Abstract
Merkel cell carcinoma (MCC) is a lethal, virus-associated cancer that lacks effective therapies for advanced disease. Agents blocking the PD-1/PD-L1 pathway have demonstrated objective, durable tumor regressions in patients with advanced solid malignancies and efficacy has been linked to PD-L1 expression in the tumor microenvironment. To investigate whether MCC might be a target for PD-1/PD-L1 blockade, we examined MCC PD-L1 expression, its association with tumor-infiltrating lymphocytes (TILs), Merkel cell polyomavirus (MCPyV), and overall survival. Sixty-seven MCC specimens from 49 patients were assessed with immunohistochemistry for PD-L1 expression by tumor cells and TILs, and immune infiltrates were characterized phenotypically. Tumor cell and TIL PD-L1 expression were observed in 49% and 55% of patients, respectively. In specimens with PD-L1(+) tumor cells, 97% (28/29) demonstrated a geographic association with immune infiltrates. Among specimens with moderate-severe TIL intensities, 100% (29/29) demonstrated PD-L1 expression by tumor cells. Significant associations were also observed between the presence of MCPyV DNA, a brisk inflammatory response, and tumor cell PD-L1 expression: MCPyV(−) tumor cells were uniformly PD-L1(−). Taken together, these findings suggest that a local tumor-specific and potentially MCPyV-specific immune response drives tumor PD-L1 expression, similar to previous observations in melanoma and head and neck squamous cell carcinomas. In multivariate analyses, PD-L1(−) MCCs were independently associated with worse overall survival (hazard ratio 3.12; 95% CI, 1.28-7.61; p=0.012). These findings suggest that an endogenous immune response promotes PD-L1 expression in the MCC microenvironment when MCPyV is present, and provide a rationale for investigating therapies blocking PD-1/PD-L1 for patients with MCC.
Keywords: Merkel cell carcinoma, PD-L1, B7-H1, immunotherapy, Merkel cell polyomavirus
INTRODUCTION
Merkel cell carcinoma (MCC) is an uncommon but aggressive cutaneous malignancy, demonstrating both epithelial and neuroendocrine features. The majority (at least ~80%) of cases are associated with the oncogenic Merkel cell polyomavirus (MCPyV).(1-3) MCC is diagnosed predominantly in the sun-exposed skin of older, fair-skinned individuals. An increased risk of developing MCC is observed in immunocompromised patients and those with other primary cancers.(4-6)
MCC has historically been difficult to treat.(7) It recurs following surgical excision of localized disease in about one-third of patients.(8) While lymphadenectomy and adjuvant radiotherapy or chemotherapy may decrease the likelihood of recurrence, a significant percentage of patients develop metastatic disease, for which no therapy has been shown to prolong survival. Commonly used chemotherapy regimens for patients with metastatic or unresectable local disease include cisplatin or carboplatin plus etoposide; topotecan; or cyclophosphamide, doxorubicin (or epirubicin) and vincristine (CAV). Though MCC is often initially chemosensitive, resistance generally develops quickly and tumor regressions are rarely durable. The 2-year survival rate for patients with American Joint Committee on Cancer (AJCC) stage IV disease is estimated to be 26%.(9) Accordingly, new therapeutic options are sorely needed for patients with MCC in the adjuvant and advanced metastatic settings.
MCC, similar to other virus-associated malignancies, is highly immunogenic, and patients with MCPyV positive tumors have improved overall survival compared to those with MCPyV negative tumors.(10) Oncoproteins such as MCPyV large and small T antigens and capsid proteins often stimulate the generation of antigen-specific T cells and antibodies in patients with MCC, and high serum antibody titers against viral capsid protein 1 (VP1) have been associated with improved progression-free survival.(11, 12) T cells specific for viral antigens can be detected within MCC tumors and in the blood. Moreover, MCC patients whose tumors harbor high numbers of CD3+, CD8+ tumor-infiltrating lymphocytes (TILs) demonstrate improved overall survival.(13, 14) Conversely, immunocompromised patients have a higher than expected rate of MCC and display a higher MCC-specific death rate.(15) These studies support an important role for the host immune system in containing MCC.
Notwithstanding the immunogenicity of MCC, the immune system often ultimately fails to control its growth. One potential explanation for this seeming contradiction is the expression of immune-inhibitory ligands in the tumor microenvironment such as programmed death ligand-1 (PD-L1, B7-H1, CD274). PD-L1 shares homology with B7.1 and B7.2, two members of the B7 immunoglobulin superfamily.(16) The major receptor for PD-L1, namely programmed death-1 (PD-1), is expressed by activated T lymphocytes and delivers inhibitory signals upon PD-L1 ligation.(17, 18) PD-L1 is physiologically expressed by both activated hematopoietic cells and inflamed epithelium as a means to down-modulate antigen-stimulated adaptive immune responses in peripheral tissues, thereby avoiding collateral damage to normal tissues.(19) However, PD-L1 may be aberrantly expressed by tumor cells, creating a shield against immune attack.(20) PD-L1 expression has been demonstrated in many different human cancers, but whether MCC exploits this mechanism of immune resistance has yet to be established.
