Abstract
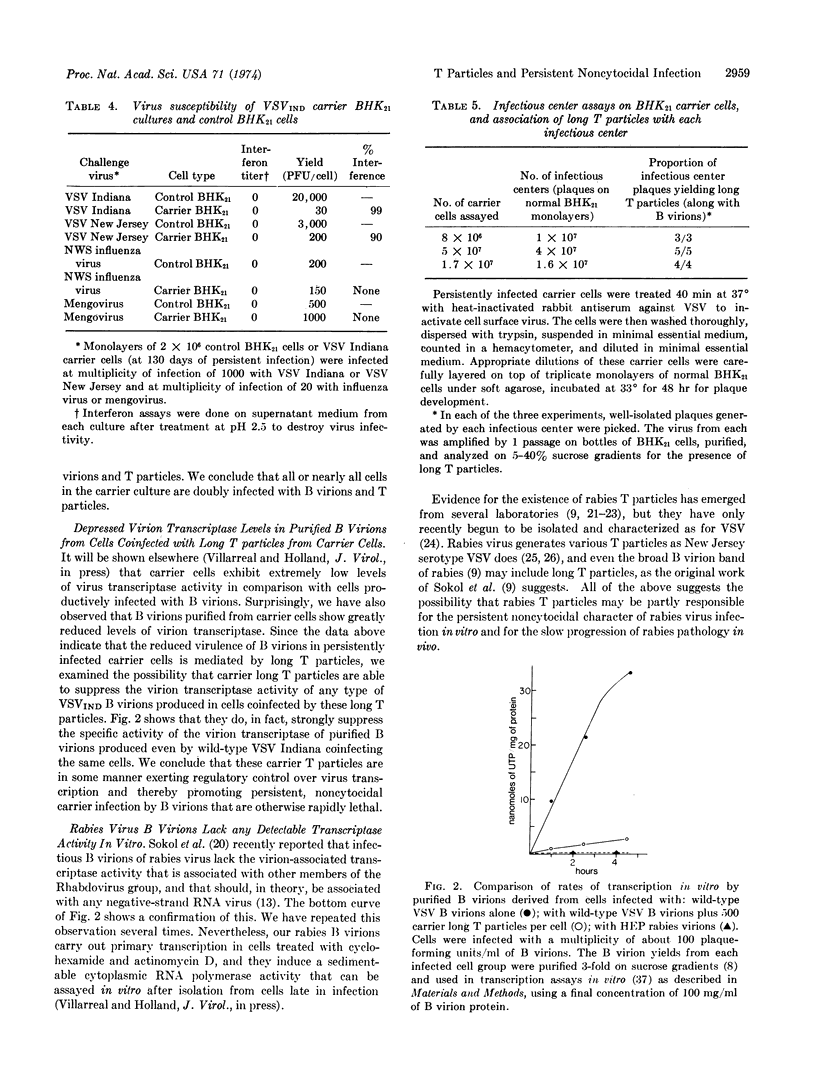
Infectious B virions of vesicular stomatitis virus were 100% lethal to BHK21 (baby hamster kidney) cells when infecting alone, and persistent noncytocidal infection could not be achieved with cloned B virions alone. However, a mixture of B virions and homologous, short, defective, interfering particles (T particles) of a temperature-sensitive mutant of the virus regularly established persistently infected, noncytocidal carrier cultures. A long T particle was generated during establishment of the carrier culture; we show that this long T particle can establish and maintain persistent noncytocidal infection even when it infects cells along with virulent wild-type B virions. This long T particle causes the production of wild-type B virions with greatly reduced virion transcriptase (EC 2.7.7.6; RNA nucleotidyltransferase) levels when coinfecting the same cells, so it appears to prevent cytopathology by regulating virus transcription. The implications of these findings for rabies and other slowly progressing noncytocidal infections are discussed.
