Abstract
A new initiative of the United States (U.S.) Dialysis Outcomes and Practice Patterns Study (DOPPS), the DOPPS Practice Monitor (DPM) provides up-to-date data and analyses to monitor trends in dialysis practice during implementation of the new Centers for Medicare and Medicaid Services (CMS) End-Stage Renal Disease (ESRD) Prospective Payment System (PPS; 2011–2014). We review DPM rationale, design, sampling approach, analytic methods, and facility sample characteristics. Using stratified random sampling, the sample of ~145 U.S. facilities provides results representative nationally and by facility type (dialysis organization size, rural/urban, free-standing/hospital-based), achieving coverage similar to the CMS sample frame at average values and tails of the distributions for key measures and patient characteristics. A publicly available Web report (www.dopps.org/DPM) provides detailed trends including demographic, comorbidity, and dialysis data, medications, vascular access, and quality of life. Findings are updated every 4 months and lagged only 3–4 months. Baseline data are from mid-2010, prior to the new PPS. In sum, the DPM provides timely, representative data to monitor the effects of the expanded PPS on dialysis practice. Findings can serve as an early warning system for possible adverse effects on clinical care and as a basis for community outreach, editorial comment, and informed advocacy.
Index Words: End-stage renal disease, Prospective Payment System, Quality Incentive Program, Dialysis Outcomes and Practice Patterns Study
Introduction
Payment for United States (U.S.) dialysis services has changed fundamentally with the new Centers for Medicare and Medicaid Services (CMS) End-Stage Renal Disease (ESRD) Prospective Payment System (PPS, 2011 to 2014) and the Quality Incentive Program (QIP, 2012), (1, 2). These payment reforms establish new financial incentives and constraints for dialysis facilities. Due to these altered incentives, there is potential for substantial changes in a broad range of clinical dialysis practices, and the need to promptly monitor access to and quality of dialysis care under the expanded PPS has been highlighted (3,4,5). The DOPPS Practice Monitor (DPM) is a new initiative to monitor trends in dialysis practice during both PPS and QIP implementation. To our knowledge, no other nationally representative data source currently exists to fulfill this role in a timely manner.
To achieve DPM aims, the DOPPS has increased U.S. enrollment from 85 to 137 dialysis facilities, with a final target sample of 145 facilities. Stratified random sampling has assured that the sample is representative nationally and by facility type (dialysis organization size, rural/urban location, etc). A key feature for the DPM is a detailed, publicly available Web report to describe trends in clinical practice, including achievement of CMS Quality Measures and clinical practice guidelines. The website was launched in November 2010 with baseline data from mid 2010, i.e. prior to implementation of the expanded PPS. Findings are presented for the nation overall and by facility type, and are updated every four months with a lag of only three to four months for most data.
By providing timely, nationally representative data for U.S. dialysis patients and facilities, the DPM is an important independent data source to report trends in dialysis practice, to evaluate the impact on clinical outcomes, and to serve as a basis for informed advocacy. In this article, we review the DPM rationale, design, sampling approach, and analytic methods. We also demonstrate that the DPM facility sample is representative of U.S. dialysis facilities overall, of facility subtypes that may respond differently to reimbursement policy change, and of patient groups with higher dialysis costs who may be most vulnerable to these changes in care.
Methods
DOPPS and DPM Sampling
Begun in 1996, the DOPPS is a prospective cohort study that has studied hemodialysis (HD) patients and practices over four study phases in 12 countries. The most recent phase, DOPPS 4, began in 2009. Detailed study methods have been published previously (6,7). These methods have been applied by the DOPPS since its inception to provide nationally representative statistics for many different types of HD practice measures, patient characteristics, and outcomes. Informed patient consent is obtained as indicated in accordance with local requirements.
Facility Sample Frame
In each DOPPS country, facilities are randomly selected from a list of all dialysis facilities treating at least 20 adult (aged ≥18) in-center chronic HD patients. For the U.S. DPM, the sample frame is based on the 2008 Dialysis Facility Compare (DFC) database (8) created from CMS data obtained through a data use agreement. This database provides demographic and quality-of-care measures for all Medicare-certified dialysis facilities in the U.S. through the end of 2007. Data from the DFC is augmented with facility patient counts obtained from the July 2008 Annual Facility Survey database (9).
Of 4,959 facilities, 4,343 (88%) served at least 20 chronic adult in-center HD patients and comprised the final sample frame of facilities eligible for DPM participation. The restriction to facilities treating at least 20 patients was a design feature to provide reliable estimates of practice at each facility, and is readily justifiable because these facilities served 97% of U.S. HD patients. From this sample frame, approximately 145 dialysis units were targeted for DPM recruitment.
Facility Sampling Strata
Random facility selection was performed within pre-specified strata of different facility types. Prior DOPPS experience has demonstrated that data from 20 randomly selected facilities yield reliable estimates to accurately represent practices within a particular facility grouping. Therefore, targets were set to select a minimum of 20 facilities per category in the following three strata:
(A) DIALYSIS ORGANIZATION SIZE
Free-standing dialysis units were classified as part of an LDO (large dialysis organization;>500 dialysis facilities), MDO (medium-size dialysis organization;11–500 facilities), and independent or SDO (small-size dialysis organization; ≤10 dialysis facilities). Currently, the LDO stratum is composed of only the two largest U.S. dialysis organizations, each having well over 1000 facilities. Since there are no standard definitions to distinguish a SDO from a MDO, the cut point at 10 facilities was used to meet the needs of the DPM sampling design.
