Abstract
Aim
The primary objective of this study was to assess whether there was an improvement in QoL for patients with brain metastases after radiotherapy treatments.
Background
Assessment of quality of life (QoL) in brain metastasis patients has become increasingly recognized as an important outcome.
Materials and methods
Patients treated for brain metastasis in our department during 2010 were included in our prospective study. QoL assessments were conducted at baseline, 1 month, and 3 months after completion of whole-brain radiotherapy (WBRT). Wilcoxon test for multiple comparisons was calculated to detect significant differences in global QoL scores.
Results
Thirty-nine patients with brain metastases completed the EORTC QLQ-C30/BN-20 questionnaire independently. Median age was 59.9 years (from 37 to 81 years). Our results report differences between the baseline and 3 months in worsening of a global health status (p = 0.034) and cognitive function (p = 0.004), as well as drowsiness (p = 0.001), appetite loss (p = 0.031) and hair loss (p = 0.005). There is a tendency for deterioration of physical function (p = 0.004), communication deficit (p = 0.012), and weakness of legs (p = 0.024), between the baseline and 1 month evaluation. There was no difference in a global cognitive status between different evaluations. Median survival time was 3 months (CI 95% 1.85; 4.15).
Conclusions
Our findings indicate a small deterioration for a global QoL status, and large deterioration for cognitive function after radiation treatments, as well as worsening of brain metastasis related symptom items. Further research is necessary to refine treatment selection for patients with brain metastases, since it may at least contribute to the stabilization of their QoL status.
Keywords: Brain metastases, Quality-of-life, Radiotherapy, Whole brain radiotherapy
1. Background
Brain metastases represent one of the most common and refractory malignancies worldwide with a rising incidence in all countries.1 Approximately 10–30% of patients with cancer develop brain metastases during the course of their illness as an indicator of disease's progression.2,3 An increasing trend toward prolonged patient survival has been reported, especially for breast, lung, and kidney, as a result of more efficient regimens.4 The results of recent studies show that early identification and aggressive treatment can often ameliorate symptoms and increase both the survival and the quality of life (QoL). During the past two decades, technical advances have been made in diagnosis and treatment of brain metastases. The cornerstones of treatment are surgery, whole brain radiotherapy (WBRT), and radiosurgery (RS).5–7 Since the majority of patients present with multiple lesions or widespread metastatic disease, WBRT is a standard treatment to provide symptomatic relief, to allow for tapering of corticosteroid's dose, and possibly improve survival. Although many trials have shown that WBRT can reduce neurological symptoms, the median survival following the diagnosis of brain metastasis is generally only 2–4 months.8 With the evolution of treatment techniques (RS and improved surgical interventions), there is a growing interest in stratifying these patients into those that should be treated aggressively due to a potential for longer survival, and those who should be treated with simple WBRT because of their poor survival potential.9 Radiation Therapy Oncology Group (RTOG) database of clinical trials has developed the two most rigorous prognostic indices, the recursive partitioning analysis (RPA) and the graded prognostic assessment (GPA). RPA has been the gold standard for more than a decade, whereas GPA is its recent refinement.10–12
It is worth noting that most patients treated for brain metastases die of extracranial disease.13 This is an important consideration because, although most studies have used overall survival as the main endpoint, survival is probably not the best parameter to measure the efficacy of the existing therapeutic modalities.14 In this context, the assessment of QoL and neurocognitive function in patients with brain metastases has become increasingly recognized as an important addition to traditional outcome measures, such as length of survival and time to disease progression. The use of QoL outcomes could provide prognostic information, allowing identification of patients who will benefit from a specific intervention, preventing overtreatment of patients, or facilitating decision making for poorer prognosis patients less likely to benefit from WBRT.15,16 Therefore, it is imperative for clinicians to be familiarized with QoL tools and utilities, since palliative therapy courses should aim to improve or at least stabilize QoL.