As new data emerge about immunotherapies targeting the PD-1/PD-L1 pathway, the potential expression of PD-L1 in the MCC tumor microenvironment is of increasing interest. Recent trials of nivolumab (BMS-936558, MDX-1106; Bristol-Myers Squibb), a blocking antibody against PD-1, and BMS-936559 (anti-PD-L1) have demonstrated the power and durability of an immune-based antitumor response in patients with treatment-refractory solid malignancies including melanoma, renal cell carcinoma, and non-small cell lung cancer.(21-24) Blockade of the PD-1/PD-L1 pathway by these immunotherapies is thought to enhance endogenous antitumor immune responses, thereby mediating tumor regression. Preliminary evidence for a correlation between tumor cell surface (“membranous”) PD-L1 expression, detected by immunohistochemistry, and the likelihood of response to nivolumab has been reported.(22, 24) A significant association between PD-L1 expression and tumor histologies likely to respond to anti-PD-1 therapy suggests that this factor might be useful in identifying different tumor types amenable to this immunotherapy. (22)
In the current study, we investigate the expression of PD-L1 in the MCC microenvironment and its association with TILs, the presence of MCPyV, and overall survival in a cohort of 49 patients. We demonstrate PD-L1 expression by tumor cells in MCPyV-positive but not MCPyV-negative MCC, and show that tumor cell PD-L1 expression is an independent positive prognostic factor. Taken together, these data provide a rationale for using PD-1/PD-L1 blockade as a novel immunotherapeutic approach in patients with MCC.
MATERIALS AND METHODS
Case selection
Following IRB approval, 67 specimens from 49 unique patients with MCC, collected from 2000-2011, were identified in the Johns Hopkins Hospital surgical pathology archives. The slides from each case were reviewed by a pathologist (JT or JV) to confirm the diagnosis of MCC and one representative paraffin block was chosen for additional studies. Clinical information gathered included patient age, sex, tumor site, disease progression and overall survival. The AJCC pathologic stage based on the original diagnostic specimens (primary lesion, lymph node biopsy, and/or distant metastasis) was determined according to the 7th ed. 2010.(9, 25)
Immunohistochemistry
Immunohistochemistry (IHC) was performed on archived, formalin-fixed, paraffin-embedded (FFPE) tissue. PD-L1 expression was assessed using the murine anti-human PD-L1 monoclonal antibody 5H1 (from Lieping Chen, Yale University) at a concentration of 2 ug/mL, as previously described.(26) IHC for CD3, CD4, and CD8 was performed according to standard automated methods. Select cases were also stained for CD68 (macrophages/histiocytes) and CK20 (Merkel cells).
PD-L1 expression by tumor cells and relationship to TILs
Percentages of tumor cells or TILs (defined in this study as immune infiltrates including lymphocytes and histiocytes) demonstrating membranous (cell surface) expression of PD-L1 were scored on one representative slide from each tumor as 0-4%, 5-9%, 10%, and at increasing 10% intervals by two independent pathologists (JT and JV) who were blinded to clinical outcomes. Scoring differences greater than 10% were adjudicated. Cases demonstrating at least 5% membranous expression of PD-L1 were considered positive. The pattern of PD-L1 expression in the tumor microenvironment was also assessed. PD-L1 expression was scored as absent (no PD-L1 expression by tumor, and no TILs or no PD-L1 expression by TILs), present on both tumor cells and TILs in immediate proximity, or present on TILs but not on tumor cells.
Characterization of immune infiltrates
The recommended surgical pathology scoring system for TILs in MCC specimens applies to primary lesions (27), thus a modified scoring system was adopted. The degree of CD3+ TILs and associated histiocytes was scored as (0), none; (1), “mild” (rare intratumoral cells, mostly perivascular); (2), “moderate” (focally present at periphery of tumor and/or intratumoral extending away from vessels); or (3), “severe” (either extending around the majority of the periphery of the tumor deposit, or diffusely present throughout tumor).(26, 28) As Merkel cells and lymphocytes can sometimes be difficult to differentiate on hematoxylin/eosin (H&E) staining alone, a CD3 immunostain was used to aid in assigning TIL score. When PD-L1 expression by lymphohistiocytic infiltrates was observed along the periphery of tumor, the proportion of the perimeter demonstrating immunostaining was scored as 0-4%, 5-9%, 10% and then at 10% intervals up to 100%. In addition, intratumoral and peritumoral CD8+ T cells were scored as “low” or “high” density with a cut point of approximately 60 CD8+ cells per typical HPF.(13) Lastly, the CD4:CD8 ratio in intratumoral and peritumoral TILs was estimated as 4:1, 3:1, 2:1, 1:1, 1:2, 1:3 or 1:4.
