Abstract
A 67 year-old Caucasian male from Arizona presented with indolent symptoms of intestinal obstruction and hydronephrosis, found at surgery to be caused by a mass involving the terminal ileum and cecum, extending into the posterior abdominal wall and obstructing the right ureter. Histopathology was diagnostic of basidiobolomycosis. PCR of tissue and sequencing identified the fungus as, Basidiobolus ranarum. During one year of posaconazole treatment, the residual mass shrank, hydronephrosis was relieved and peripheral eosinophilia resolved.
Keywords: Basidiobolomycosis, Posaconazole
1. Introduction
Basidiobolomycosis is a rare fungal infection caused by Basidiobolus ranarum, a member of the subphylum Entomophthoramycotina, previously included in the Zygomycete class [1]. While the organism may be found in soil throughout the world, basidiobolomycosis has been most commonly reported in tropical areas of Africa and Southeast Asia and in the tropical and subtropical regions of Asia, Australia and South America [1]. Typically, the infection manifests as a nodular, subcutaneous disease assumed to be related to minor trauma with an inoculated leaf or insect [1]. However, gastrointestinal basidiobolomycosis has been increasingly recognized; a recent review reported 44 cases worldwide including 19 in the United States [2]. Patients with gastrointestinal basidiobolomycosis often present with non-specific symptoms such as abdominal pain or distension from an obstructed viscus [2], [3]. Laboratory studies are notable for leukocytosis and peripheral eosinophilia [2] and imaging may reveal a gastrointestinal mass, frequently with extension to surrounding organs [3]. Histologically, characteristic features include granulomatous inflammation with tissue eosinophilia and broad, pauci-septate hyphae surrounded by a dense eosinophilic cuff, known as the Splendore–Hoeppli phenomenon. However, unlike mucormycosis, basidiobolomycosis does not demonstrate vascular invasion in tissue [4]. Treatment includes both surgery to relieve obstruction as well as prolonged antifungal therapy, usually with itraconazole, although the optimal regimen has not been established [2], [3]. Here, we present a case of gastrointestinal basidiobolomycosis treated with posaconazole after the disease recurred following surgical excision. To our knowledge, this is the first reported case using posaconazole in the management of this infection.
2. Case
A 67 year-old Caucasian male from Apache Junction, Arizona presented to a local hospital with a few weeks of increasing dull, right-sided abdominal pain (day 0). Abdominal computed tomography (CT) demonstrated a right abdominal mass with ureteral obstruction and hydronephrosis (Fig. 1A). Routine blood chemistry was notable for acute kidney injury, with serum creatinine (Cr) 3.3 mg/dL. Colonoscopy (day+5) revealed an obstructing lesion in the distal ileum. A percutaneous nephrostomy and ureteral stent were placed (day+7) with resultant improvement in serum Cr to 1.6 mg/dL, and the patient underwent resection of the abdominal mass as well as partial colectomy with ileocolic anastomosis (day+16).
Fig. 1.
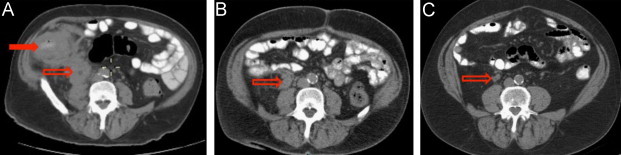
Serial abdominal imaging (computed tomography): (A) ileocecal mass (closed arrow) and periureteral mass (open arrow) with inflammatory changes and hydroureter prior to surgery (day 0); (B) recurrent periureteral mass (open arrow) three months after surgical excision (day+104); and (C) residual periureteral scar (open arrow) after one year of posaconazole therapy (day+524).