(B) HOSPITAL-based / NON-HOSPITAL-based
All dialysis units were categorized as hospital-based or free-standing, non-hospital based dialysis units. All hospital-based units in CMS data were SDO/independent facilities.
(C) RURAL / NON-RURAL LOCATION
Based on their zip code, dialysis units were grouped into three categories according to the 2004 census tract-based rural-urban commuting area (RUCA) codes (10), as well as the approximate travel time from the center of each zip code to the nearest urbanized area. Facilities were grouped into the three categories of: (i) Remote Rural, population <10,000 with >45 minutes travel time to the nearest urbanized area, (ii) Non-remote Rural, population <10,000 with <45 minutes travel time, and (iii) Non-rural, population ≥10,000. As there is no standard method of combining RUCA codes, we chose our definition of rurality to be consistent with common applications.
Patient Sampling
Data are collected from 20–40 randomly selected chronic in-center HD patients enrolled in the study at each participating study site, using a selection algorithm that selects a larger number of patients with increasing size of the dialysis unit to yield an overall average of ~30 study patients per facility. Patients departing the study (e.g., due to death, transfer to other facilities, switch to peritoneal dialysis or home HD, transplantation, or recovery of renal function) are replaced at four month intervals. Replacements are determined by random selection of patients new to the facility since the prior selection, providing prevalent cross-sections that are representative of patients in each facility at every four-month interval (6).
Sampling Weights
Facility sampling weights, patient sampling weights, and post-stratification weights are applied to report data that are representative at the national and facility stratum levels (11). To obtain ≥20 facilities in each stratum category, over-sampling of facilities occur in some categories (Table 1). The fraction of facilities within each stratum is termed the facility sampling fraction. The reciprocal of the facility sampling fraction is the facility sampling weight. Within each facility, data are collected on a random sample of 20–40 chronic adult patients. The fraction of all the facility’s chronic adult HD patients that participate in the study at a sampling selection time point is termed the patient sampling fraction. The reciprocal of the patient sampling fraction serves as the patient sampling weight. Additionally, post-stratification weights are computed for each patient to correct for disproportionate consent rates among basic demographic variables, years with ESRD, and diabetes as ESRD cause, which are collected on all facility patients.
Table 1.
Distribution of DPM facilities by facility characteristics, showing intentional oversampling in some strata
| (A) Facility Characteristics Used for Stratified Sampling of DPM Facilities | |||
|---|---|---|---|
|
| |||
| Facility Characteristic | Categories | N (%) of CMS facilities | N (%) of facilities in US DOPPSa |
| Dialysis organization size | LDO | 2704 (62.3) | 68 (49.6) |
| MDO | 633 (14.6) | 22 (16.1) | |
| SDO | 1006 (23.2) | 47 (34.3) | |
|
| |||
| Rurality | Non-rural | 3923 (90.3) | 82 (59.9) |
| Rural (Local) | 180 (4.1) | 26 (19.0) | |
| Rural (Remote) | 240 (5.5) | 29 (21.2) | |
|
| |||
| Hospital-based | Hospital-based | 235 (5.4) | 19 (13.9) |
| Free-standing | 4108 (94.6) | 118 (86.1) | |
|
| |||
| (B) Facility Characteristics NOT Used for Sampling of DPM Facilities | |||
|
| |||
| Profit status | For-profit | 3574 (83.0) | 91 (67.4) |
| Non-profit | 732 (17.0) | 44 (32.6) | |
|
| |||
| Geographic region | Central | 1010 (23.3) | 43 (31.4) |
| East | 951 (21.9) | 39 (28.5) | |
| South | 1205 (27.8) | 27 (19.7) | |
| West | 1177 (27.1) | 28 (20.4) | |
|
| |||
| Total | N=4343 | N=137 | |
LDO=large dialysis organization (>500 units); MDO=medium dialysis organization (11–500 units); SDO=small or independent dialysis organization (≤10 units). Rurality as defined in text.
As of September 2010
With the exception of hospital based facilities (19 recruited to date), the minimum study target of 20 facilities per selection stratum has been achieved in all sampling strata.
Data Elements and Reporting
DOPPS Data Elements
Patient-level study data are limited to routinely-collected clinical data. Data are collected by manual entry and/or electronic extraction from patient medical records. At study entry, data include demographics, detailed comorbidities, vascular access, medication prescription and dose, and laboratory values. Patient-level data updated every four months include: laboratory values (recorded monthly); medication prescription and dose (prescription and dose for mineral metabolism and anemia-related medications are updated monthly); vascular access procedures, other clinical procedures, and hospitalizations (with cause and procedures); date and causes of death; and reason for study departure. Patient self-reported data (e.g., QoL, depression symptoms, satisfaction with care, and others) are collected yearly. Facility survey data related to practice preferences, services offered, and staffing are collected yearly from the facility medical director and nurse study manager. DOPPS data collection instruments are updated annually.