Detection of Merkel cell polyomavirus
The presence of MCPyV in tumor samples was detected by quantitative real-time PCR (qPCR) performed on genomic DNA extracted from FFPE tissues, as published.(29, 30) Ten consecutive 10 um thick unstained sections were cut from a representative paraffin block for each case. The first and last sections were stained with H&E to confirm persistence of tumor on the deepest sections, and a dermatopathologist (JT) marked the malignant focus to be isolated. Genomic DNA was extracted from a tumor focus of approximately 2 mm diameter using the Pinpoint Slide DNA Isolation System™ (Zymo Research Irvine, CA) according to the manufacturer's protocol. After proteinase K digestion, genomic DNA was purified using the QIAamp DNA Mini Kit (Qiagen Valencia, CA), according to the manufacturer's “Blood and Body Fluid Spin” protocol. MCPyV was detected with qPCR for the viral capsid protein 1 (VP1) and viral T antigen 3 (LT3) genes.(29, 30) The LT3 primers localize to the first exon and thus detect both large and small T antigens. qPCR was performed in triplicate for each sample, and the B-actin gene was used as a normalizer for gene abundance. Molecular grade water was used as a non-template control. Standard curves were developed by diluting the MCPyV-positive cell line MKL-1. In those instances where only one of the two viral genes VP1 or the LT3 was detected, the samples were considered positive for MCPyV. The lowest value we reliably detected was a MCPyV to B-actin ratio of 0.09, which is in keeping with assays reported by others.(14, 31)
Statistical analysis
Associations of PD-L1 expression with clinicopathologic features were evaluated with Fisher's exact test and Wilcoxon-Mann Whitney tests. For patients with multiple tumor samples, the samples were treated as independent observations for the clinicopathologic associations. Overall survival was calculated from the date of the original diagnostic biopsy to the date of last follow-up or death with the Kaplan-Meier method and compared with the log-rank test. Multivariate survival analyses were performed using the Cox proportional hazards model. Survival analyses for patients with multiple specimens were conducted using the sample that had the highest value for the parameter in question. Statistical analyses were performed using the R statistical package (version 2.15.1). All tests were two-sided and p values <0.05 were considered significant.
RESULTS
Patient characteristics and tumor specimens
To assess factors associated with PD-L1 expression in the tumor microenvironment, we studied 49 patients with MCC whose clinicopathologic characteristics are summarized in Table 1. Patients were predominantly male (65%) with a median age of 65 years. Forty-nine percent of patients presented with metastatic disease (AJCC stage III-IV), while 51% had localized disease (AJCC stage I-II).(25) Overall, 49% of patients had PD-L1+ tumor cells, and 55% had PD-L1+ infiltrating immune cells, in at least one tumor specimen examined. Expression of PD-L1 in the tumor microenvironment, by either tumor cells or infiltrating immune cells, did not correlate with patient gender, age, or pathologic stage at the time of diagnosis. The median follow-up time for the total cohort of 49 patients was 50 months (range 4-142 months), with a median follow-up of 25 months (range 4-127 months) for those who died and 87 months (range 15-142) for patients still alive. At the end of the follow-up period, 23 patients had died, 13 had disease progression but were alive, and 13 had no evidence of disease.
Table 1.
Relationship of PD-L1 expression by tumor cells and infiltrating immune cells to patient demographics and clinicopathologic features
| Tumor cells |
Infiltrating immune cells |
||||||
|---|---|---|---|---|---|---|---|
| All Patients n=49 | PD-L1 (−) n=25 | PD-L1+a n=24 | p-valueb | PD-L1 (−) n=22 | PD-L1 + a n=27 | p-valueb | |
| Gender, n (%) | |||||||
| Female | 17 (35) | 8 (32) | 9 (38) | 0.769 | 8 (36) | 9 (33) | >0.99 |
| Male | 32 (65) | 17 (68) | 15 (62) | 14 (64) | 18 (67) | ||
| Age at primary diagnosis | |||||||
| Mean (SD) | 65.94 (12.63) | 65.52 (12.62) | 66.38 (12.89) | 64.23 (12.92) | 67.33 (12.45) | ||
| Median (range) | 65 (30,90) | 70 (42,90) | 64.5 (30,90) | 0.734 | 64.5 (42,90) | 69.5 (30,90) | 0.329 |
| Pathologic stage at diagnosis, n (%) | |||||||
| IA | 19 (39) | 12 (48) | 7 (29) | 0.457 | 10 (45) | 9 (33) | 0.593 |
| IB | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | ||
| IIA | 4 (8) | 2 (8) | 2 (8) | 1 (5) | 3 (11) | ||
| IIC | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | ||
| IIIA | 8 (16) | 2 (8) | 6 (25) | 2 (9) | 6 (22) | ||
| IIIB | 12 (24) | 7 (28) | 5 (21) | 7 (32) | 5 (19) | ||
| IV | 4 (8) | 2 (8) | 2 (8) | 2 (9) | 2 (7) | ||
Tumor cells or associated infiltrating immune cells were considered PD-L1+ if ≥5% of cells had membranous (cell surface) PD-L1 expression. In this table, patients with multiple specimens are considered PD-L1+ if any specimen was positive.
Fisher's exact test for categorical variables, Wilcoxon-Mann Whitney test for continuous variables
Sixty-seven individual tumor specimens were available from 49 patients for assessment of PD-L1 and CD3 expression by IHC (39 patients had a single specimen, and 10 patients each had 2-5 specimens). Forty and 39 specimens from 34 patients had both TILs present and tissue available for additional characterization of immune cell subsets by IHC, respectively. Forty-two specimens from 32 patients yielded sufficient material for MCPyV DNA detection by qPCR. For the purpose of the survival analysis on those patients with multiple tumor samples, the patient was considered “positive” for a given parameter if any tumor specimen from that patient was positive.
