Abstract
A 32 year old female presented with a 4 year history of on and off dry scaly lesions in the webspaces. KOH mount of the skin scrapings was positive for fungal hyphae. The fungus was isolated on Sabouraud dextrose agar and identified as Cylindrocarpon lichenicola. The patient was treated and responded to itraconazole.
Keywords: Cylindrocarpon lichenicola, Immunocompetent, Fusarium
1. Introduction
Cylindrocarpon species are saprophytic fungus known to inhabit an extensive range of woody and herbaceous plants and commonly present in the soi1 [1]. They occur on dead plant substrate or act as weak pathogens of plants [2,3]. They have been rarely associated with human disease [2]. They are known to cause post traumatic keratitis [4,5] and, have been implicated in mycetoma following injury [6,7], athlete's foot [8], peritonitis in a case of continuous ambulatory peritoneal dialysis [7], localised invasive lesion in a case of AML [2], disseminated infection in neutropenic patients [9]. The human infecting species include C. cyanescens, C. destructans, C. lichenicola and C. vaginae [1]. Among these Cylindrocarpon lichenicola is the only one known to induce invasive disease besides localised infection [10]. We hereby report a case of localised cutaneous lesion caused by this fungus in an immunocompetent patient.
2. Case
A 38 year old lady living in rural area of Sullia, Southern India visited Dermatology outpatient department (OPD) of our hospital (day 0). She complained of chronic paronychia with exacerbations and remissions ever since 4 years along with nail discolouration, now presented with acute episode of paronychia since 1 month. She also complained of itching in the webspaces of both hands since 3½–4 years. There is no diurnal variation of the symptoms. Patient gave history of exposure to water and detergent for 1–2 h every day. She gave no history of trauma but she has been working in the fields to clear the weeds and other bushes regularly on weekly basis with her bare hands. There might have been minute abrasions in her hand due to her work which the patient felt was insignificant. Not a diabetic or there is no history of atopy. No such complaints in other family members. Patient has been receiving on and off treatment since 4 years from various local hospitals but has not responded to any treatment.
On examination dry scaly lesions were noted bilaterally in all webspaces and skin over the proximal interphalangeal joints (Fig. 1). Lesions did not extend beyond webspaces as in case of scabies. No autoinoculation to any other cutaneous sites which rules out dermatophytic infections. There were no maceration or any white discharge like contact dermatitis and secondary infection with candida. No induration on palpation. Webspace scrapings and pus from paronychia was collected and sent to microbiology laboratory immediately for microscopy and culture investigations (bacterial and fungal culture). Patient was started on fluconazole 150 mg/weekly and ciprofloxacin 500 mg twice daily for one week and asked to revisit the OPD.
Fig. 1.
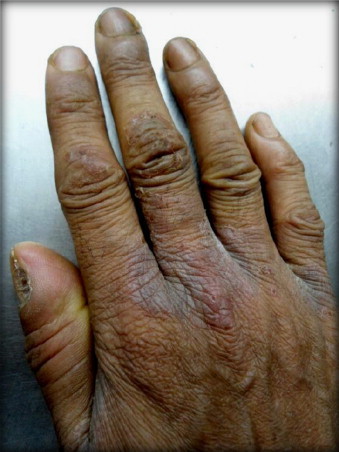
Dry scaly lesions in webspaces and skin over the proximal interphalangeal joints.
KOH mount of the scrapings, revealed numerous septate fungal hyphae. Specimen were inoculated on Sabourauds Dextrose Agar (SDA) (Hi-Media Laboratories Ltd, Mumbai) and incubated at 37° C and 25° C. Growth was observed on SDA by 4 days (day +3) at 25° C. Macroscopic features resembled Fusarium species. But microscopically features were different. The isolate was subsequently identified as C. lichenicola based on macroscopic and microscopic characters [2,11]. Colonies on SDA exhibited rapid growth reaching a diameter of 30 mm by 4 days (day +3) at 25° C. Colonies had white floccose aerial mycelium that became pale brown with age (Fig. 2). The reverse of the fungal colony on SDA exhibited diffusible pale reddish brown pigmentation. On slide culture hyphae were slender, hyaline and septate, conidiophore were long, simple or poorly branched (Fig. 3). Macroconidia produced from simple, subulate condiogenous cell (phialides). Macroconidia were colourless, ellipsoid or obovate to cylindrical, with a blunty rounded apex, distinctly truncate at the base with offset basal pedicel. Macroconidia lack the distinctive foot cell. They were borne singly or in clusters (Fig. 3), they have septa which are predominantly three but occassionaly upto five septa may be present (Fig. 4). Microconidia were absent. On day+13, abundant chlamydospore, 8–15 μm in diameter were produced. They occurred singly or in clumps most part formed terminally on short lateral branches or few intercalary [1] (Figs. 3 and 5). They were thick walled, globose, hyaline to pale brown [11] and usually 2–4 celled [1]. Further when grown at different temperature C. lichenicola revealed poor growth at 35° C and showed no growth at 42° C.