DPM Web Reports
Data on the DPM website are updated every four months with a lag of three to four months from the time of data collection to appearance on the Web report. The DPM provides national and stratum-specific descriptive statistics and trends in the following nine clinical areas: demographics, comorbidities, anemia, mineral and bone disorder (MBD), dialysis prescription and dose, vascular access, cardiovascular medications, nutrition, and quality of life (QoL). Demographic and comorbidity data are reported annually. Vascular access use is reported every four months. Medication use is reported monthly for anemia and MBD medications, and once every four months for other medications. Erythropoietin Stimulating Agents (ESA) and IV iron dose are also reported. Laboratory data are reported for each month as the mean of the last two to three monthly values, except for less frequently measured labs such as parathyroid hormone (PTH). QoL is reported annually as physical and mental component summary scores. Examples of detailed data reported are provided for anemia and MBD measures in Box 1. Sample graphical presentations for DPM data are provided in Figure 1. Altogether >1300 charts and tables are provided on the DPM website. Highlighted graphics are downloadable.
Box 1. Detailed list of data elements reported in the DPM for anemia and mineral and bone disorder.
| Anemia Measures |
|---|
ESA use
|
ESA dose
|
Hemoglobin
|
Iron use
|
IV iron dose
|
Serum ferritin
|
Serum TSAT
|
| Mineral and Bone Disorder Measures |
Serum calcium, total
|
Serum calcium, albumin-corrected
|
Serum phosphorus
|
Serum PTH
|
PTH Measurement
|
Phosphate binder
|
Vitamin D Analog
|
Cinacalcet
|
Specifications of variables for reporting purposes are reported in the Methods section. Each item will be reported on the website for the nation overall and by facility type.
Note: Conversion factors for units: serum calcium in mg/dL to mmol/L, ×0.2495; serum phosphorus in mg/dL to mmol/L, ×0.3229; hemoglobin in g/dL to g/L, ×10; iron in μg/dL to μmol/L, ×0.179; No conversion necessary for ferritin in ng/mL and μg/L or parathyroid hormone (PTH) in pg/mL and ng/L.
Figure 1. Examples of graphics used in the DPM reports.
Panel A: Sample Box-and-Whisker Chart (Modified)
At each time period on the X axis, the central dot represents the 50th percentile (median) of the distribution, the vertical line below the central dot connects the 10th and 25th percentile values, and the vertical line above the central dot connects the 75th and 90th percentile values. Additional statistics (mean, standard deviation, etc.) are provided in a separate data table (not shown). Values at each month are based on the average of at least two measurements obtained during the prior three months. Conversion factors for units: mg/dL to mmol/L, ×0.3229.
Panel B: Sample “Stacked” Column Chart
Segment heights represent the proportion of observations in each category at the time period indicated on the X axis. Values at each month are based on the average of at least two measurements obtained during the prior three months. Conversion factors for units: mg/dL to mmol/L, ×0.3229.
Trends are monitored for variables reported on the DPM website. Tests of location (T tests, quantile regression) that account for repeated measures are used to assess significant differences between baseline (i.e. pre-2011) values and post-PPS (i.e. 2011–2014) values. After sufficient data are obtained in 2011 to establish post-PPS trends, additional modeling techniques, including longitudinal mixed models and spline regression, will be used to quantify the effect of PPS implementation on trends in monitored variables. An Executive Summary provided with each DPM update highlights notable findings.
Specific clinical data are not provided on the website, but will form the basis for multivariable analyses and peer-reviewed publications. These include death, hospitalizations, clinical procedures and other events, facility survey data, and selected patient self-reported data.
Evaluating the DPM sample
To assess the extent to which the sample of DPM facilities is representative of U.S. HD facilities overall, 2007 CMS data for the 137 DPM facilities are compared with the 2007 CMS data for all remaining U.S. HD facilities treating at least 20 in-center chronic HD patients (N=4,206) (8). Analyses are limited to chronic HD patients aged ≥18 years. Comparisons are performed for eight patient characteristics or laboratory measures. These items are chosen because they are part of the 2012 CMS QIP; are identified as important in a recent Government Accountability (GAO) report (3); or are otherwise expected to be potentially affected by the new PPS. Comparisons are tested using a weighted T test.
Additionally, we sought to demonstrate the reliability of DOPPS data versus the CMS database. Baseline data from a prevalent cross-section of patients in all 66 DOPPS phase 3 U.S. facilities (study dates: 2005–2008) are expressed at the facility level using summary methods comparable to CMS. To form the comparison group, we select a record from the CMS dialysis facility reports database that most closely matches the date when the facility was recruited into the DOPPS. Group-wise comparisons (DOPPS phase 3 vs. CMS) are conducted using the two-sample T test and the Wilcoxon rank-sum test.