PD-L1 expression in the MCC microenvironment
Previous studies of melanomas and of squamous cell carcinomas of the head and neck (HNSCC) have shown that PD-L1 can be expressed by multiple components of the tumor microenvironment, including tumor cells themselves and infiltrating immune cells (lymphocytes and macrophages/histiocytes).(26, 28) Theoretically, PD-L1 expression by any cell type could exert local immunosuppressive effects, although previous reports suggest that tumor-expressed membrane PD-L1 correlates with response to anti-PD-1 therapy.(22) In the current study of MCC, membranous PD-L1 expression by tumor cells was observed in 29 of 67 specimens (43%). Among 29 specimens containing PD-L1+ tumor cells, 100% harbored immune infiltrates, in contrast to only 47% of 38 specimens with PD-L1(−) tumor cells (p<0.001; Table 2A). All but one specimen harboring PD-L1+ tumor cells was characterized by a geographic co-localization of these cells with infiltrating immune cells (Figure 1); in a single specimen, tumor cell PD-L1 expression was geographically remote from a moderate immune infiltrate (Figure 2). When observed in association with TILs, focal tumor expression of PD-L1 was seen in ≤10% of the total tumor area represented on the slide (mean=5%, SD ± 0.94; median 5%, range 5-10%); expression was located predominantly at the tumor periphery and/or perivascularly within the tumor where infiltrating immune cells were most often seen. There was a strong correlation between PD-L1 expression by tumor cells and by the associated immune infiltrate (Table 2B; p<0.001), indicating an immune-active environment at the interface of these different cell populations. A greater proportion of immune cells than tumor cells demonstrated PD-L1 expression (mean=36%, SD ± 30.0; median=30%, range 5-100%).
Table 2A.
Correlation of PD-L1 expression by tumor cells with the presence of immune infiltrates in 67 MCC specimens
| Tumor site | n | Tumor PD-L1(−), n (%) |
Tumor PD-L1+, n (%)a |
p-valuec | ||
|---|---|---|---|---|---|---|
| TIL(−) | TIL +b | TIL (−) | TIL + b | |||
| Primary | 29 | 8 (50) | 8 (50) | 0 (0) | 13 (100) | 0.003 |
| Metastasis | 38 | 12 (55) | 10 (45) | 0 (0) | 16 (100) | < 0.001 |
| All | 67 | 20 (53) | 18 (47) | 0 (0) | 29 (100) | < 0.001 |
Tumor cells were considered PD-L1+ if ≥5% of cells had membranous (cell surface) PD-L1 expression.
TILs were considered positive if infiltrates were mild, moderate, or severe (scores 1-3; see Methods).
Fisher's exact test
Figure 1.
Geographic co-localization of PD-L1+ tumor cells with infiltrating immune cells in MCC. The predominant pattern of tumor cell PD-L1 expression is at the interface with infiltrating immune cells. At this interface, MCC cells (CK20 immunostain demonstrating a characteristic perinuclear dot-like staining pattern), CD3+ T cells, and associated CD68+ macrophages all demonstrated PD-L1 expression. This pattern was most often observed in geographic foci with a high density of CD8+ T cells. Green arrows show lymphocytes expressing PD-L1, and the yellow arrow demonstrates a PD-L1+ tumor cell cluster immediately adjacent to the immune infiltrate. Original magnification 400x, all panels.
Figure 2.
Constitutive PD-L1 expression in MCC. Only one among 29 PD-L1+ MCC specimens demonstrated diffuse membranous expression by tumor cells that was out of proportion to the scant number of infiltrating CD3+ T lymphocytes. Orange arrows point to CD3+ T lymphocytes. Original magnification 400x, all panels.
Table 2B.
Correlation of PD-L1 expression by tumor cells with PD-L1 expression by adjacent immune infiltrates in 67 MCC specimens
| Tumor site | n | Tumor PD-L1(−), n (%) |
Tumor PD-L1+, n (%)a |
p-valueb | ||
|---|---|---|---|---|---|---|
| TIL PD-L1(−) | TIL PD-L1+a | TIL PD-L1(−) | TIL PD-L1+ | |||
| Primary | 29 | 12 (75) | 4 (25) | 0 (0) | 13 (100) | < 0.001 |
| Metastasis | 38 | 21 (95) | 1 (5) | 0 (0) | 16 (100) | < 0.001 |
| All | 67 | 33 (87) | 5 (13) | 0 (0) | 29 (100) | < 0.001 |
Tumor cells or associated infiltrating immune cells were considered PD-L1+ if ≥5% of cells had membranous (cell surface) PD-L1 expression.
Fisher's exact test
In 38 specimens that were negative for tumor cell PD-L1 expression, 18 (47%) contained TILs (Table 2A) but were characterized by only a mild infiltrate (score 1). In contrast, among 29 specimens with PD-L1+ tumor cells, all were associated with TILs, and TIL intensities were moderate (score 2) to severe (score 3) (not shown; p<0.001). Similarly, when TIL intensity was studied in relation to TIL PD-L1 expression, PD-L1 expression was significantly associated with increasing intensity of the immune infiltrate (not shown; p<0.001). Neither tumor nor TIL PD-L1 expression correlated significantly with tumor site (primary lesion versus metastasis).