2. Aim
Few studies have focused on QoL and cognitive assessments as primary outcome. The primary objective of this study was to assess QoL for patients with brain metastases measured 1 and 3 months after radiation therapy treatments. Secondary objectives were to evaluate disease survival and cognitive impairment after treatment.
3. Materials and methods
All patients with diagnosed brain metastases referred to receive WBRT during the year 2010 in the Radiotherapy Department at Instituto Português de Oncologia de Lisboa – Francisco Gentil were included in our prospective study. Ethics approval was obtained from the hospital research ethics board. Computerized tomography (CT) and/or magnetic resonance imaging (MRI) scanning of the brain was mandatory. Patients with language barrier or significant cognitive impairment were excluded. Suitable patients were considered for RS at an affiliated hospital. The dose of 30 Gy in ten fractions is a standard WBRT regimen at our center for patients with brain metastases. Proxies were not used because they have been shown to have a poor concordance with self-reports in the setting of brain metastases.
All patients were prescribed dexamethasone at varying doses during radiotherapy and were given a tapering schedule after completion of WBRT. Outcomes of patients were measured up to September 2011. All patients were asked to complete the European Organization of Radiotherapy treatments QoL questionnaire (EORTC QLC 30/BN20) independently at different times of evaluation.17 QoL assessments were conducted at baseline (before first day of WBRT), 1 month, and 3 months after completion of WBRT. Overall survival (OS) was calculated using Kaplan–Meier estimators and Mantel–Cox log rank test was used to detect significant differences between subgroups. QoL results are presented as mean scores and were compared between time points and between subgroups of patients using the Wilcoxon test to detect significant differences in global QoL scores. Neurocognitive function was assessed by the Mini Mental State Examination (MMSE) at each QoL evaluation. Patients were grouped according to the RPA and GPA classification. QLQ-C30 and BN20 instruments have been developed by the EORTC Quality of Life Study Group for measuring the QoL of cancer patients in clinical trials.17 The QLQC30 contains 30 items and covers the domains of physical, role, emotional, cognitive, and social function, as well as a global health status and several symptoms. The BN20 questionnaire is a brain-specific module to be used in conjunction with QLQ-C30 and contains 20 items, grouped into four domains (future uncertainty, visual disorder, motor dysfunction and communication deficit) as well as seven single items (headaches, seizures, drowsiness, hair loss, itchy skin, weakness of legs, bladder control). Questionnaire data was processed according to the procedures outlined in the EORTC QLQ-C30 scoring manual.18 Taking into account language validation and the more extended experience in the EORTC QLQ-C30/BN20, we chose this questionnaire as the best option to evaluate our patients’ QoL outcomes.
4. Results
From January to December 2010, 46 patients with brain metastases were referred for consideration of WBRT. Of these, 39 patients were included in the study. Seven patients were excluded because of language barrier, cognitive impairment impeding ability to participate, and a very low performance status.
No patient declined participation. Sixty-two percent of patients were female, and 38% were male, with 59.9 years median age (range, 37–81). Median baseline KPS score was 60 (range, 50–100). Patient demographics are shown in Table 1. The most common primary cancers were breast (41%) and lung (35.9%). Median time from primary diagnosis until brain metastasis diagnosis was 6.2 months. Seven patients were submitted to previous surgery with brain lesion resection before WBRT. Thirty one percent of patients presented with a single brain metastasis, 15.4% had two lesions, and 53.8% had three or more lesions. Median diameter of the largest lesion was 16.9 mm, the second largest was 9.1 mm, and third one, 6.8 mm. Twenty percent of patients were taking antiseizure medication. Forty-five percent of patients were taking dexamethasone at the time of initial consultation, with 21.6% having been prescribed 16 mg per day. All patients were prescribed dexamethasone at varying doses during radiotherapy. The median time between brain metastasis diagnoses and initial radiation therapy/surgery was 2 weeks, with a range from 6 days to 18 weeks. The most commonly used radiotherapy dose fractionation schedule was 30 Gy in ten fractions, with a median treatment duration of 13 days. Out of the 39 patients, 71.8% were treated with isolated WBRT, 17.9% had WBRT after metastasectomy and 10% were treated with RC after WBRT. All patients completed the EORTC QLQ-C30 and BN20 instruments at baseline, 24 (61.5%) at 1 month, and 19 (48.7%) at 3 months.
