA) or the QiaQuick Gel extraction kit (QIAGEN Sciences) and then either sequenced directly or cloned into the Escherichia coli vector pCR 2.1 (Invitrogen, Carlsbad, CA) and the recombinant plasmid sequenced. Sequencing was performed by the UC Davis College of Biological Sciences DNA Sequencing Facility (Davis, CA). Resulting sequences were compared and aligned with those deposited in the NCBI GenBank database using the Basic Local Alignment Search Tool (BLAST) [9], CLUSTAL OMEGA (http://www.ebi.ac.uk/Tools/msa/clustalo), and EMBOSS Needle (http://www.ebi.ac.uk/Tools/psa/emboss_needle/nucleotide.html).
The nested endpoint PCR yielded products from only the fetal lung tissue. Direct sequencing of the fetal lung Nest 3 PCR product yielded a 167 base sequence that was 100% identical to those of Coccidioides in GenBank as determined by BLAST. The product of the Nest 2 PCR reaction was cloned and four clones sequenced. The sequence of one clone differed from the others at a single site (C versus T). The ribosomal RNA gene and internal transcribed spacer are present in multiple copies in fungi. As such, intragenomic variation within these regions become apparent when the PCR products are cloned and the recombinant plasmids sequenced [10]. However, both sequences had 99% identity to Coccidioides (accession AB232885 and others) in GenBank. Species determination was made using the method of Tintlenot et al. rather than comparison with the sequences that are present in GenBank [11]. The amplified sequences contained bases at each phylogenetically informative site that was consistent with C. posadasii (Table 1). Sequences have been submitted to GenBank and assigned accession numbers KF373786 and KF373787.
Table 1.
Determination of Coccidioides species using phylogenetically informative sites within the ITS1 and ITS2 region.
3. Discussion
To our knowledge, this is the first case of abortion due to coccidioidomycosis in camelids and the first confirmed case of C. posadasii infection in animals in southern California, an area where C. immitis is more commonly reported [1,2]. The diagnosis of disseminated coccidioidomycosis in the fetus, placenta, and the dam was based on gross and microscopic lesions and supported by serology of the dam. Real-time and nested endpoint PCR and DNA sequencing were used to confirm the species of Coccidioides involved, i.e. C. posadasii, in fetal tissues. Multiple attempts to identify Coccidioides spp. DNA in maternal tissues by PCR were unsuccessful, perhaps due to the paucity of spherules found within the lesions. However, considering the presence of placental lesions, the disseminated infection in the dam and the fetus, and the identification of C. posadassi in the latter, it is most likely that the dam was infected by the same species of Coccidioides.
Abortion due to Coccidioides infection has been reported in two mares [6,12] and there is only sporadic anecdotal evidence of occurrence in other domestic animals. While pregnancy is considered to be a risk factor for severe disseminated coccidioidomycosis of the mother in humans, fetal or neonatal coccidioidomycosis is uncommon [1,3,5]. Reports of human neonatal coccidioidomycosis have suggested that aspiration of infected vaginal secretions during the birth may be the mode of transmission [13,14]. Previously, it was presumed that transplacental infection did not occur in women since even when extensive coccidioidal placentitis was observed, there was no observed disease in the fetus [13–16]. However, a more recent report supports the possibility of transplacental transmission [5]. In the present case, the presence of disseminated coccidioidomycosis in the dam, the severe lesions and presence of numerous spherules in the placenta, and disseminated coccidioidomycosis in the aborted fetus indicate in utero infection, either via aspiration of contaminated amniotic fluid or by hematogenous spread. Placental insufficiency and/or fetal disease may have resulted in the abortion.
The placenta in both mares and South American camelids is classified as “diffuse epitheliocorial”, whereas in humans it is “discoid hemochorial”, taking into account the shape and area of contact between fetal and maternal tissue and the maternal layers retained [17–19]. It is not known if the different types of placenta increase or decrease the probability of transplacental Coccidioides infection and determination would require additional studies.
The identification of C. posadasii as the cause of the fetal infection is of epidemiological significance, as the region where this abortion occurred (i.e. Southern California) is usually considered endemic for C. immitis. The aborted dam in this case had never been in areas considered endemic for C. posadasii, such as Arizona, southern New Mexico or western Texas. C. posadasii should therefore be considered as a possible cause of disease in South American camelids living in Southern California. The true prevalence of C. posadasii in animals in California needs to be further investigated.
Conflict of interest statement
The authors declared that they received no financial support for their research and/or authorship of this article.