Characterization of infiltrating immune cells related to PD-L1 expression
TILs were further characterized according to CD4+ and CD8+ subsets, and the association of the CD4:CD8 ratio and the CD8 density with PD-L1 expression by tumor and by TILs was studied in both the intratumoral and peritumoral compartments (Table 3). CD4+ T cells were present at equal or greater numbers than CD8+ T cells in peritumoral immune infiltrates in 35/39 (90%) specimens, while the inverse was seen in intratumoral infiltrates, with CD8+ T cells predominating in 23/39 (59%) of specimens. PD-L1 expression by both tumor cells and TILs was strongly associated with an intratumoral CD8-predominant TIL phenotype (p=<0.001 and p=0.003, respectively); in contrast, there was no significant difference between the composition of peritumoral immune infiltrates in PD-L1+ vs. PD-L1(−) tumors. When CD8+ T cell density was enumerated in the intratumoral and peritumoral compartments, a high density (>60 per HPF) of CD8+ cells was associated with PD-L1 expression by tumor cells (p=0.015 and p<0.001, respectively) and by TILs (p=0.043 and p<0.001, respectively). Taken together, these findings are compatible with a role for CD8+ T cells in mediating PD-L1 expression in the MCC microenvironment, and are consistent with our previous findings in melanoma and head and neck squamous cell carcinoma (HNSCC).(26, 28)
Table 3.
Relationship of TIL subsets to tumor and TIL PD-L1 expression
| Tumor |
TILs |
||||||
|---|---|---|---|---|---|---|---|
| All Specimens n=39a | PD-L1 (−) n=15 | PD-L1 + n=24 | p-valueb | PD-L1 (−) n=11 | PD-L1 + n=28 | p-valueb | |
| Intratumoral CD4:CD8, n (%) | |||||||
| CD4 ≥ CD8 | 16 (41) | 12 (80) | 4 (17) | < 0.001 | 9 (82) | 7 (25) | 0.003 |
| CD8 > CD4 | 23 (59) | 3 (20) | 20 (83) | 2 (18) | 21 (75) | ||
| Peritumoral CD4:CD8, n (%) | |||||||
| CD4 ≥ CD8 | 35 (90) | 15 (100) | 20 (83) | 0.146 | 11 (100) | 24 (86) | 0.309 |
| CD8 > CD4 | 4 (10) | 0 (0) | 4 (17) | 0 (0) | 4 (14) | ||
| All Specimens n=40a | PD-L1 (−) n=15 | PD-L1 + n=25 | p-valueb | PD-L1 (−) n=11 | PD-L1 + n=29 | p-valueb | |
|---|---|---|---|---|---|---|---|
| CD8 Intratumoral, n (%) | |||||||
| ≤ 60/HPF | 31 (78) | 15 (100) | 16 (64) | 0.015 | 11 (100) | 20 (69) | 0.043 |
| > 60/HPF | 9 (22) | 0 (0) | 9 (36) | 0 (0) | 9 (31) | ||
| CD8 Peritumoral, n (%) | |||||||
| ≤ 60/HPF | 14 (35) | 12 (80) | 2 (8) | <0.001 | 10 (91) | 4 (14) | < 0.001 |
| > 60/HPF | 26 (65) | 3 (20) | 23 (92) | 1 (9) | 25 (86) |
39 specimens had TILs present and tissue available for CD4 staining. 40 specimens had TILs present and material for CD8 staining.
Fisher's exact test
Association of viral status with immune infiltrates and PD-L1 expression
Among 42 MCC specimens available for detection of MCPyV, 34 (81%) were positive. There were six patients who had multiple specimens available for viral testing, and there was concordance in viral status among specimens from individual patients. Specifically, two patients had a primary tumor and two metastases for analysis, all of which were positive. Two patients had a primary tumor and one metastasis for analysis, both of which were positive in one patient and both of which were negative in the other patient. Two patients had only metastases available, one with four different lesions and one with two different lesions, all of which were positive for virus. When analyzed by patient, 78% (25/32) of patients had MCPyV positive tumors, which is in keeping with the reported prevalence rates of approximately 80-90%. (1, 29, 30, 32)
The association between the presence of MCPyV, inflammatory infiltrates and PD-L1 expression was assessed. There was a significant association of the presence of virus with tumor PD-L1 expression, and the presence of virus with a brisk (moderate or severe) inflammatory response: 50% of MCPyV+ specimens were PD-L1+ and had a moderate-severe immune infiltrate, compared to 0% of MCPyV(−) specimens. The association of TILs with MCPyV+ tumors is reminiscent of findings from Sihto and colleagues.(14) Similarly, HPV+ HNSCC are more likely to be inflamed than their HPV(−) counterparts.(28) These findings suggest the hypothesis that an immune response to viral antigens creates a local pro-inflammatory environment driving tumor PD-L1 expression. PD-L1 expression by the inflammatory cell population alone did not associate significantly with the presence of virus.