Table 1.
Patient characteristics at baseline (WBRT – whole brain radiotherapy; RPA – recursive partitioning analysis; GPA – graded prognostic assessment).
| Number of patients (n) | Percentage (%) | ||
|---|---|---|---|
| Median age (years) | 59.9 (37–81) | ||
| Primary tumor | Lung | 14 | 36.0 |
| Breast | 16 | 41.0 | |
| Melanoma | 2 | 5.1 | |
| Colorectal cancer | 2 | 5.1 | |
| Others | 5 | 12.8 | |
| RPA classification | I | 15 | 38.5 |
| II | 5 | 12.8 | |
| III | 19 | 48.7 | |
| GPA classification | 0–1 | 20 | 51.3 |
| 1.5–2.5 | 16 | 41.0 | |
| 3 | 3 | 7.7 | |
| 3.5–4 | 0 | 0 | |
| Karnofsky | 90–100 | 11 | 28.2 |
| performance status | 89–71 | 9 | 23.1 |
| <70 | 19 | 48.7 | |
| Number of lesions | 1 | 12 | 30.8 |
| 2–3 | 11 | 28.2 | |
| >3 | 16 | 41.0 | |
| Fractionation of WBRT | 30 Gy/10Fr | 37 | 94.9 |
| 20 Gy/5Fr | 2 | 5.1 | |
| Initial steroid dose (dexamethasone) | 4 mg | 21 | 56.8 |
| 5–15 mg | 8 | 21.6 | |
| >16 mg | 8 | 21.6 | |
| Extra-cranial | Not progressive | 13 | 33.3 |
| Tumor status | Progressive | 26 | 66.6 |
In QoL assessment, differences were observed from baseline evaluation to 1 month after treatment evaluation in worsening of global health status (p = 0.009), physical function (p = 0.004), cognitive function (p = 0.004), communication deficit (p = 0.012), drowsiness (p < 0.001), hair loss (p = 0.001), and weakness of legs (p = 0.024). Between 1 and 3 months after treatment, there was no statistical difference detected in QoL assessment. Between baseline and 3 months after completion of radiation treatments, differences were observed in deterioration of global health status (p = 0.034), cognitive functioning (p = 0.004), appetite loss (p = 0.031), drowsiness (p = 0.001), and hair loss (p = 0.005). All comparisons were made using the Wilcoxon test (Table 2 and Fig. 1). There were no differences in global cognitive status (MMSE) between baseline (median 24.9) and 3 months after radiation treatment (median 24.1). Twenty seven (69.2%) death events occurred during the study. The median survival time of the entire cohort is 3 months (CI 95% 1.85; 4.15).
Table 2.
Statistically significant changes in QoL before, and after whole brain radiotherapy (1–3 months).