Histopathologic examination of the resected mass demonstrated broad, aseptate hyphae, tissue eosinophilia and the Splendore–Hoeppli phenomenon (Fig. 2). Because preoperative diagnosis was presumed to be malignant, no tissue was sent for culture. However, based on the histologic appearance, tissue was sent to the Centers for Disease Control and Prevention where the identification of the fungus as Basidiobolus ranarum was established by DNA extraction from formalin-fixed, paraffin-embedded tissue and polymerase chain reaction (PCR) amplification of the ITS-4 region of the ribosomal RNA gene with subsequent sequencing of the PCR product [5]. The top seven matches in the BLAST search of a 213bp PCR fragment from ITS-4 had 94–96% identity with Basidiobolus ranarum and other Basidiobolus species that are now considered synonymous with Basidiobolus ranarum.
Fig. 2.
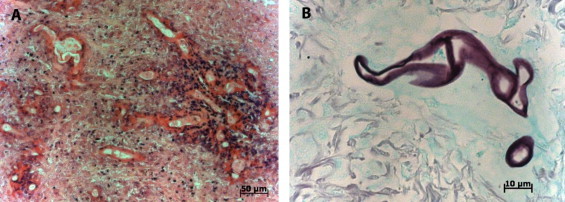
Histopathology of surgically resected abdominal mass: (A) hematoxylin and eosin stain, demonstrating hyphae with surrounding eosinophilic cuffing (Splendore–Hoeppli phenomenon); and (B) Gomori methenamine silver stain, showing pauci-septate hyphae.
On day+21, the patient was given fluconazole at 200 mg by mouth per day but he discontinued the drug after several months due to alopecia. The ureteral stent was removed on day+80. Follow-up abdominal CT three months after surgical resection (day+104) revealed return of the hydronephrosis with a right periureteral mass (Fig. 1B). He was referred to the National Institutes of Health (NIH) for further care of progressive gastrointestinal basidiobolomycosis. After providing informed consent, the patient was admitted to an NIH Institutional Review Board-approved protocol.
Past medical history was notable for diabetes mellitus (insulin-dependent) and cardiomyopathy, with placement of an automatic implantable cardioverter-defibrillator.
On presentation to our clinic (day +139), his chief complaint was easy fatigability; he had no specific abdominal or urinary symptoms. He was afebrile with normal vital signs, and physical exam was unremarkable apart from a well-healed, midline abdominal scar. Laboratory studies were notable for elevated C-reactive protein (9.25 mg/L; normal value<3 mg/L) and erythrocyte sedimentation rate (43 mm/h; normal value: 0–25 mm/h), as well as anemia (hemoglobin 10.1 g/dL; normal value: 13.7–17.5 g/dL), peripheral eosinophilia (1.05 K/uL; normal value: 0.04–0.54 K/uL) and reduced renal function (serum Cr 1.73 mg/dL; normal value: 0.77–1.19 mg/dL). Positron emission tomography with Fluorine-18-Fluorodeoxyglucose/CT scan of the abdomen showed tracer uptake in the right periureteral mass as well as right hydronephrosis, consistent with persistent infection.
The patient was started on posaconazole (200 mg by mouth four times per day) (day+139) but this was reduced to three times per day shortly thereafter by the patient due to alopecia, dry mouth and dry skin. Six months later (day+319), he was seen in follow up and was felt to be responding well to therapy, as evidenced by a reduction in the size of the right periureteral mass and improved hydronephrosis on CT scan, stable renal function, and normalization of inflammatory markers, hemoglobin and eosinophilia. He reported good adherence to posaconazole therapy without significant side effects; posaconazole trough level was considered adequate at 2.26 mcg/mL.
After one year of posaconazole therapy (day+524), repeat CT scan showed near resolution; there was a residual, 2 cm spiculated mass in the right retroperitoneum consistent with scar tissue, with no hydronephrosis or lymphadenopathy (Fig. 1C). Posaconazole was discontinued and the patient was discharged to the care of his local physicians for management of his chronic medical conditions.
3. Discussion
The pathogenesis of and risk factors for gastrointestinal basidiobolomycosis remain poorly understood. Although Basidiobolus ranarum may be found in soil worldwide, the gastrointestinal form of the disease has been reported predominately in the United States (US) and Saudi Arabia, with some additional cases in Brazil and Iran [2]. Notably, 17 of the 19 US patients were residents of Arizona [2], as was the case with our patient. Thus, there is suspicion that the arid climate common to these locations may be a contributing factor although the underlying mechanism remains unclear.