Results
Description of the U.S. DOPPS Facility Sample
As of September 2010, 291 U.S. HD facilities had been contacted for participation in the DOPPS as part of the DPM initiative, of which 137 (47%) had agreed and were actively participating. The distribution of these units by facility characteristics is shown in Table 1. The distribution illustrates the purposive over-sampling method used to monitor clinical practice in certain facility types that are less common in the U.S, including SDO/independent, hospital-based, and rural units. The stratified random selection scheme also resulted in a sufficient number of participating units in other (non-sampling) facility strata, including profit status and geographic region, to provide meaningful practice data in these facility strata.
Comparison to Facilities Declining Participation
Among the 53% of sampled facilities declining participation, burden to facility staff was the most common reason cited. Weighted comparisons of 2007 CMS data between the facilities declining (“declined”) and actively participating (“DPM”) are presented in Table 2. Facilities that declined participation had a mean facility standardized mortality ratio (SMR) value 0.06 points higher than DPM participating facilities (0.95 vs. 0.89; p=0.2), as well as a higher percentage of African Americans (35.9% vs. 26.1%, p=0.03).
Table 2.
Comparisons of DPM sample with CMS sample frame
| Sample | Facility N | SMR | % with age >65 | % African American | % with Hgb 10–12 g/dL | % with URR >65% | % with catheter >90 days | % with Medicaid | % on PD | |
|---|---|---|---|---|---|---|---|---|---|---|
| All facility typesa | DPM | 137 | 0.89 | 45.6 | 26.1 | 55.0 | 95.5 | 11.7 | 21.1 | 5.9 |
| Declined | 154 | 0.95 | 44.0 | 35.9* | 54.8 | 93.5 | 10.2 | 22.9 | 4.6 | |
|
| ||||||||||
| Non-DPM | 4206 | 0.95 | 45.1 | 35.8** | 53.5 | 95.7 | 11.5 | 23.8 | 5.0 | |
|
| ||||||||||
| LDO | DPM | 68 | 0.88 | 44.3 | 29.1 | 47.0 | 96.5 | 10.8 | 21.7 | 6.0 |
| Non-DPM | 2636 | 0.96 | 44.3 | 38.1* | 47.5 | 96.5 | 11.0 | 23.8 | 4.9 | |
|
| ||||||||||
| MDO | DPM | 22 | 0.98 | 50.7 | 8.7 | 67.4 | 92.9 | 13.1 | 16.0 | 0.1 |
| Non-DPM | 611 | 0.92 | 45.4 | 37.4** | 63.5 | 94.2 | 12.2 | 24.3† | 4.8** | |
|
| ||||||||||
| SDO | DPM | 28 | 0.83 | 45.5 | 29.8 | 71.3 | 94.9 | 12.9 | 22.5 | 9.0 |
| Non-DPM | 743 | 0.94† | 47.3 | 28.8 | 63.5* | 94.2 | 12.0 | 23.7 | 4.4 | |
|
| ||||||||||
| Non-Rural | DPM | 69 | 0.89 | 45.0 | 27.1 | 54.6 | 95.9 | 11.5 | 20.6 | 6.2 |
| Non-DPM | 3638 | 0.95 | 44.9 | 35.9* | 53.1 | 95.6 | 11.4 | 23.4 | 5.1 | |
|
| ||||||||||
| Rural (Local) | DPM | 25 | 0.82 | 48.4 | 29.2 | 51.2 | 96.7 | 13.1 | 26.0 | 1.4 |
| Non-DPM | 150 | 0.97† | 46.8 | 42.4† | 50.7 | 96.9 | 11.1 | 27.4 | 0.9 | |
|
| ||||||||||
| Rural (Remote) | DPM | 24 | 0.89 | 50.2 | 15.5 | 54.3 | 91.1 | 10.8 | 25.0 | 2.3 |
| Non-DPM | 202 | 0.88 | 45.3 | 38.5** | 49.6 | 96.4 | 10.2 | 29.3 | 2.0 | |
|
| ||||||||||
| Free-standing | DPM | 118 | 0.87 | 45.4 | 26.5 | 54.4 | 95.7 | 11.5 | 21.1 | 5.8 |
| Non-DPM | 3990 | 0.95† | 45.0 | 36.3** | 52.9 | 95.7 | 11.4 | 23.9 | 4.8 | |
|
| ||||||||||
| Hospital-based | DPM | 19 | 0.88 | 48.2 | 18.6 | 65.9 | 91.6 | 14.7 | 22.2 | 8.7 |
| Non-DPM | 216 | 0.86 | 47.0 | 27.2 | 65.5 | 94.7† | 14.5 | 23.1 | 8.1 | |
Note: Conversion factors for units: hemoglobin in g/dL to g/L, ×10.
“DPM” includes participating DPM facilities as of Sep 2010; “declined” includes sampled facilities that declined to participate or left the study prior to Sep 2010; “Non-DPM” includes facilities in the CMS sample frame that had not been selected for DPM participation as of Sep 2010. Values shown are means of CMS facility-level data. p values are for T tests comparing “Non-DPM” to “DPM” and comparing “Declined” to “DPM”, .”
p 0.05 to <0.10;
p 0.01 to <0.05;
p<0.01 (p≥0.10 if not shown)
Mean DPM values are weighted to provide national or stratum-specific estimates.