| Baseline (T0) |
1 month evaluation (T1) |
3 months evaluation (T2) |
T0 vs. T1 | T1 vs.T2 | T0 vs. T2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | p-Value | p-Value | p-Value | ||
| EORTCQLQ C30 | Global health status | 39 | 47.1 | 27.1 | 24 | 37.0 | 27.2 | 19 | 40.3 | 21.6 | 0.009 | 0.551 | 0.034 |
| Physical function | 39 | 60.9 | 30.9 | 24 | 49.4 | 28.3 | 19 | 59.6 | 30.0 | 0.004 | 0.530 | 0.068 | |
| Cognitive functioning | 39 | 68.4 | 25.6 | 24 | 44.9 | 25.8 | 19 | 38.0 | 26.7 | 0.004 | 0.205 | 0.004 | |
| Appetite loss | 39 | 12.8 | 22.4 | 24 | 26.1 | 34.8 | 19 | 39.2 | 44.5 | 0.256 | 0.160 | 0.031 | |
| EORTC QLQ BN20 | Communication deficit | 39 | 23.9 | 24.7 | 24 | 33.8 | 27.5 | 19 | 29.6 | 17.0 | 0.012 | 0.875 | 0.125 |
| Drowsiness | 39 | 22.2 | 29.0 | 24 | 66.7 | 31.8 | 19 | 74.1 | 33.4 | <0.001 | 0.160 | 0.001 | |
| Hair loss | 38 | 4.4 | 17.6 | 24 | 40.6 | 37.5 | 19 | 25.9 | 26.9 | 0.001 | 0.196 | 0.005 | |
| Weakness of legs | 39 | 40.2 | 33.5 | 24 | 63.8 | 33.2 | 19 | 61.1 | 30.8 | 0.024 | 0.860 | 0.077 | |
Statistically significant differences are described in bold values.
Fig. 1.
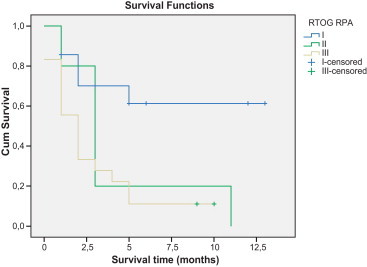
Overall survival outcomes according to recursive partitioning analysis (RPA).
Fig. 1 shows survival curves of patients divided by the RPA class. Almost half of the patients were classified as class III (19 patients), 12.8% were class II (5 patients), and 38.5% were class I (15 patients). During follow-up, 17 patients (89.5%) of the 19 patients classified in RPA III died as did all patients classified in RPA II. In RPA class I, death occurred in 5 patients (35%). Median OS on RPA II and RPA III was 3 (CI95% 1.83; 4.17) and 2 months (CI 1.85; 4.15), respectively. Since the death rate in RPA I was lower than 50%, we could not determine a median OS in this sub-group of patients.
Fig. 2 shows survival curves of patients divided by the GPA class. Seventeen (89.5%) of the 19 patients classified in GPA 1 and 9 (60%) of the 15 patients in GPA 2 died during follow up. All 3 patients classified in GPA 3 were alive during follow-up. Median OS for GPA 1 and GPA 2 was 2 months (CI95%: 1.08; 2.92) and 5 months (IC95%: 1.55; 8.45), respectively.
Fig. 2.
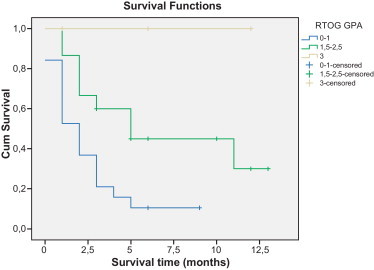
Overall survival outcomes according to graded prognostic assessment (GPA).
5. Discussion
Recently, QoL has become an increasingly important outcome in cancer trials. Several trials on brain metastasis have been published that included an evaluation of study population's QoL.13,19–23 Although there are numerous QoL questionnaires, no standard questionnaire is currently used to access QoL in patients with brain metastases. Nowadays, the use of these different questionnaires does not allow for a comparison of QoL trials. A standard tool would be beneficial for comparisons across trials and allowing meta-analysis.