Fig. 2.
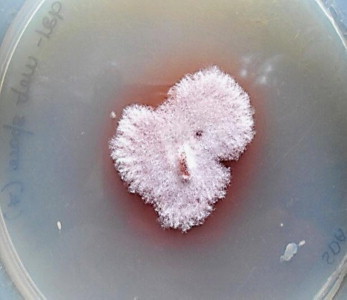
Colony of C. lichenicola on SDA after 4 days of incubation at 25° C.
Fig. 3.
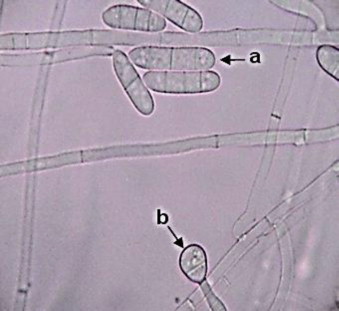
Slide culture showing (a) macroconidia and (b) terminal multicelled chlamydospore. Magnification, ×400.
Fig. 4.
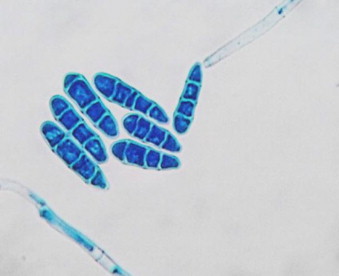
Lactophenol cotton blue mount showing cluster of macroconidia of C. lichenicola produced from a long simple conidiophore. Magnification, ×400.
Fig. 5.
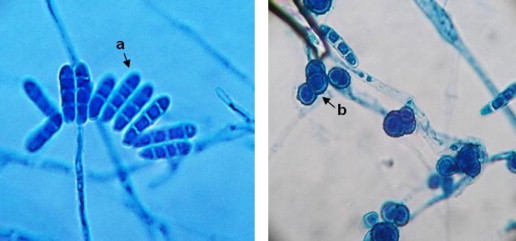
Lactophenol cotton blue mount showing (a) cluster of macroconidia with rounded apical cells and (b) intercalary multicelled chlamydospore of C. lichenicola. Magnification, ×400.
Patient revisited the hospital after 2 months (day +60). Paronychia had resolved but there was no improvement in the lesions of the webspaces. As the causative fungus was now identified patient was requested for other investigations which included haematological profile, X-ray of the hands and tests for HIV status. Reports showed no neutropenia, all other blood parameters were within normal limits, no bone involvement and tests for HIV were negative. Patient was now started on itraconazole 200 mg/day and asked to review after 1 month (day +90). On revisit after a month (day +90) the lesions had completely healed.
3. Discussion
Cylindrocarpon lichenicola was originally reported by Massalongo as Fusarium lichenicola and previous cases were reported under the name Cylindrocarpon tonkinense. Cylindrocarpon belong to the phylum ascomycetes under the family nectriaceae in the order hypocreales and class sardariomycetes [10]. It contains over 35 species and six varieties arranged into four groups depending upon the presence and absence of chlamydoconidia and microconidia[1,12]. C. lichenicola has a cosmopolitan geographic distribution. Even though uncommon in temperate regions it occurs frequently in tropic climate [2]. Cylindrocarpon species are soil fungus that may be saprophytic or facultative plant pathogen [13]. Cylindrocarpon thrives in the soil of Southern India, where temperature and humidity remain relatively high all year around [14]. Our patient has been working with bare hands in the fields. This might have caused minute abrasion on the skin sufficient to inoculate the fungus. Traumatic implantation of fungus contaminated plant material is known to cause the disease. This fungus has demonstrable keratinolytic properties and hence causes cutaneous lesions [15]. Patient being a nondiabetic, with normal neutrophil count and immunocompetent, the fungus appears to have remained at the inoculated sites with chronic cutaneous lesions without dissemination.
Cylindrocarpon species resembles Fusarium species morphologically and taxonomically, with both sharing telomorphs in the Genus Nectria and sometimes C. lichenicola misidentified as Fusarium solani. The spectrum of infections caused by Cylindrocarpon in humans is similar to F. solani infections including post traumatic keratitis, disseminated infections in immunocompromised host, peritonitis in patients on peritoneal dialysis and cutaneous lesions [13]. However, C. lichenicola has some specific morphological features which help in their identification as well as their differentiation from F. solani. The isolate differed microscopically from F. solani by forming macroconidia which are predominantly straight rather than curved, by having rounded apical cells rather than tapering and by having basal cells with truncate and offset rather than attenuated pedicel (foot cell), by lacking microconidia, by having pigmented chlamydoconidia, and by the formation of a brown rather than cream coloured colony on SDA [2]. Macroconidia lack the distinctive foot cell characteristic of Fusarium species [6]. Till date no mortality has been reported with Cylindrocarpon infections but they often lead to chronic infective conditions.