It is suspected that gastrointestinal basidiobolomycosis is acquired through ingestion of soil, animal feces or food contaminated by these materials [3]. The organism has been found in decaying plant material, compost heaps, and leaves of deciduous trees, and as intestinal carriage among lizards and other amphibians [6]. Our patient did report living in an “open” home with frequent visualization of lizards and geckos, although he did not recall any close contact with the animals. A case-control study conducted by Lyon et al. found only use of ranitidine to be a statistically significant risk factor, ostensibly because reduced gastric acidity leads to increased post-pyloric survival of the organism [3]. The study also observed that patients with gastrointestinal basidiobolomycosis had a longer pack-year-history of tobacco, and were more likely to have a vegetable garden, participate in “occupational digging”, or own a dog, though none of these risk factors reached clinical significance, possibly due to the low number of patients in each group (7 case patients, 28 control subjects) [3]. Interestingly, two case patients in that series lived in the same trailer park, further suggesting an environmental source of the infection (rather than an immunologic predisposition, for example) [3].
Supporting the supposition of a gastrointestinal portal of entry is the frequent finding on pathology of intestinal thickening with transmural inflammation [4], [7]. On endoscopy, cobblestoning of the intestinal mucosa has been described, similar to Crohn's disease [7], [8], [9]. Conversely, the superficial mucosa has been described as intact in several cases [3]. This may be analogous to the finding of epidermal preservation in cases of subcutaneous basidiobolomycosis [10], suggesting that the organism gains entry through a mucosal surface, but spreads through submucosal layers. There have been scant reports of intraabdominal basidiobolomycosis arising from a cutaneous focus [10], [11], but the majority of cases appear to start with an intramural mass with contiguous spread to surrounding organs, as occurred in our patient.
Although the gastrointestinal form of basidiobolomycosis is an increasingly reported manifestation of this unusual infection, the diagnosis often proves elusive due to the non-specific clinical presentation. In a recent review, abdominal pain was the most common presenting symptom (84%), followed by an abdominal mass (43%) and constipation (39%); fever was only present in 32% of cases [2]. Based on the radiographic appearance of an abdominal (usually colonic) mass with spread to surrounding organs, including fistulization, the disease often mimics colon cancer or Crohn's disease which may delay diagnosis [2], [8], [9].
Peripheral eosinophilia, as was present in our case, appears to be a prominent clinical feature; in a review of pediatric cases by El-Shabrawi and Kamal, all had elevated inflammatory markers and eosinophilia [12]. More recently, the review by Vikram et al. found peripheral eosinophilia in 76% of cases [2]. As in our patient, normalization of eosinophilia and inflammatory markers generally correlates with clinical improvement [3], [7]; it follows that persistence of peripheral eosinophilia should prompt the clinician to evaluate for an ongoing source of infection.
For definitive diagnosis, culture of Basidiobolus ranarum is considered the gold standard. Macroscopically, colonies are yellow–gray in color with a waxy appearance and many radial folds [3]. In tissue, pauci-septate hyphae may be seen (similar to mucormycosis but with no angioinvasion) [4]. There is often granulomatous inflammation with marked tissue eosinophilia and the Splendore–Hoeppli phenomenon, i.e. collections of eosinophilic granular material surrounding the fungal elements [2], [3], [4]. On culture, Basidiobolus ranarum may produce zygospores with a characteristic beaked prominence [3].
In culture negative cases, serology for Basidiobolus ranarum may have a role; based on limited studies, the sensitivity is estimated to be 50% [2] and titers may correlate with response to therapy [13]. Recently, sequencing of a PCR product from tissue has been increasingly utilized as an alternative means of diagnosis [14], [15]. In our case, the DNA for PCR was extracted from a paraffin-embedded surgical specimen, since no cultures were sent at the time of surgery due to low clinical suspicion for infection.