Literature review showed that certain parameters of QoL deteriorate after WBRT.14,24,25 These findings have led authors to question whether patients with poor prognosis benefit from radiotherapy in terms of effect on QoL and symptom experience. For patients with better prognosis, some studies have shown certain parameters of QoL significantly improved after WBRT.15,16 Yaneva and Semerdijeva20, used the EORTC QLQC30 in a patients population with KPS > 70 who underwent WBRT, with significant improvements in functional indicators, symptoms, and health-related QoL. These results differ from the findings of Gerrad et al.14, who also used the QLQ-C30 questionnaire. Their population had KPS < 70, more than 60 years, or a primary site other than the breast. Our results reported p values statistically significant between baseline and 3 months for a small deterioration of global health status and large deterioration of cognitive function, as well as worsening of drowsiness, appetite loss and hair loss. There was a tendency for deterioration of physical function and weakness of legs (Table 2). In all other QoL domains, no statistically significant changes were obtained. Although a considerable improvement in QoL was not evident, WBRT may have contributed to the stabilization of the QoL status. It is of note that no correlation was observed between steroid intake and the worsening of appetite loss item, since all patients were with corticosteroid intake of at least 4 mg/day. Reporting of steroid use in assessing brain metastasis patients was non-uniform. There is not sufficient literature concerning additional benefits of steroid therapy with WBRT; nevertheless, corticosteroids are recommended to provide temporary relief of symptoms related to increased intracranial pressure and edema secondary to brain metastasis.26,27
Steinmann et al.23 prospectively studied QoL of 46 patients with previously untreated brain metastases at start of treatment and 3 months after treatment. QoL deteriorated in most domains, significantly in drowsiness, hair loss and weakness of legs. The scores for headaches and seizures were slightly better after 3 months. Assessment by proxies also suggested the worsening of QoL. Initial QoL at baseline was better in those alive than in those deceased at 3 months evaluation, significantly for the physical function and symptom scales of fatigue and pain, motor dysfunction, communication deficit and weakness of legs.
In our study, almost half of the patients (19 patients) had poor performance status, reflected in the low median survival of 3 months for the total population of patients. This is consistent with the findings of retrospective studies from other centers.9,10,13,14,28 This low median survival emphasizes once more the need to refine treatment selection for patients with brain metastases.
It is well known that prognostic tools are useful to guide tailored strategies for cancer. Many factors, including but not limited to performance status (KPS), age, extracranial disease and primary tumor status, have been identified as prognostically relevant in brain metastasis patients outcome. Other factors, such as number, size, location of intracranial lesions, histology of the primary malignancy and interval between primary tumor diagnosis and detection of brain disease have been less considered.29 Niemec et al.30 also reported that such factors as adenocarcinoma histology and female sex were prevalent in long-term survivors of brain metastases from lung cancer. RPA was the first and most commonly used scoring system to classify brain metastasis survivorship categories,10 although other systems have also been developed. The GPA score, beside including age, KPS, and extracranial metastasis, also assigned the number of brain metastases as a scoring parameter. Some authors described this particular scoring system as the most objective, quantitative and easiest to be used.31,32 Villa et al.33 decided to prospectively analyze the GPA index score, and compared it to other published prognostic indices, including RPA, to assess the prediction performances of those prognostication systems. Their data did not suggest a greater prognostic power of one scoring system over another, and stated that GPA class may be more difficult to use for daily prognostication of brain metastasis patients. Our results suggest that both RPA class 3 patients and GPA 0–1 do poorly, with a median survival of 2 months in both groups. This data is consistent with other studies.14,24,25 In our experience, both GPA and RPA were a useful predictive models, nevertheless, as the authors explained, caution should be exercised by treating physicians to use these prognostic models and to comprehensively integrate other health, familial and socioeconomical related parameters to this very heterogeneous population of patients with brain metastases.
Neurocognitive function is also an important concern for brain metastasis patients. In our study, we used MMSE to assess cognitive impairment, and to validate the response to our questionnaires. There was no statistically significant difference between sets in MMSE score results. Although the MMSE is the most frequently used measure of the neurocognitive function in the studies, it is less sensitive to mild neurocognitive impairment and may not identify subtle improvements.34,35 In addition, the MMSE has not been as thoroughly evaluated in patients with brain metastases compared with patients with primary brain tumors. Li et al.36 concluded in their study with patients who had been treated with radiosensitizer (gadolinium) and WBRT that there is a correlation between the neuro-cognitive function and QoL, and that efforts to prevent the worsening of neurocognitive function could help maintain QoL.