Comparison to U.S. Dialysis Units Overall
We analyzed 2007 CMS data to compare the distribution of eight variables in DPM facilities (“DPM”) with their distribution among the CMS non-DPM sample frame (N=4,206). The DPM sample did not differ appreciably from the CMS sample frame (p>0.10) for six of the eight selected variables. DPM facilities have fewer African American patients than the CMS non-DPM sample (26.1% vs. 35.8%, p=0.001). The mean SMR was lower for the DPM sample (0.89 vs. 0.95 in the CMS, non-DPM sample), although this difference was not statistically significant (p = 0.10).
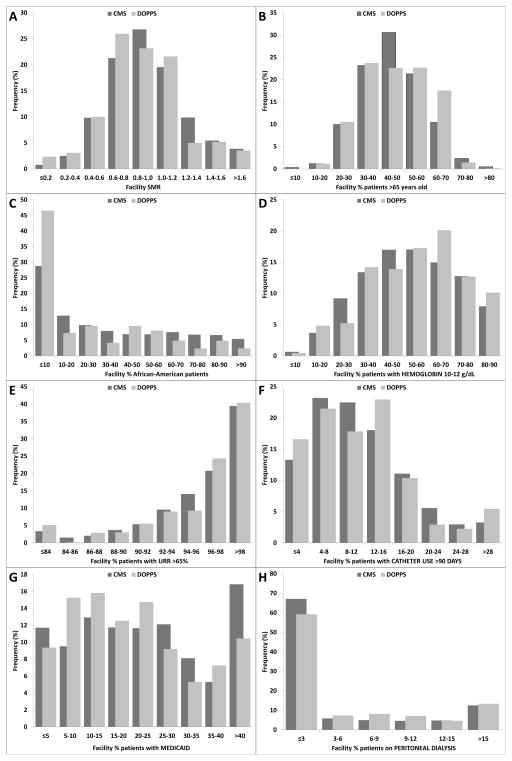
The weighted distributions of the facility variables in the DPM sample and CMS non-DPM sample are shown in Figure 2A–H. The DPM achieves sampling coverage similar to that in the CMS sample frame at the tails of each of the distributions, in addition to similar mean values (Table 2). This is important since practice changes in response to the new PPS may have different effects on practice at the tails of the distribution of a particular patient characteristic or practice area. Evaluation of unweighted distributions demonstrates that, for the eight facility variables, the DPM sample provides at least 24 facilities below the 20% percentile and at least 19 facilities above the 80% percentile of each variable’s distribution. Thus, the DPM has an adequate facility sample (≥20 facilities) to report trends in care and to test auxiliary hypotheses specifically among facilities in the lowest and highest 20% of the overall CMS distribution.
Figure 2.
Figures 2A–H. Distributions of CMS data for facilities in the DOPPS sample compared to the CMS facility sample frame
Values were calculated for eight patient/clinical measures among a prevalent cross-section of HD patients ≥18 years old in the 2008 Dialysis Facility Compare data base, based on 2007 CMS data. Each panel displays the distribution of values for the indicated measure across dialysis facilities in the DPM facility sample (N=137 facilities) and the CMS facility sample frame (N=4,206) of facilities treating ≥20 chronic in-center HD patients. Conversion factor for hemoglobin in g/dL to g/L, ×10.
Several differences between the DPM sample and the CMS sample frame are worth comment.
The overall fraction of African American patients is lower, and the fraction of facilities with <10% African American patients is higher, in the DPM facility sample than in the CMS sample frame. This result is partially attributable to purposely oversampling remote-rural facilities, although DPM facilities also display a lower prevalence of African American patients in the rural (non-remote) and non-rural facility strata. However, nearly one-fourth of DPM facilities have at least 50% African American patients (Figure 2C). Thus, the DPM can readily report trends in care for African American patients, as well as for facilities with a high proportion of African American patients. The overall fractions of Asian and Native American patients in the DPM facilities (3.7% and 2.4%, respectively) are comparable to the overall CMS sample frame (3.6% and 1.8%, respectively).
The mean SMR for the DPM sample is lower than for the CMS sample frame. However, the DPM facility sample provides representation across the range of SMRs in the CMS sample frame (Figure 2A–H).
Reliability of the U.S. DOPPS data
To test the assumption that data from the DOPPS sample of 20–40 patients per facility are representative of these facilities overall, we compared DOPPS data with CMS data in the 66 facilities participating in U.S. DOPPS phase 3 (2005–2008) (Table 3). Differences in patient characteristics (aged >65, African American, catheter use) were small and not statistically significant (p>0.10). The small differences between DOPPS and CMS data for Hgb and urea reduction ratio (URR) targets (p>0.10 and p=0.07, respectively) are likely due to differences in measure specification. CMS data are expressed as patient-annualized averages whereas the DOPPS estimates are based on two monthly values obtained approximately four months apart for each patient in the facility cross-sectional sample.
Table 3.