Keywords: virus transcription, rabies
Full text
PDF
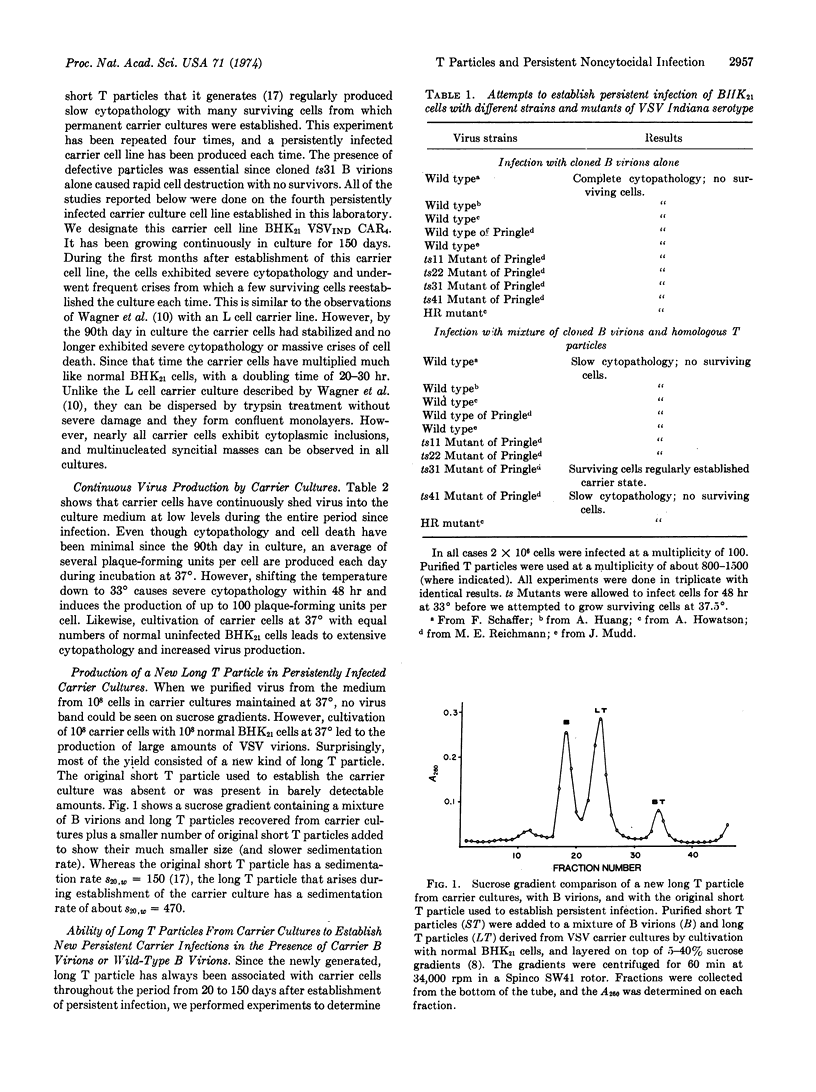

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick J., Brown F. An interfering component of rabies virus which contains RNA. J Gen Virol. 1974 Jan;22(1):147–151. doi: 10.1099/0022-1317-22-1-147. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson T. E., Brody J. A., Sever J. L., Dyken M. L., Cannon J. Measles antibody titers in multiple sclerosis patients, siblings, and controls. JAMA. 1970 Mar 23;211(12):1985–1988. [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. P., Gray R. H., Payne F. E. Replication of measles virus: distinct species of short nucleocapsids in cytoplasmic extracts of infected cells. J Virol. 1974 Mar;13(3):721–728. doi: 10.1128/jvi.13.3.721-728.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Howatson A. F. In vitro transcriptase activity of vesicular stomatitis virus B and T particles: analysis of product. Intervirology. 1973;1(3):168–175. doi: 10.1159/000148843. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Leavitt R. W., Kingsbury D. T., Holland J. J. Natural selection of mutants of vesicular stomatitis virus by cultured cells of Drosophila melanogaster. J Gen Virol. 1973 Sep;20(3):341–351. doi: 10.1099/0022-1317-20-3-341. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevec L., Kang C. Y. Homotypic and heterotypic interference by defective particles of vesicular stomatitis virus. Nature. 1970 Oct 3;228(5266):25–27. doi: 10.1038/228025a0. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Villarreal L. P., Kohne D., Lesnaw J., Holland J. J. RNA polymerase activity and poly(A) synthesizing activity in defective T particles of vesicular stomatitis virus. Virology. 1974 Mar;58(1):240–249. doi: 10.1016/0042-6822(74)90158-5. [DOI] [PubMed] [Google Scholar]
- Roy P., Repik P., Hefti E., Bishop D. H. Complementary RNA species isolated from vesicular stomatitis (HR strain) defective virions. J Virol. 1973 Jun;11(6):915–925. doi: 10.1128/jvi.11.6.915-925.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. II. Effect of measles antibody on persistently infected HeLa sublines and recovery of a HeLa clonal line persistently infected with incomplete virus. J Bacteriol. 1966 Dec;92(6):1805–1811. doi: 10.1128/jb.92.6.1805-1811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Synthesis of poly(A) in vitro by purified virions of vesicular stomatitis virus. Nat New Biol. 1973 Nov 7;246(149):17–19. doi: 10.1038/newbio246017a0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Yoshino K., Taniguchi S., Arai K. Autointerference of rabies virus in chick embryo fibroblasts. Proc Soc Exp Biol Med. 1966 Nov;123(2):387–392. doi: 10.3181/00379727-123-31496. [DOI] [PubMed] [Google Scholar]