Acknowledgments
We thank Ms. J. Beingesser and E.J. Hurley for excellent technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Brown J., Benedict K., Park B.J., Thompson G.R., III Coccidioidomycosis: epidemiology. Clin. Epidemiol. 2013;5:185–197. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M.C., Koenig G.L., White T.J., Taylor J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94(1):73–84. [PubMed] [Google Scholar]
- 3.Bercovitch R.S., Catanzaro A., Schwartz B.S., Pappagianis D., Watts D.H., Ampel N. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin. Infect. Dis.: An Off. Publ. Infect. Dis. Soc. Am. 2011;53(4):363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 4.Fowler M.E., Pappagianis D., Ingram I. Coccidioidomycosis in llamas in the United States: 19 cases (1981–1989) J. Am. Vet. Med. Assoc. 1992;201(10):1609–1614. [PubMed] [Google Scholar]
- 5.Charlton V., Ramsdell K., Sehring S. Intrauterine transmission of coccidioidomycosis. Pediatr. Infect. Dis. J. 1999;18(6):561–563. doi: 10.1097/00006454-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Stoltz J.H., Johnson B.J., Walker R.L., Pappagianis D. Coccidioides immitis abortion in an Arabian mare. Vet. Pathol. 1994;31(2):258–259. doi: 10.1177/030098589403100217. [DOI] [PubMed] [Google Scholar]
- 7.Baptista-Rosas R.C., Catalán-Dibene J., Romero-Olivares A.L., Hinojosa A, Cavazos T., Riquelme M. Molecular detection of Coccidioides spp. from environmental samples in Baja California: linking Valley Fever to soil and climate conditions. Fungal Ecol. 2012;5(2):177–190. [Google Scholar]
- 8.Martin K.J., Rygiewicz P.T., Fungal-specific P.C.R. primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005;5:28. doi: 10.1186/1471-2180-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 10.Simon U.K., Weiss M. Intragenomic variation of fungal ribosomal genes is higher than previously thought. Mol. Biol. Evol. 2008;25(11):2251–2254. doi: 10.1093/molbev/msn188. [DOI] [PubMed] [Google Scholar]
- 11.Tintelnot K., De Hoog G.S., Antweiler E., Losert H., Seibold M., Brandt M.A. Taxonomic and diagnostic markers for identification of Coccidioides immitis and Coccidioides posadasii. Med. Mycol.: Off. Publ. Int. Soc. Hum. Anim. Mycol. 2007;45(5):385–393. doi: 10.1080/13693780701288070. [DOI] [PubMed] [Google Scholar]
- 12.Langham R.F., Beneke E.S., Whitenack D.L. Abortion in a mare due to coccidioidomycosis. J. Am. Vet. Med. Assoc. 1977;170(2):178–180. [PubMed] [Google Scholar]
- 13.Spark R.P. Does transplacental spread of coccidioidomycosis occur? Report of a neonatal fatality and review of the literature. Arch. Pathol. Lab. Med. 1981;105(7):347–350. [PubMed] [Google Scholar]
- 14.Bernstein D.I., Tipton J.R., Schott S.F., Cherry J.D. Coccidioidomycosis in a neonate; maternal-infant transmission. J. Pediatr. 1981;99(5):752–754. doi: 10.1016/s0022-3476(81)80403-9. [DOI] [PubMed] [Google Scholar]
- 15.Cohen R. Placental coccidioides; proof that congenital coccidioides is nonexistent. Arch. Pediatr. 1951;68(2):59–66. [PubMed] [Google Scholar]
- 16.McCaffree M.A., Altshuler G., Benirschke K. Placental coccidioidomycosis without fetal disease. Arch. Pathol. Lab. Med. 1978;102(10):512–514. [PubMed] [Google Scholar]
- 17.David M., Iturrizaga F.T.V., Santos Tatiana C., Bombonato Pedro P., Geixeira Dulcinea G., Maria A.Miglino. Vol. 27. Pesquisa Veterinária Brasileira; 2007. The Materno-Fetal Interface in Llama (Lama Guanicoe Glama) pp. 221–228. [Google Scholar]
- 18.Pasquini S. Pasquini. placental types. In: Pasquini S., Pasquini, editors. Anatomy of Domestic Animals. 7th ed. Sudz publishing; Pilot Point, Texas, USA: 1996. [Google Scholar]
- 19.Dockery P., Bermingham J., Jenkins D. Structure-function relations in the human placenta. Biochem. Soc. Trans. 2000;28(2):202–208. doi: 10.1042/bst0280202. [DOI] [PubMed] [Google Scholar]