Comparison of DOPPS data with CMS 2005–2007 facility-level data for facilities in US DOPPS Phase 3
| Sample | Facility N | % aged >65 | % African American | % with Hgb 10–12 g/dLa | % with URR >65%b | % with catheterc |
|---|---|---|---|---|---|---|
| DOPPS Phase 3 | 66 | 44.0 | 37.3 | 50.4 | 91.9 | 25.8 |
| CMS 2005–2007 | 66 | 45.6 | 34.3 | 52.9 | 94.7† | 28.2 |
Hgb= hemoglobin; URR=urea reduction ratio;
0.05<p<0.10 for two-sample T test (p≥0.10 if not shown)
Note: Conversion factors for units: hemoglobin in g/dL to g/L, ×10.
CMS 2005–2007 results use CMS data for the year in which the DOPPS facility began participating in US-DOPPS phase 3; DOPPS phase 3 results use DOPPS data collected for a prevalent cross-section of study patients in each US-DOPPS phase 3 facility at the time of initiating DOPPS phase 3 data collection between 2005–2007.
Hgb - Among Medicare patients with either ESA reimbursement claims (CMS result) or reported ESA prescription (DOPPS result). CMS results are expressed as percentage of patient-months during the year. DOPPS results are based on the mean of two monthly values obtained approximately four months apart.
URR - Among Medicare patients only. CMS results are expressed as percentage of patient-months during the year. DOPPS results are based on the mean of two monthly values obtained approximately four months apart.
Catheter - CMS results are expressed as percentage of patient-months during the year; DOPPS results are based on vascular access in use at study entry.
Discussion
On January 1, 2011, CMS fundamentally changed payment for dialysis services for Medicare patients with ESRD by making a single bundled payment (the expanded PPS) for each dialysis treatment to cover dialysis-related items and services (1). This has replaced the prior system, that made a single payment for dialysis and other composite rate services but paid separately for most drugs, laboratory tests, and other services provided by dialysis facilities. An option was provided to allow for a four-year transition period to the new PPS by 25% per year from 2011 to 2014. However, two separate surveys indicated that >90% of facilities were planning to choose 100% payment under the new PPS in January 2011 (12,13). CMS will also implement a QIP in January 2012, initially based on claims-based quality measures (Hgb<10 g/dL, Hgb>12 g/dL, URR≥65%) (2). Together, the expanded PPS and the QIP are intended to encourage facilities to operate efficiently while assuring high-quality care.
The DPM is a new U.S. DOPPS initiative designed to provide the community contemporary data and analyses that will monitor trends in dialysis practices from before to after PPS and QIP implementation. Facility recruitment for the DPM has been highly successful, with nearly 50% of contacted facilities participating in the study. As of September 2010, 137 facilities were participating with a final study goal of 145 facilities. The target of at least 20 facilities for each of the DPM facility strata has been recruited (with the exception of 19 hospital-based facilities to date), (Table 1). The DPM facility sampling approach has yielded a recruited sample that is representative of U.S. dialysis facilities overall, of key facility subtypes, and of particular patient groups. These distributions illustrate the capacity of the DPM to report trends in care not only for average facilities, but also among facilities performing at the tails of the distribution for particular patient characteristics or clinical practices.
The value of the DPM sample is the imperative to closely monitor trends in practice with the newly implemented PPS and the upcoming QIP. This payment reform will undoubtedly change dialysis practice, but many of the effects are difficult to predict due to the variety of financial incentives in the final rule (and upcoming rules) (3,14). On one hand, financial constraints may place pressures to limit care, perhaps by favoring staffing reductions to the extent possible or shifting time-consuming care away from the dialysis unit (e.g. to the emergency room or hospital). On the other hand, pressure to operate at capacity may incentivize programs to improve care by reducing skipped treatments (e.g., patient education, coordination of transportation) and decreasing avoidable, high cost hospitalizations (e.g. replacing central catheters with arteriovenous fistulae). Incentives may also increase training for and use of home dialysis therapies. Though the DPM is a study of in-center HD, it will monitor trends in patients switching from in-center HD to peritoneal dialysis or home HD and vice versa. Additionally, the QIP provides a mechanism for rewarding quality of care, initially based on measures of anemia and dialysis adequacy which have been proposed for 2012 (2). However, there is also a risk that such programs may divert attention from other clinical areas that are not being measured or rewarded (15).
Specific areas of clinical care will initially face greater pressures than others. As examples, we summarize here possible changes in the management of anemia and MBD. Box 1 lists many of the data elements in anemia and MBD that the DPM website will report. Regarding anemia management, average Hgb levels have fallen over the past several years after the publication of multi-center clinical trials and updated Food and Drug Administration guidance (16,17,18), and may fall even further. Several DOPPS observations support the probability that average ESA doses will decrease, while iron dosing may increase. First, ESA doses (for the same Hgb levels) have been consistently higher in the U.S. than in other DOPPS countries (19,20). However, some of this difference is accounted for by measurable differences in patient case-mix and the extent to which the remaining difference is discretionary and therefore modifiable (i.e., not due to patient factors) is unclear. Second, the DOPPS recently reported effects of the 2006 change in ESA reimbursement among dialysis patients in Japan, from a separately billable item to inclusion in a bundled dialysis treatment payment. With bundling, average ESA dose decreased by 12%, IV iron use per four months increased from 32% to 41% of patients, and Hgb levels remained essentially unchanged (21). While it is tempting to speculate that changing incentives in the U.S. may drive similar practice changes as seen in Japan, anemia management practices differ substantially between the U.S. and Japan, with much lower ESA and iron dosing, substantially lower IV iron use, and lower Hgb levels and target ranges in Japan versus the U.S.