In vitro resistance to amphotericin B and itraconazole is observed [2,7,13]. Inspite of this, patients have responded to treatment with amphotericin B [2] and itraconazole [13]. Patients of acute myeloid leukaemia with disseminated infection with C. lichenicola responded to amphotericin B on marrow regeneration [2,9]. The therapeutic option in this case was amphotericin B or itraconazole. Amphotericin B is known to cause severe nephrotoxicity and also lesions were localised not disseminated, patient was advised 1 month of treatment with itraconazole for which she responded.
Summerbell and Schroers performed sequencing of large subunit region of ribosomal DNA and revealed that C. lichenicola is well-instituted within a clade of typical F. solani. As a result C. lichenicola known to be by its original name F. lichenicola [16]. Antifungal susceptibility testing and molecular studies are not done due to financial constraints.
Conflict of interest
There are none.
Acknowledgements
We are deeply thankful to Dr. K.V. Chidananda, Director, K.V.G. Medical College & Hospital, Sullia, for being supportive during the work up of this case.
References
- 1.Booth C., Clayto Y.M., Usherwood M. Cylindrocarpon species associated with mycotic keratitis. Proceedings of the Indian Academy of Sciences. 94. 1985;1985;22(3)(3):433–436. 433–436. [Google Scholar]
- 2.Iwen P.C., Tarantolo S.R., Sutton D.A., Rinaldi M.G., Hinrichs S.H. Cutaneous infections caused by Cylindrocarpon lichenicola in a patients with acute leukemia. Journal of Clinical Microbiology. 2000;38(9):3375–3378. doi: 10.1128/jcm.38.9.3375-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brayford D. Cylindrocarpon. In: Singleton L.L., Mihail J.D., Rush M., editors. Methods for research on soilborne phytopathogenic fungi. St Paul (MN): APS Press, 1993, p. 103–6.
- 4.Kaliamurthy J., Jesudasan C., Prasanth D.A., Thomas P.A. Keratitis due to Cylindrocarpon lichenicola. Journal of Postgraduate Medicine. 2006;52:155–157. [PubMed] [Google Scholar]
- 5.Gaujoux T., Borsali E., Gavrilov J.C., Touzeau O., Goldschmidt P., Despiau M.O. Fungal keratitis caused by Cylindrocarpon lichenicola. Journal Francais d'Ophtalmologie. 2012;35(5):356. doi: 10.1016/j.jfo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Zoutman D.E., Singler L. Mycetoma of the foot caused by Cylindrocarpon destructans. Journal of Clinical Microbiology. 1991;29(9):1855–1859. doi: 10.1128/jcm.29.9.1855-1859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma R., Farmer C.K., Gransden W.R., Ogg C.S. Peritonitis in continuous ambulatory peritoneal dialysis due to Cylindrocarpon lichenicola infection. Nephrology Dialysis Transplantation. 1998;13:2662–2664. doi: 10.1093/ndt/13.10.2662. [DOI] [PubMed] [Google Scholar]
- 8.Lancy B., Blanc C., Lapalu J. Cylindrocarpon: a new athlete's foot agent. Bulletin Trimestriel de la Société Mycologique de France. 1985;14:73–76. [Google Scholar]
- 9.James E.A., Orchard K., Mcwhinney P.H., Warnock D.W., Johnson E.M., Mehta A.B. Disseminated infection due to Cylindrocarpon lichenicola in a patient with acute myeloid leukemia. Journal of Infection. 1997;34:65–67. doi: 10.1016/s0163-4453(97)80012-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu D. Cylindrocarpon. In: Liu D., editor. Molecular detection of human fungal pathogens. CRC Press, Taylor & Francis Group; LLC: 2011. pp. 411–415. [Google Scholar]
- 11.Brayford D. Cylindrocarpon lichenicola. CMI description of pathogenic fungi and bacteria no. 926. Mycopathologica. 1987;100:125–126. doi: 10.1007/BF00467104. [DOI] [PubMed] [Google Scholar]
- 12.Booth C. The genus Cylindrocarpon. Mycological paper no. 104. Kew Surrey (England): Commonwealth Mycological Institute; 1996.
- 13.Villalobas H.R., Georgala A., Heymans H.B.C., Pye G., Crokaert F., Aoun M. Disseminated infection due to Cylindrocarpon (Fusarium) lichenicola in a neutropenic patient with acute leukaemia: Report of a case and review of the literature. European Journal of Clinical Microbiology & Infectious Diseases. 2003;22:62–65. doi: 10.1007/s10096-002-0851-9. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian C.V. Indian Council of Agricultural Research; New Delhi: 1971. Hypomycetes: an account of indian species. [Google Scholar]
- 15.Kaben U., Beck R., Tintelnot K. Keratomycosis due to Cylindrocarpon lichenicola. Mycoses. 2006;49:9–13. doi: 10.1111/j.1439-0507.2006.01318.x. [DOI] [PubMed] [Google Scholar]
- 16.Summerbell R.C., Schroers H.J. Analysis of relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. Journal of Clinical Microbiology. 2002;40(8):2866–2875. doi: 10.1128/JCM.40.8.2866-2875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