We do recognize that our number of patients is quite limited, especially those who completed all tree questionnaires (19 patients). Specific effects in subgroups of patients (as in RPA/GPA group, or MMSE evaluation) may only be detectable in a much larger study. During our study, we noticed that QLQ-C30/BN-20 were time consuming, taking sometimes over 20 min to complete, which was an issue of considerable importance especially on severely ill patients with low performance status. Steinmann et al.23 validated a shortened version of QLQ-C30 in their study. QLQC15-PAL is used in a palliative-care setting; containing only 15 items which can be completed in a much shorter time. In addition, practicability and compliance appeared better in this questionnaire version. This could be a useful tool for future standard QoL evaluation.
6. Conclusions
In conclusion, our findings indicate a small deterioration for global QoL status and large deterioration for cognitive functioning after radiation treatments, as well as worsening of brain metastasis related symptom items. Our data suggest that the RPA index, as well as the new GPA index, are valid prognostic indices. The low survival report reflects the poor outcome of these patients. Further research is necessary to refine treatment selection for patients with brain metastases, since it may at least contribute to the stabilization of their QoL status.
Conflict of interest
Authors declare that they do not have conflict of interest with any financial organization.
References
- 1.Zhang X., Zhang W., Cao W.D., Cheng G., Liu B., Cheng J. A review of current management of brain metastases. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-2019-2. [DOI] [PubMed] [Google Scholar]
- 2.Gravrilovic I., Posner J. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C., Spanne O., Mehta M., Grosu A., Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases, what has changed in the last 20 years? Cancer. 2011 doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 4.Schouten L.J., Rutten J., Huveneers H.A., Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and the lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 5.Ellis T., Neal T., Chan M. The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol. 2012 doi: 10.1155/2012/952345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetz P., Ebinu J., Roberge D., Zadeh G. Current standards in the management of cerebral metastases. Int J Surg Oncol. 2012 doi: 10.1155/2012/493426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scoccianti S., Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2011 doi: 10.1016/j.radonc.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Berk L. An overview of radiotherapy trials for the treatment of brain metastases. Oncology. 1995;9(11):1205–1212. [PubMed] [Google Scholar]
- 9.Craighead P., Chan A. Defining treatment for brain metastases patients: nihilism versus optimism. Support Care Cancer. 2010 doi: 10.1007/s00520-010-1068-6. [DOI] [PubMed] [Google Scholar]
- 10.Gaspar L., Scott C., Murray K., Murray K., Curan W. Validation of RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 11.Sperduto P., Berkey B., Gaspar L., Mehta M., Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1960 patients in the RTOG database. Int J Rad Oncol Biol Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 12.Nieder C., Nestle U., Motaref B., Walter K., Niewald M., Schnabel K. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Rad Oncol Biol Phys. 2000;46(2):297–302. doi: 10.1016/s0360-3016(99)00416-2. [DOI] [PubMed] [Google Scholar]
- 13.Lutterbach J., Bartelt S., Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol. 2002;128:417–425. doi: 10.1007/s00432-002-0354-1. [DOI] [PubMed] [Google Scholar]