MBD management will also likely change but in ways that are less predictable at this time because financial incentives regarding MBD management are less apparent and the 2012 QIP will not include MBD measures (2,22). Inclusion in the new PPS of all IV renal meds and their oral equivalents may change vitamin D analog prescription practices, such as switching to other vitamin D analogs and/or to other medication classes (e.g. calcimimetics) for managing PTH (1). Additionally, bundling of dialysis-related laboratory tests may result in less frequent measurement of PTH and other assays. Yet more dramatic practice changes can be expected closer to the time when payment for ESRD-related oral-only drugs, including phosphate binders and calcimimetics, are incorporated into the PPS in January 2014.
The DPM is responsive to identified priorities in the GAO report (3). As stated in that report, CMS should monitor access to and quality of dialysis care promptly after the new PPS is implemented. Preliminary CMS plans for monitoring the new PPS build on existing initiatives (e.g., the ESRD networks, surveyors, and DFC). The DPM complements these efforts by providing more timely and detailed information on trends in dialysis practices and quality of care. The GAO report also highlighted groups of beneficiaries with above average dialysis costs, including patients with Medicaid, African American race, and/or younger age, and cautioned that these patients may be most vulnerable. The DPM provides ample facility representation across the full spectrum of facilities treating these patient groups (Table 2, Figure 2), and thus, the DPM is a resource to readily follow trends in their care.
The DPM will also monitor access to care. Relevant data (not all available on the website) includes trends in total census size, patient case mix, medical coverage, and home modality use in the representative sample of DPM facilities, as well as trends possibly reflecting access to care such as changes in “adherence to care” (e.g. skipped treatments), use of medical procedures, and outcomes such as withdrawal from dialysis. The DPM is not designed to monitor other aspects of access to care, including overall or regional trends in ESRD incidence/prevalence and number of dialysis facilities.
A hypothesis motivating the DPM design is that responses to the new PPS will vary by facility type, potentially affecting patient care. For example, SDO and independent facilities may be at different risk than LDO facilities due to smaller volumes of patients and treatments, disadvantages in negotiating with pharmacy and laboratory vendors, and new administrative burdens (23,24). In addition, previous research has identified differences in anemia management practices by for-profit status and organizational affiliation (25,26,27). Hospital-based chronic dialysis facilities, most of which are independently owned, could face additional challenges. Choice among dialysis facilities for rural-area patients is often limited. Thus, closure of rural facilities or “cherry picking” of patients could limit access to care for these patients. Twenty-nine of the 240 facilities defined as rural-remote in the CMS sample are participating in the DPM, providing a unique opportunity to monitor practices and outcomes in this facility stratum. The DPM will report clinical trends in each of these, and other, facility types.
To follow effects of the new PPS on clinical outcomes and their costs, the DOPPS will capture detailed clinical event data. These include outpatient (e.g., vascular access) procedures, elective hospitalizations (e.g., blood transfusions, parathyroidectomy), urgent hospital admissions, and patient survival. The website will not report on trends in most clinical events because many are relatively uncommon and require a longer observation period and statistical adjustment for carefully assessing whether changes over time may not be due to random error. Patient-reported outcomes, including QoL and satisfaction with care, will also be collected. The DPM will serve as a unique source for following these QoL measures. The DPM will also be a source for facility-level survey data such as trends in facility services, staffing, etc. as the PPS is implemented.
Because the DPM is based on a sample of facilities and patients, it will require eventual confirmation with national CMS data. However, a great strength of the DPM sample is that it has been selected to provide national representation (unlike LDO data). The DPM is an efficient means to serve as an early warning system, as well as a reliable source for many data that will not be captured across the population via other data sources (e.g. patient-reported outcomes, vascular access care, facility services, etc.). Although the DOPPS previously has observed close agreement of its data with those reported by the U.S. Renal Data System and Fistula First Initiative, linkage with CMS data is planned for the DPM as a means to provide later confirmation of DPM findings.
A potential limitation is that practices may already have changed somewhat during 2010 in anticipation of the new PPS. National CMS data for comparisons of 2009 to 2010 will become available for analysis in late 2011, when DPM data from both 2010 and 2011 are available on the DPM website.
In sum, the DPM will fill an important need, providing a source of timely, representative data and tracking the effects of the expanded PPS on dialysis practice. Findings from the DPM reports can serve as an early warning system for possible adverse effects on clinical care and as a basis for patient and dialysis community outreach, editorial comment, and informed advocacy. Similar efforts are being considered for the international DOPPS to monitor practice changes that may follow implementation of national policy revisions in other countries participating in the DOPPS.
Acknowledgments
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications. The DPM, a new DOPPS initiative, is supported by Amgen. Editorial assistance was provided by Shauna Leighton of the Arbor Research Collaborative for Health.