An optimal treatment regimen for this uncommon infection has not yet been established. Most patients have received a combination of surgical and medical therapies [2]. Although there have been some reports of clinical improvement with antifungal therapy alone [2], [8], [16], the long term prognosis in such cases remains unclear. Of the antifungal agents, itraconazole has been used most frequently (73%), followed by amphotericin (22%), ketoconazole (8%) and voriconazole (5%) [2], [3]. Potassium iodide and trimethroprim/sulfamethoxazole may also have some clinical efficacy [11]. On average, antifungal therapy has been implemented for 8 months, and overall survival is estimated at 80% [2]. Notably, the use of amphotericin has been associated with several clinical failures [2], [17], [18], and susceptibility testing has confirmed amphotericin resistance in some of these cases [2], [7].
The use of posaconazole in this infection has several potential advantages. The drug does not require dose adjustment for hepatic or renal insufficiency. It is well tolerated with few side effects and its absorption is not hampered by medications that affect gastric acidity [19]. However, possible limitations include cost, the lack of an intravenous formulation (for hospitalized patients), and the need for therapeutic monitoring due to variable absorption [19]. To our knowledge, this is the first reported case of gastrointestinal basidiobolomycosis treated with posaconazole in addition to surgical therapy. Moreover, the case demonstrates the importance of antifungal therapy in this infection as both clinical, laboratory and radiographic improvement (including relief of urinary obstruction) were only achieved following initiation of posaconazole.
Conflict of interest
There are none.
Acknowledgments
We would like to thank Mary Brandt, Chief of the Mycotic Diseases Branch at the Centers for Disease Control and Prevention, for helping to facilitate the molecular diagnosis in this case. We also appreciate the assistance of Janyce Sugui in preparing the microscopy images. The work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases at the National Institutes of Health. The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
References
- 1.Kontoyiannis D.P., Lewis R.E. Agents of mucormycosis and entomophthoramycosis. In: Mandell G.L., Bennett J.E., Dolin R., editors. Mandell, Douglas and Bennett's principles and practices of infectious diseases. Elsevier; Philadelphia: 2010. p. 3257–69. [Google Scholar]
- 2.Vikram H.R., Smilack J.D., Leighton J.A., Crowell M.D., De Petris G. Emergence of gastointestinal basidiobolomyosis in the United States, with a review of worldwide cases. Clinical Infectious Diseases. 2012;54(12):1685–1691. doi: 10.1093/cid/cis250. [DOI] [PubMed] [Google Scholar]
- 3.Lyon G.M., Smilack J.D., Komatsu K.K., Pasha T.M., Leighton J.A., Guarner J. Gastrointestinal basidiobolomycosis in Arizona: clinical and epidemiological characteristics and review of the literature. Clinical Infectious Diseases. 2001;32(10):1448–1455. doi: 10.1086/320161. [DOI] [PubMed] [Google Scholar]
- 4.Yousef O.M., Smilack J.D., Kerr D.M., Ramsey R., Rosati L., Colby T.V. Gastrointestinal basidiobolomycosis. Morphologic findings in a cluster of six cases. American Journal of Clinical Pathology. 1999;112(5):610–616. doi: 10.1093/ajcp/112.5.610. [DOI] [PubMed] [Google Scholar]
- 5.Munos-Cadavid C., Rudd S., Zaki S.R., Patel M., Moser S.A., Brandt M.E. Improving molecular detection of fungal DNA in formalin-fixed paraffin-embedded tissues: comparison of five tissue DNA extraction methods using panfungal PCR. Journal of Clinical Microbiology. 2010;48(6):2147–2153. doi: 10.1128/JCM.00459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gugnani H.C. A review of zygomycosis due to Basidiobolus ranarum. European Journal of Epidemiology. 1999;15(10):923–929. doi: 10.1023/a:1007656818038. [DOI] [PubMed] [Google Scholar]