Association of PD-L1 expression in the MCC microenvironment with overall survival
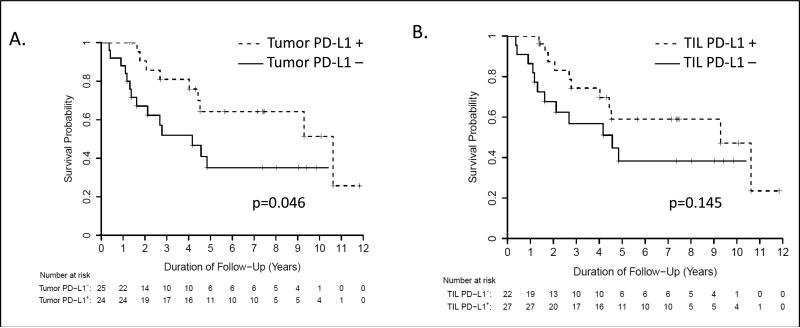
PD-L1 expression in the tumor microenvironment appears to reflect antitumor immunity and adaptive immune resistance by the tumor, creating a negative feedback loop to mediate T cell inhibition.(26) Negative associations of tumor PD-L1 expression with prognosis have been reported for several cancers.(33-35) However, we have reported a positive correlation of PD-L1 expression with improved overall survival in patients with metastatic melanoma.(26) Thus, the association of both tumor cell and TIL PD-L1 expression with survival in 49 patients with MCC was assessed in the current study. By univariate analysis, there was a significant association of tumor cell PD-L1 expression (p = 0.046), but not TIL PD-L1 expression, with improved overall survival (Figure 3). To further analyze this association, we used a Cox multivariate proportional hazards model and controlled for age and whether the specimen studied was a primary vs. metastatic lesion. The failure of MCC to express PD-L1 was independently associated with worse overall patient survival by multivariable analysis (hazard ratio = 3.12; 95% confidence interval: 1.28 to 7.61, p = 0.012).
Figure 3.
Correlation of PD-L1 expression with overall survival in 49 patients with MCC. (A) Kaplan-Meier survival curve showing improved survival for MCC patients whose tumor cells demonstrated membranous PD-L1 expression. (B) TIL and associated histiocyte PD-L1 expression was not significantly associated with improved outcomes.
DISCUSSION
In 2008, Feng and colleagues reported the association of MCC with a previously undescribed virus, now called Merkel cell polyomavirus (MCPyV).(1) Further studies have demonstrated that MCPyV is directly carcinogenic; that is, viral DNA is integrated into the tumor genome and the cells that undergo malignant transformation are the same cells infected by the virus.(2) Consequently, neoantigens in the form of oncoproteins are expressed by tumor cells, creating an opportunity for immune recognition and tumor elimination. Previous studies indicate that the natural history of MCC is impacted by the resultant host immune response. However, approximately 10-20% of MCCs are generally not reported to be associated with MCPyV infection, dividing MCC into 2 groups that may differ in the tumor-host immune system interaction. Our results support the notion that the immune microenvironment differs between virus positive and virus negative MCC, since the latter does not express PD-L1, a major component of the PD-1 pathway that down-modulates the amplitude of T cell activation within the tumor. In contrast, we find that roughly half of virus positive MCCs express the immunosuppressive ligand PD-L1 on multiple cell types (tumor cells, lymphocytes, macrophages/histiocytes) in the tumor microenvironment. Further, we also demonstrate features of geographic co-localization of tumor cell PD-L1 expression with TILs, similar to our previous studies of melanoma and HNSCC, suggesting that cytokine secretion by tumor-reactive immune cells may promote PD-L1 expression to create a negative feedback loop protecting tumor cells from immune destruction. These findings provide a rationale for future studies evaluating the potential role of PD-1/PD-L1 immune checkpoint blockade therapy in treating MCC.
The natural history of malignancies linked with viral infections, which are frequently associated with chronic inflammation, is often influenced by the immunologic state of the host. The results of the current study contribute to the emerging theme that the immune regulatory PD-L1/PD-1 axis may play a significant role in the development and progression of virus-associated cancers. Intratumoral expression of PD-L1 and/or PD-1 has been demonstrated in human papillomavirus (HPV)-associated HNSCC (28, 36), hepatitis B virus (HBV)-related hepatocellular carcinoma (37), Epstein-Barr virus-associated nasopharyngeal cancer (38), and adult T-cell leukemia/lymphoma linked to human T-cell leukemia virus-1 (39). In each of these cancers, the PD-L1/PD-1 axis is thought to be up-regulated as a part of host's innate and adaptive immune response to the presence of virus, yet expression of these molecules enables tumor evasion from cytotoxic T cell attack. However, the balance between immune activation and tolerance may depend on tumor type. For example, in this study we demonstrated an association between MCPyV DNA, a brisk immune infiltrate, local expression of PD-L1, and improved patient survival, indicating a degree of MCC containment by the host immune system. We postulate that high lymphocyte infiltration and consequent PD-L1 up-regulation in a subset of virus-positive tumors reflects both enhanced antigenicity associated with viral infection and virus independent factors, such as E-selectin expression on vascular endothelium that promotes lymphocyte egress.(40) Of note, Rodig and colleagues, in a recently published study using ultra-sensitive IHC and quantitative molecular techniques, demonstrated that MCPyV DNA was detectable in almost all MCC specimens analyzed.(3) It may be that integration of MCPyV DNA into host DNA – and subsequent expression of oncogenic viral proteins – beyond a certain threshold is required for immune activation of the tumor microenvironment.