Footnotes
Conflict of Interest
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications. The DPM, a new DOPPS initiative, is supported by Amgen. The authors have no other conflicts to declare.
References
- 1.Medicare Program; End-Stage Renal Disease Prospective Payment, Final Rule and Proposed Rule. Federal Register. 2010 Aug 12;75:155. 49030–49214. [PubMed] [Google Scholar]
- 2.Medicare Program; End-Stage Renal Disease Quality Incentive Program, Proposed Rule. Federal Register. 2010 Aug 12;75:155. 49215–49232. [Google Scholar]
- 3.United States Government Accountability Office. End-stage Renal Disease: CMS Should Monitor Access to and Quality of Dialysis Care Promptly after Implementation of New Bundled Payment System. United States Government Accountability Office; Washington, D.C: 2010. [Google Scholar]
- 4.MedPAC. Comment Letter: CMS proposed rule entitled Medicare Program; End-stage renal disease prospective payment system. 2009 Dec 16; Available at http://www.medpac.gov.
- 5.Sedor JR, Watnick S, Patel UD, et al. ASN End-Stage Renal Disease Task Force: perspective on prospective payments for renal dialysis facilities. JASN. 2010;21(8):1235–1237. doi: 10.1681/ASN.2010060656. [DOI] [PubMed] [Google Scholar]
- 6.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5Suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(Suppl74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. Dialysis Facility Compare. Available from: http://www.cms.gov/DialysisFacilityCompare/
- 9.Department of Health and Human Services, Centers for Medicare and Medicaid Services. Annual Facility Survey. 2008 Jul; Form CMS-2744A (02/04) [Google Scholar]
- 10.WWAMI Rural Health Research Center at the University of Washington, Seattle WA. Available at: http://depts.washington.edu/uwruca/index.php.
- 11.Kish L. Survey Sampling. Vol. 1965 New York: Wiley; 1965. [Google Scholar]
- 12.The Moran Company. Memorandum to Kidney Care Council: Modeling the Transition Budget Neutrality Adjustor for the ESRD Prospective Payment System (PPS) Final Data. 2010 Nov 2; Available at: http://www.nephronline.com/uploaded/transition%20adjustor.pdf.
- 13.National Renal Administrators Association. Letter to Donald Berwick, MD, Administrator for the Centers for Medicare & Medicaid Services. 2010 Oct 21; Available at: http://www.nephronline.com/uploaded/nraa_berwick_ltr_10-21-10-1.pdf.
- 14.Sargent JA. The dialysis industry faces bundling. [Accessed October 21, 2010];Renal Business Today Web site. 2010 Oct 20; Available at http://www.renalbusiness.com/articles/2010/10/the-dialysis-industry-faces-bundling.aspx.
- 15.Casalino LP, Alexander GC, Jin L, Konetzka RT. General internists’ views on pay-for-performance and public reporting of quality scores: a national survey. Health Affairs. 2007;26(2):492–499. doi: 10.1377/hlthaff.26.2.492. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 18.KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin targets. Am J Kidney Dis. 2007;50:479–512. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:94–111. doi: 10.1053/j.ajkd.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 20.McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int. 2010;78:215–223. doi: 10.1038/ki.2010.108. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa T, Bragg-Gresham JL, Pisoni RL, et al. Changes in anemia management and hemoglobin levels following revision of a bundling policy to incorporate recombinant human erythropoietin. Kidney Int. 2011;79(3):340–346. doi: 10.1038/ki.2010.382. [DOI] [PubMed] [Google Scholar]
- 22.Eckardt KU, Kasiske B. Kidney Disease Improving Global Outcomes (KDIGO): clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76(Suppl):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Bhat JG, Bhat P. The 2009 proposed rule for prospective ESRD payment: perspectives from a for-profit small dialysis organization. Am J Kidney Dis. 2010;55(2):231–3. doi: 10.1053/j.ajkd.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Sadler JH. The 2009 proposed rule for prospective ESRD payment: perspectives from a not-for-profit small dialysis organization. Am J Kidney Dis. 2010;55(2):229–30. doi: 10.1053/j.ajkd.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Thamer M, Zhang Y, Kaufman J, Cotter D, Dong F, Hernán MA. Dialysis facility ownership and epoetin dosing in patients receiving hemodialysis. JAMA. 2007;297(15):1667–1674. doi: 10.1001/jama.297.15.1667. [DOI] [PubMed] [Google Scholar]
- 26.Collins AJ, Ebben JP, Gilbertson DT. EPO adjustments in patients with elevated hemoglobin levels: provider practice patterns compared with recommended practice guidelines. Am J Kidney Dis. 2007;49:135–142. doi: 10.1053/j.ajkd.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Hirth RA, Turenne MN, Wheeler JRC, Ma Y, Messana JM. Do resource utilization and clinical measures still vary across dialysis chains after controlling for the local practices of facilities and physicians? Medical Care. 2010;48(8):726–732. doi: 10.1097/MLR.0b013e3181e3570a. [DOI] [PubMed] [Google Scholar]