- 14.Gerrad G., Prestwich R., Edwards A. Investigating the palliative efficacy of whole-brain radiotherapy for patients with multiple-brain metastases and poor prognostic features. Clin Oncol. 2003;15(7):422–428. doi: 10.1016/s0936-6555(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 15.Addeo R., Caraglia M., Faiola V. Concomitant treatment of brain metastases with whole brain radiotherapy and temozolamide is active and improves QoL. BMC Cancer. 2007;7:18. doi: 10.1186/1471-2407-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott C., Suh J., Stea B., Nabid A., Hackman J. Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral plus whole brain radiation therapy for brain metastases. Am J Clin Oncol. 2007;30(6):580–587. doi: 10.1097/COC.0b013e3180653c0d. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson N., Ahmedzai S., Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Fayers P.M. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer. 2011;37:1331–1334. doi: 10.1016/s0959-8049(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 19.Moinpour C.M., Feilq P., Metch B., Hayden K.A., Meykens F.L., Jr., Crowley J. Quality of life end points in cancer clinical trials: review and recommendations. J Natl Cancer Inst. 1989;81(7):485–495. doi: 10.1093/jnci/81.7.485. [DOI] [PubMed] [Google Scholar]
- 20.Yaneva M.P., Semerdijeva M.A. Assessment of the effect of palliative radiotherapy for cancer patients with intracranial metastases using EORTC-QoL-C30 questionnaire. Folia Med. 2006;48(2):23–29. [PubMed] [Google Scholar]
- 21.Wong J., Hird A., Zhang L. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125–1131. doi: 10.1016/j.ijrobp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Murray K.J., Scott C., Greenberg H.M. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39(3):571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 23.Steinmann D., Shafer C., Oorschot B. Effects of radiotherapy for brain metastases on quality of life (QoL) Strahlenther Onkol. 2009;185:190–197. doi: 10.1007/s00066-009-1904-0. [DOI] [PubMed] [Google Scholar]
- 24.Wong J., Hird A., Kirou-Mauro A., Napolskikh J., Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25–43. doi: 10.3747/co.v15i5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow E., Davis L., Holden L., Tsao M., Danjoux C. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18–23. doi: 10.1016/j.jpainsymman.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Millar B.M., Bezjak A., Tsao M., Sturdza A., Laperriere N. Defining the impact and contribution of steroids in patients receiving whole-brain irradiation for cerebral metastases. Clin Oncol. 2004;16(5):339–344. doi: 10.1016/j.clon.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Ryken T., MecDermott, Robinson P. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bezjak A., Adam J., Barton R. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002;38:487–496. doi: 10.1016/s0959-8049(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 29.Lagerwaard F.J., Levendag P.C., Nowak P.J., Eijkenboom W.M., Hanssens P.E., Schmitz P.I. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43(4):795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 30.Niemec M., Glogowski M., Tyc-Szczepniak D., Wierzchowski M., Kepka L. Characteristics of long-term survivors of brain metastases from lung cancer. Rep Pract Oncol Radiother. 2011;16:49–53. doi: 10.1016/j.rpor.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rades D., Dunst J., Schild S.E. A new scoring system to predicting the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2008;184(5):251–255. doi: 10.1007/s00066-008-1831-5. [DOI] [PubMed] [Google Scholar]
- 32.Rades D., Dziggel L., Haatanen T. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys. 2011;80(4):1122–1127. doi: 10.1016/j.ijrobp.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Villa S., Weber C., Moretones C. Validation of the new graded prognostic assessment scale for brain metastases: a multicenter prospective study. Radiat Oncol. 2011;6:23. doi: 10.1186/1748-717X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman M.A., Tremont-Lukats I., Meyers C.A. Neurocognitive and functional assessment of patients with brain metastases. Am J Clin Oncol. 2003;26:273–279. doi: 10.1097/01.COC.0000020585.85901.7C. [DOI] [PubMed] [Google Scholar]
- 35.Olson R., Tyldesley S., Carolan H., Parkinson M., Chhanabhai T., McKenzie M. Prospective comparison of the prognostic utility of the Mini Mental State examination and the Montreal Cognitive Assessment in patients with brain metastases. Support Care Cancer. 2011;19:1849–1855. doi: 10.1007/s00520-010-1028-1. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Bentzen S.M., Renschler M., Mehta M.P. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]