Increased levels of PD-1+ TILs cells have recently been correlated with a favorable clinical outcome in HNSCC.(36) In contrast, in patients with HBV+ hepatocellular carcinoma, high levels of circulating PD-1+ and PD-L1+ cells were associated with a worse prognosis.(37) In virus-associated malignancies, PD-L1 expression likely reflects both a host reaction to the tumor, and the chronic inflammatory environment triggered by the presence of virus. Because PD-L1/PD-1 checkpoint blockade has been shown to reverse virus-specific T-cell exhaustion and facilitate virus clearance (41, 42), it is an attractive therapeutic option not only for virus-associated malignancies, but also for treating the underlying infection.
As more knowledge is gained about the expression patterns of PD-L1 in various human cancers, it appears that different cancers may demonstrate distinct characteristics reflecting the cell type or anatomic site of origin, the specific inflammatory trigger (e.g., virus or tumor neoantigens), the resultant cytokine milieu, cellular subpopulations dominating the immune infiltrate, and/or activation of aberrant molecular signaling pathways. For instance, in the current study, PD-L1 expression was predominantly seen in the associated lymphohistiocytic infiltrates and was focal when observed in the tumor cells. We have also observed cases of colorectal carcinoma with prominent PD-L1 expression by tumor-associated macrophages and minimal tumor cell surface expression.(23) In contrast, as previously reported in melanoma; PD-L1 expression was seen in both the tumor and infiltrating immune cells, in more equal proportions.(26) We have reported PD-L1+ tumor cells to be geographically associated with TILs in the majority of MCCs, melanomas, and HPV+ HNSCCs; however, in singular cases we observed PD-L1 expression out of proportion to infiltrating immune cells, suggesting oncogene-driven expression as described in association with PTEN loss in glioblastoma multiforme (43) and in HPV(−) HNSCC (28). Separate models of innate (constitutive) and adaptive tumor immune resistance via PD-L1 expression have been proposed.(44) These are not mutually exclusive; it is possible that both of these mechanisms can be operative independently or concordantly, and their relative contributions may vary by tumor type. Studies investigating the interplay between oncogenic-driver pathways in different tumor types, PD-L1 expression, and different populations of infiltrating immune cells are currently underway in our laboratories.
In both melanoma and HNSCC, interferon (IFN−)gamma has been highlighted as a major cytokine driving PD-L1 expression.(26, 28) A number of other factors, including LAG-3, IL-17, IL-10, IL-6, IL-21 and other common gamma chain cytokines have also been identified in association with PD-L1 expression.(45-48) Although we did not directly investigate soluble factors associated with PD-L1 expression in MCC, we anticipate that IFN-gamma plays a strong role. MCC-specific TILs have previously been shown to secrete IFN-gamma (11), and Nghiem and colleagues also demonstrated that in MCC patients with better prognosis, tumors contained CD8+ lymphocytic infiltrates and overexpressed IFN-gamma mRNA.(13) Consistent with these findings, when tumor cell PD-L1 expression was observed in our cohort, it was in geographic association with CD8+ TILs, and it was shown to be a positive prognostic factor.
In summary, a growing body of evidence suggests that the natural history of MCC is significantly impacted by the immunologic state of the host. Here, we demonstrate that PD-L1 expression by MCC is an independent prognostic factor predicting improved overall survival in a cohort of patients from a single institution. The results of our study indicate that MCC tumor cells and the surrounding microenvironment display elements of immune activation that may be augmented with immune checkpoint blocking agents such as anti-PD-1 and anti-PD-L1, and provide a rationale for exploring these therapies in a disease that currently lacks effective treatment options for advanced disease.
Table 4.
Viral status related to TIL intensity and PD-L1 expression by tumor cells and TILs
| All specimens, n=42 n (%) | MCPyV(−), n=8 n, (%) | MCPyV+, n=34 n, (%) | p-valueb | |
|---|---|---|---|---|
| Tumor PD-L1(−) | 25 (60) | 8 (100) | 17 (50) | 0.0135 |
| Tumor PD-L1 + | 17 (40) | 0 (0) | 17 (50) | |
| TIL score 0,1a | 25 (60) | 8 (100) | 17 (50) | 0.0135 |
| TIL score 2,3 | 17 (40) | 0 (0) | 17 (50) | |
| TIL PD-L1(−) | 21 (50) | 6 (75) | 15 (44) | 0.238 |
| TIL PD-L1 + | 21 (50) | 2 (25) | 19 (56) |
TILs (lymphocytes and associated histiocytes) were scored as (0), none; (1), “mild”; (2), “moderate”; or (3), “severe” as described in Methods.
Fisher's exact test
ACKNOWLEDGEMENTS
We thank Dr. Drew Pardoll (Johns Hopkins University School of Medicine) for critical review of the manuscript, insights and support. We also thank Dr. Lieping Chen (Yale University School of Medicine) for helpful discussions and for providing the anti-PD-L1 monoclonal antibody 5H1.
Research support for this study was provided by the National Institutes of Health [R01 CA142779 (JMT, SLT), R01DK081417 (RAA), R01DK080736 (RAA) and P30 CA006973 (BSL, HW, DS, RAA, SLT)], the Barney Family Foundation (EJL, JMT, SLT), the Laverna Hahn Charitable Trust (EJL, JMT, SLT), and the Commonwealth Foundation (JMT, HX).