- 7.Zavasky D.M., Samowitz W., Loftus T., Segal H., Carroll K. Gastrointestinal zygomycotic infection caused by Basidiobolus ranarum: case report and review. Clinical Infectious Diseases. 1999;28(6):1244–1248. doi: 10.1086/514781. [DOI] [PubMed] [Google Scholar]
- 8.Nemenqani D., Yaqoob N., Khoja H., Al Saif O., Amra N.K., Amr S.S. Gastrointestinal basidiobolomycosis: an unusual fungal infection mimicking colon cancer. Archives of Pathology and Laboratory Medicine. 2009;133(12):1938–1942. doi: 10.5858/133.12.1938. [DOI] [PubMed] [Google Scholar]
- 9.Saadah O.I., Farouq M.F., Daajani N.A., Kamal J.S., Ghanem A.T. Gastrointestinal basidiobolomycosis in a child; an unusual fungal infection mimicking fistulising Crohn's disease. Journal of Crohn's and Colitis. 2012;6(3):368–372. doi: 10.1016/j.crohns.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Radjou A.N., Rajesh N.G. Intestinal obstruction due to Basidiobolus ranarum: an unusual case. Indian Journal of Medical Microbiology. 2011;29(2):186–188. doi: 10.4103/0255-0857.81790. [DOI] [PubMed] [Google Scholar]
- 11.Choonhakarn C., Intraburan K. Concurrent subcutaneous and visceral basidiobolomycosis in a renal transplant patient. Clinical and Experimental Dermatology. Jul. 2004;2004;2929(4)(4):369–372. 369–372. doi: 10.1111/j.1365-2230.2004.01533.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Shabrawi M.H., Kamal N.M. Gastrointestinal basidiobolomycosis in children: an overlooked emerging infection? Journal of Medical Microbiology. 2011;60(7):871–880. doi: 10.1099/jmm.0.028670-0. [DOI] [PubMed] [Google Scholar]
- 13.Pasha T.M., Leighton J.A., Smilack J.D., Heppell J., Colby T.V., Kaufman L. Basidiobolomycosis: an unusual fungal infection mimicking inflammatory bowel disease. Gastroenterology. 1997;112(1):250–254. doi: 10.1016/s0016-5085(97)70242-7. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Muñoz M.T., Fernández-Barredo S., Martínez-Díaz R.A., Pérez-Gracia M.T., Ponce-Gordo F. Development of a specific polymerase chain reaction assay for the detection of Basidiobolus. Mycologia. 2012;104(2):585–591. doi: 10.3852/10-271. [DOI] [PubMed] [Google Scholar]
- 15.El-Shabrawi M.H., Kamal N.M., Jouini R., AL-Harbi A., Voigt K., Al-Malki T. Gastrointestinal basidiobolomycosis: an emerging fungal infection causing bowel perforation in a child. Journal of Medical Microbiology. 2011;60(9):1395–1402. doi: 10.1099/jmm.0.028613-0. [DOI] [PubMed] [Google Scholar]
- 16.Al Jarie A., Al Azraki T., Al Mohsen I., Al Jumaah S., Almutawa A., Mohd Fahim Y. Basidiobolomycosis: case series. Journal of Medical Mycology. 2011;21(1):37–45. doi: 10.1016/j.mycmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.van den Berk G.E., Noorduyn L.A., van Ketel R.J., van Leeuwen J., Bemelman W.A., Prins J.M. A fatal pseudo-tumour: disseminated basidiobolomycosis. BMC Infectious Diseases. 2006;15(6):140. doi: 10.1186/1471-2334-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan Z.U., Khoursheed M., Makar R., Al-Waheeb S., Al-Bader I., Al-Muzaini A. Basidiobolus ranarum as an etiologic agent of gastrointestinal zygomycosis. Journal of Clinical Microbiology. 2001;39(6):2360–2363. doi: 10.1128/JCM.39.6.2360-2363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagappan V., Deresinski S. Posaconazole: a broad-spectrum triazole antifungal agent. Clinical Infectious Diseases. 2012;54(12):1685–1691. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]