Footnotes
Conflict of Interest Disclosures:
EJL, JGV, ML, LTK, BSL, HW, SKN, TSW, DS: none
HX: consultant for Amplimmune
RAA: consultant (uncompensated), research funding, and travel reimbursement from Bristol-Myers Squibb
SLT: consultant (uncompensated), research funding, and travel reimbursement from Bristol-Myers Squibb; consultant for Amplimmune (spouse); consultant for Jounce Therapeutics; patent royalties distributed through institution (spouse) from Amplimmune Inc. and Bristol-Myers Squibb
JMT: consultant (uncompensated), research funding, and travel reimbursement from Bristol-Myers Squibb
REFERENCES
- 1.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gjoerup O, Chang Y. Update on human polyomaviruses and cancer. Adv Cancer Res. 2010;106:1–51. doi: 10.1016/S0065-230X(10)06001-X. [DOI] [PubMed] [Google Scholar]
- 3.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all merkel cell carcinomas harbor merkel polyomavirus. J Clin Invest. 2012;122(12):4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1545–9. doi: 10.1158/1055-9965.EPI-05-0895. [DOI] [PubMed] [Google Scholar]
- 5.Ascoli V, Minelli G, Kanieff M, Frova L, Conti S. Merkel cell carcinoma: A population-based study on mortality and the association with other cancers. Cancer Causes Control. 2011;22(11):1521–7. doi: 10.1007/s10552-011-9826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gass JK, Chan SK, Rytina E, Greenberg DC, Burrows NP. Multiple primary malignancies in patients with merkel cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24(5):601–3. doi: 10.1111/j.1468-3083.2009.03458.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang TS, Byrne PJ, Jacobs LK, Taube JM. Merkel cell carcinoma: Update and review. Semin Cutan Med Surg. 2011;30(1):48–56. doi: 10.1016/j.sder.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of merkel cell carcinoma: Case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8(3):204–8. doi: 10.1007/s10434-001-0204-4. [DOI] [PubMed] [Google Scholar]
- 9.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with merkel cell polyomavirus infection in merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938–45. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 11.Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in merkel cell carcinomas and blood. Clin Cancer Res. 2011;17(21):6671–80. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touze A, Le Bidre E, Laude H, Fleury MJ, Cazal R, Arnold F, et al. High levels of antibodies against merkel cell polyomavirus identify a subset of patients with merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29(12):1612–9. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 13.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29(12):1539–46. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor infiltrating immune cells and outcome of merkel cell carcinoma: A population-based study. Clin Cancer Res. 2012;18(10):2872–81. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 15.Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–6. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 17.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 21.Topalian SL, Brahmer JR, Hodi FS, McDermott DF, Smith DC, Gettinger S, et al. Anti-programmed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients with advanced solid tumors: Clinical activity, safety, and molecular markers. Ann Oncol. 2012;23(suppl 9):ix152–ix174. [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective re-induction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-2625. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edge SB. American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging handbook : From the AJCC cancer staging manual. Springer; New York: 2010. [Google Scholar]
- 26.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Co-localization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao P, Balzer BL, Lemos BD, Liegeois NJ, McNiff JM, Nghiem P, et al. Protocol for the examination of specimens from patients with merkel cell carcinoma of the skin. Arch Pathol Lab Med. 2010;134(3):341–4. doi: 10.5858/134.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loyo M, Schussel J, Colantuoni E, Califano J, Brait M, Kang S, et al. Detection of merkel cell virus and correlation with histologic presence of merkel cell carcinoma in sentinel lymph nodes. Br J Cancer. 2012;106(7):1314–9. doi: 10.1038/bjc.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loyo M, Guerrero-Preston R, Brait M, Hoque MO, Chuang A, Kim MS, et al. Quantitative detection of merkel cell virus in human tissues and possible mode of transmission. Int J Cancer. 2010;126(12):2991–6. doi: 10.1002/ijc.24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in merkel cell carcinoma. Clin Cancer Res. 2011;17(14):4806–13. doi: 10.1158/1078-0432.CCR-10-3363. [DOI] [PubMed] [Google Scholar]
- 32.Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, et al. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33(9):1378–85. doi: 10.1097/PAS.0b013e3181aa30a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 36.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 37.Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ, et al. Upregulation of circulating PDL1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011;6(9):e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23(10):1393–403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 39.Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23(2):375–82. doi: 10.1038/leu.2008.272. [DOI] [PubMed] [Google Scholar]
- 40.Afanasiev OK, Nagase K, Simonson W, Vandeven N, Blom A, Koelle DM, et al. Vascular E-selectin expression correlates with CD8 lymphocyte infiltration and improved outcome in merkel cell carcinoma. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 42.Yao S, Chen L. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol Med. 2006;12(6):244–6. doi: 10.1016/j.molmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 44.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41(2):413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41(8):2314–22. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]
- 47.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 48.Young GD, McMiller TL, Xu H, Chen S, Berger AE, Fan J, et al. AACR Annual Meeting. Washington, DC: 2013. Differential expression of immuno-regulatory genes associated with PD-L1 display: Implications for clinical blockade of the PD-1/PD-L1 pathway in melanoma. (abstract #446) [DOI] [PMC free article] [PubMed] [Google Scholar]